Phylogenetic Relationship of Plant MLO Genes and Transcriptional Response of MLO Genes to Ralstonia solanacearum in Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Species
2.2. Identification of Plant MLO Genes
2.3. Variation Analysis of Plant MLO Gene Sequences
2.4. Chromosome Localization of Plant MLO Genes
2.5. Systematic Cluster Analysis of Plant MLO Genes
2.6. Promoter and miRNA Analysis of MLO Genes in Tomato
2.7. Digital Expression Analysis of MLO Genes in Tomato
2.8. Expression Analysis of MLO Genes in Response to R. solanacearum in Tomato
3. Results
3.1. Basic Characteristics of MLO Genes
3.2. Sequence Variation of MLO Genes
3.3. Distribution of MLO Genes in Genomes
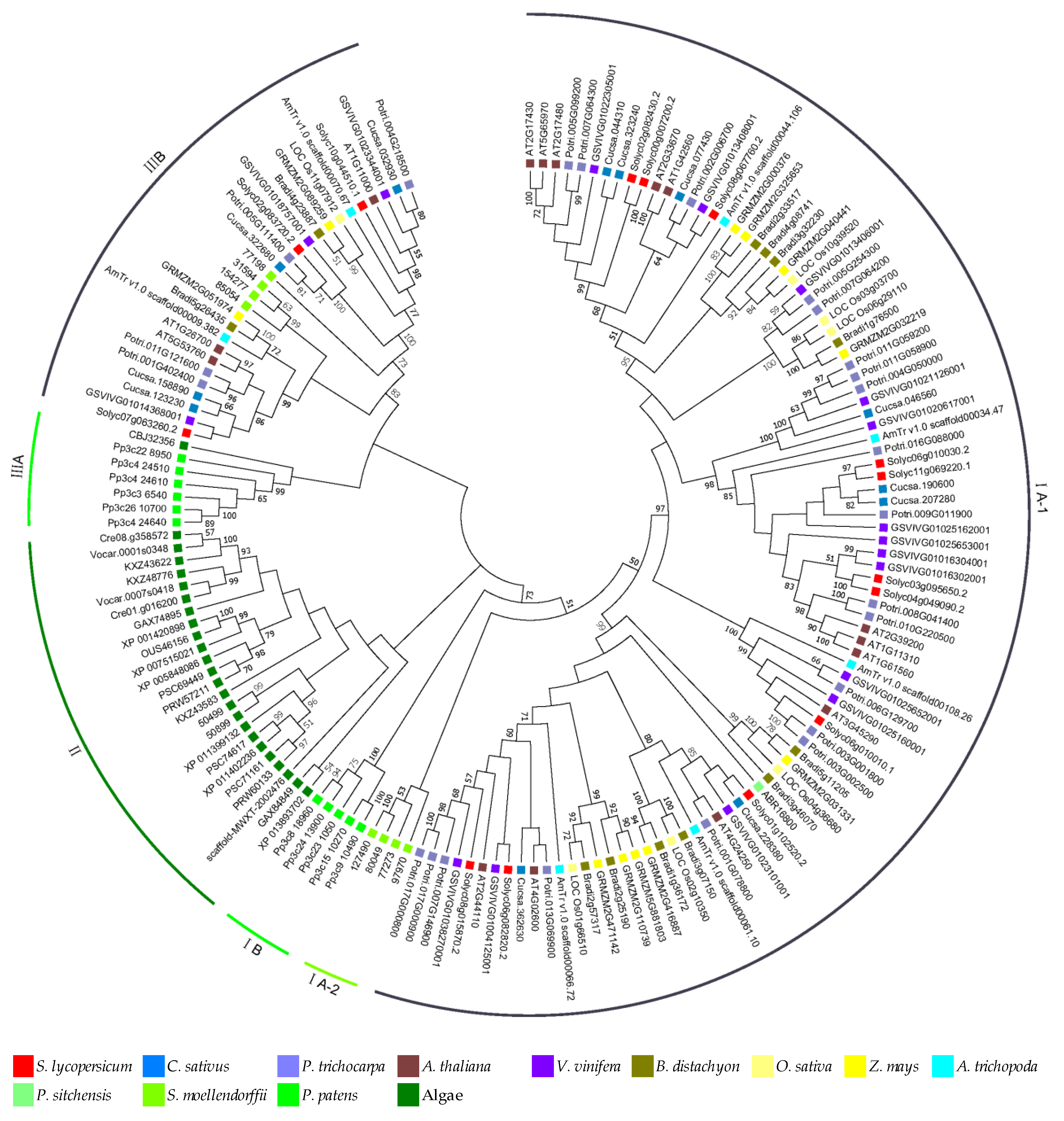
3.4. Phylogenetic Relationships of MLO Genes
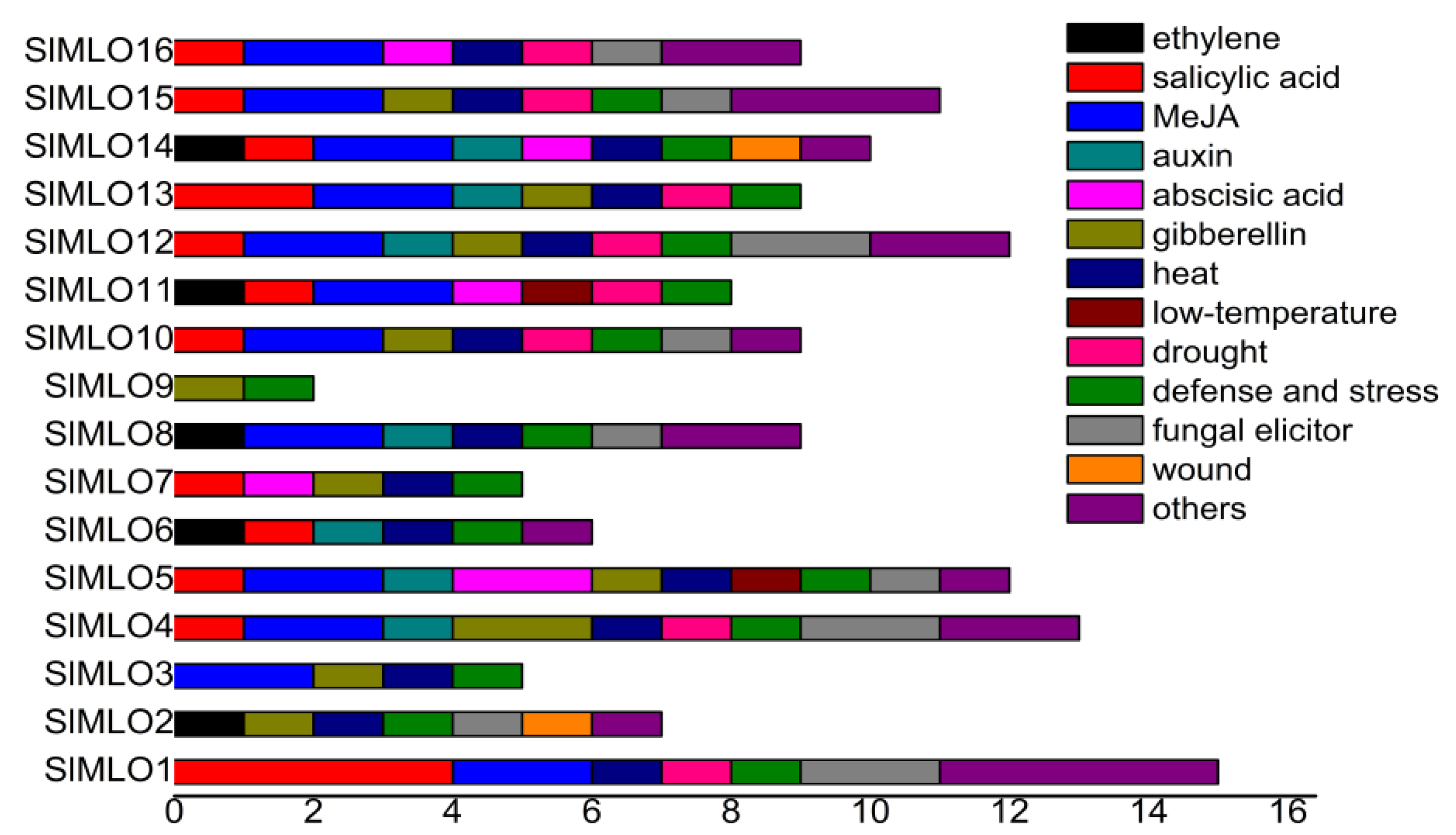
3.5. Promoter Elements of SlMLO Genes
3.6. miRNAs Targeting SlMLO Genes
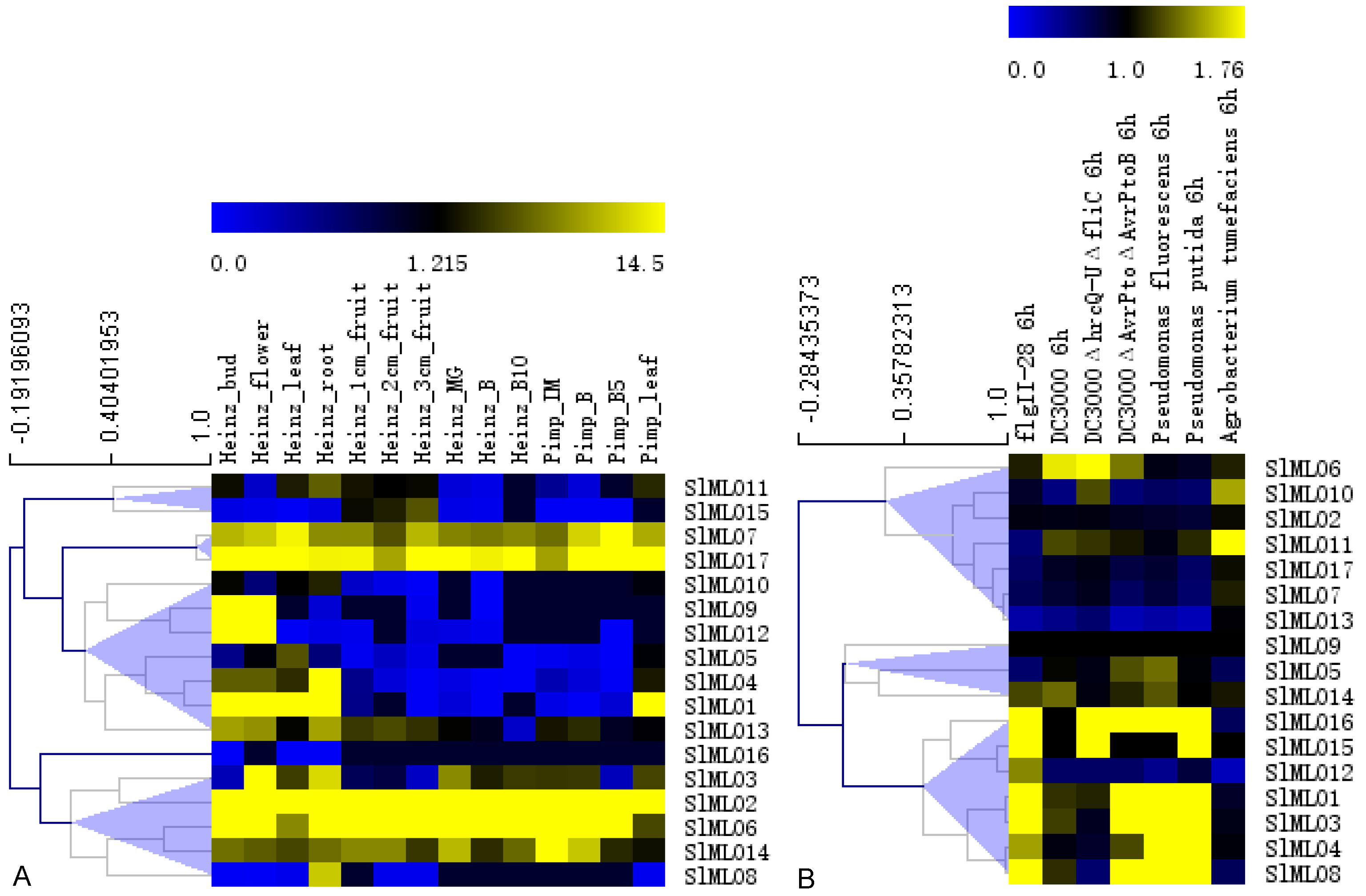
3.7. Digital Expression of SlMLO Genes
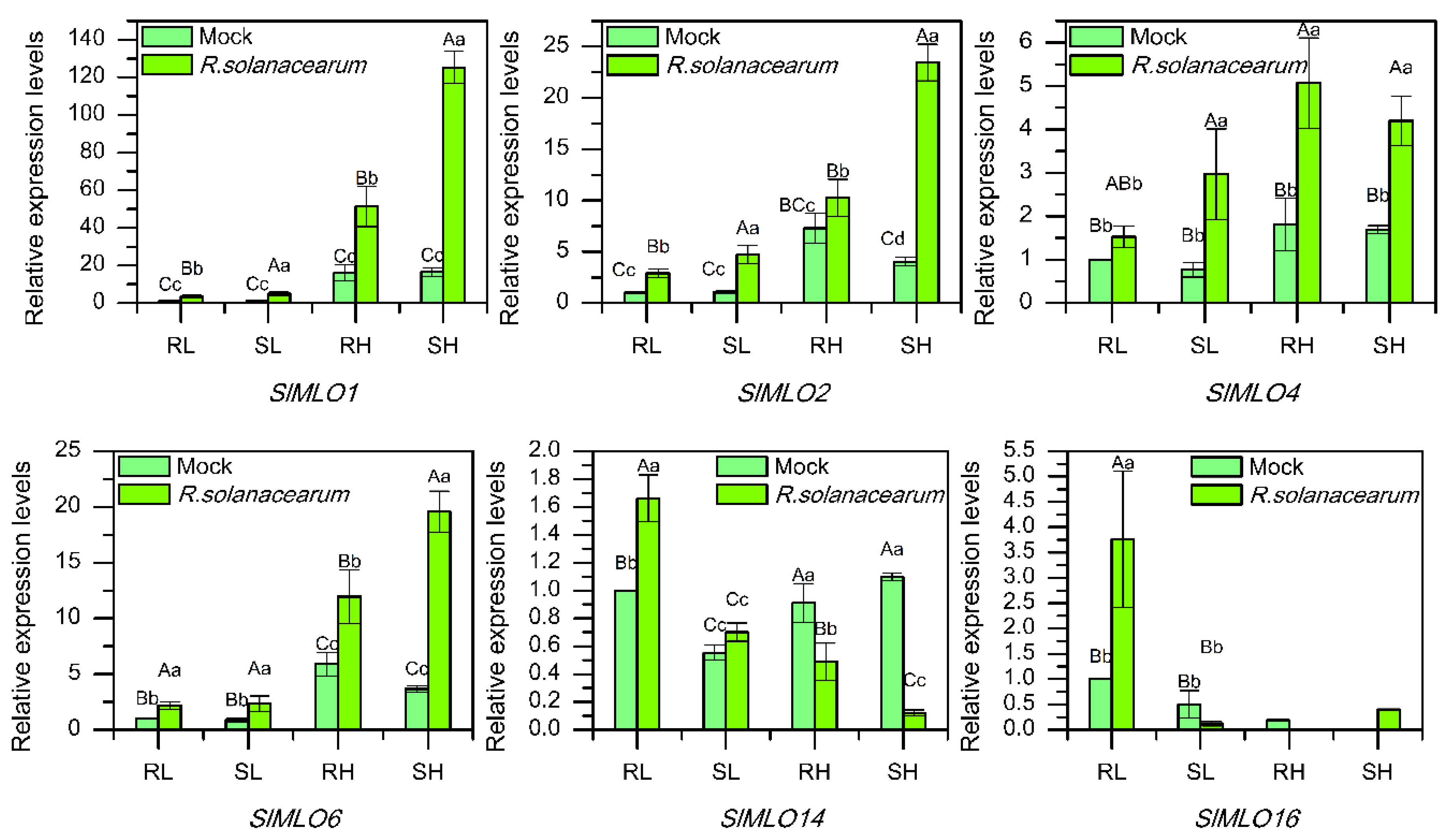
3.8. Transcriptional Response of SlMLO Genes to R. solanacearum
4. Discussion
4.1. MLO Genes had Specific Sequence Characteristics in Different Species
4.2. Duplication Was Widespread in Plant MLO Genes
4.3. Numerous Homologs Were Ascertained in the Phylogenetic Relationships
4.4. MLO Genes Could Respond to Various Biotic Stresses in Tomato
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, D.S.; Hwang, B.K. The pepper MLO gene, CaMLO2, is involved in the susceptibility cell-death response and bacterial and oomycete proliferation. Plant. J. 2012, 72, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Choi, H.W.; Hwang, B.K. Pepper mildew resistance locus O interacts with pepper calmodulin and suppresses Xanthomonas AvrBsT-triggered cell death and defense responses. Planta 2014, 240, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, S.C. Functional roles of the pepper MLO protein gene, CaMLO2, in abscisic acid signaling and drought sensitivity. Plant. Mol. Biol. 2014, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.T.; Vo, K.T.X.; Park, H.; Jeon, J.-S.; Jung, K.-H. A systematic view of the MLO family in rice suggests their novel roles in morphological development, diurnal responses, the light-signaling pathway, and various stress responses. Front. Plant. Sci. 2016, 7, 1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo-Garcia, J.; Gruner, K.; Reinstädler, A.; Kemen, A.; Kemen, E.; Cao, L.; Takken, F.L.W.; Reitz, M.U.; Schäfer, P.; O’Connell, R.J.; et al. The powdery mildew-resistant Arabidopsis mlo2 mlo6 mlo12 triple mutant displays altered infection phenotypes with diverse types of phytopathogens. Sci. Rep. 2017, 7, 9319. [Google Scholar] [CrossRef]
- Howlader, J.; Park, J.-I.; Kim, H.-T.; Ahmed, N.U.; Robin, A.H.K.; Sumi, K.R.; Natarajan, S.; Nou, I.-S. Differential expression under Podosphaera xanthii and abiotic stresses reveals candidate MLO family genes in Cucumis melo L. Trop. Plant. Biol. 2017. [Google Scholar] [CrossRef]
- Jørgensen, J.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 1992, 63, 141–152. [Google Scholar] [CrossRef]
- Kim, M.C.; Lee, S.H.; Kim, J.K.; Chun, H.J.; Choi, M.S.; Chung, W.S.; Moon, B.C.; Kang, C.H.; Park, C.Y.; Yoo, J.H.; et al. Mlo, a modulator of plant defense and cell death, is a novel calmodulin-binding protein. J. Biol. Chem. 2002, 277, 19304–19314. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhu, H. Molecular evolution of the MLO gene family in Oryza sativa and their functional divergence. Gene 2008, 409, 1–10. [Google Scholar] [CrossRef]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulze–Lefert, P.; et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef]
- Zheng, Z.; Appiano, M.; Pavan, S.; Bracuto, V.; Ricciardi, L.; Visser, R.G.F.; Wolters, A.-M.A.; Bai, Y. Genome-wide study of the tomato SlMLO gene family and its functional characterization in response to the powdery mildew fungus Oidium neolycopersici. Front. Plant. Sci. 2016, 7, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feechan, A.; Jermakow, A.M.; Torregrosa, L.; Panstruga, R.; Dry, I.B. Identification of grapevine MLO gene candidates involved in susceptibility to powdery mildew. Funct. Plant. Biol. 2008, 35, 1255–1266. [Google Scholar] [CrossRef]
- Zhou, S.J.; Jing, Z.; Shi, J.L. Genome-wide identification, characterization, and expression analysis of the MLO gene family in Cucumis sativus. Genet. Mol. Res. 2013, 12, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Pessina, S.; Pavan, S.; Catalano, D.; Gallotta, A.; Visser, R.G.F.; Bai, Y.; Malnoy, M.; Schouten, H.J. Characterization of the MLO gene family in Rosaceae and gene expression analysis in Malus domestica. BMC Genom. 2014, 15, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, C.; Chand, R.; Singh, V.K.; Singh, A.K.; Kushwaha, C. Identification and characterisation of Mlo genes in pea (Pisum sativum L.) vis-à-vis validation of Mlo gene-specific markers. Turk. J. Biol. 2016, 40, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ma, Q.; Dou, L.; Liu, Z.; Peng, R.; Yu, S. Genome-wide characterization and comparative analysis of the MLO gene family in cotton. Plant. Physiol. Biochem. 2016, 103, 106–119. [Google Scholar] [CrossRef]
- Filiz, E.; Vatansever, R. Genome-wide identification of mildew resistance locus O (MLO) genes in tree model poplar (Populus trichocarpa): Powdery mildew management in woody plants. Eur. J. Plant. Pathol. 2018, 152, 95–109. [Google Scholar] [CrossRef]
- Polanco, C.; SaÂenz de Miera, L.E.; Bett, K.; PeÂrez de la Vega, M. A genome-wide identification and comparative analysis of the lentil MLO genes. PLoS ONE 2018, 13, e0194945. [Google Scholar] [CrossRef]
- Win, K.T.; Zhang, C.Y.; Lee, S. Genome-wide identification and description of MLO family genes in pumpkin (Cucurbita maxima Duch.). Hortic. Environ. Biotechnol. 2018, 59, 397–410. [Google Scholar] [CrossRef]
- Appiano, M.; Catalano, D.; Martínez, M.S.; Lotti, C.; Zheng, Z.; Visser, R.G.F.; Ricciardi, L.; Bai, Y.; Pavan, S. Monocot and dicot MLO powdery mildew susceptibility factors are functionally conserved in spite of the evolution of class-specific molecular features. BMC Plant. Biol. 2015, 15, 257. [Google Scholar] [CrossRef] [Green Version]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant. Cell 2003, 15, 809–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, X.; Yue, J.X.; Tian, D.; Chen, J.-Q. Recent duplications dominate NBS-encoding gene expansion in two woody species. Mol. Genet. Genomics 2008, 280, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, Y.; Liu, J.; Peng, X.; Niu, X.; Fei, Z.; Cao, S.; Liu, Y. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 2012, 287, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. BioRxiv 2018. [Google Scholar] [CrossRef]
- Devoto, A.; Hartmann, H.A.; Piffanelli, P.; Elliott, C.; Simmons, C.; Taramino, G.; Goh, C.-S.; Cohen, F.E.; Emerson, B.C.; Schulze-Lefert, P.; et al. Molecular phylogeny and evolution of the plant-specific seven-transmembrane MLO family. J. Mol. Evol. 2003, 56, 77–88. [Google Scholar] [CrossRef]
- Kusch, S.; Pesch, L.; Panstruga, R. Comprehensive phylogenetic analysis sheds light on the diversity and origin of the MLO family of integral membrane proteins. Genome Biol. Evol. 2016, 8, 878–895. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, L.; Li, H.; Sahu, S.K.; Wang, H.; Xu, Y.; Xian, W.; Song, B.; Liang, H.; Cheng, S.; et al. Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nat. Plants 2019. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, J. Genome-wide analysis of NBS-LRR genes in sorghum genome revealed several events contributing to NBS-LRR gene evolution in grass species. Evol. Bioinform. 2016, 12, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Cheng, Y.; Feng, K.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Yang, Y.; et al. Genome-wide identification and expression profiling of tomato hsp20 gene family in response to biotic and abiotic stresses. Front. Plant. Sci. 2016, 7, 1215. [Google Scholar] [CrossRef] [Green Version]
- Schauser, L.; Wieloch, W.; Stougaard, J. Evolution of NIN-like proteins in Arabidopsis, rice and Lotus japonicas. J. Mol. Evol. 2005, 60, 229–237. [Google Scholar] [CrossRef]
- Deshmukh, R.; Singh, V.K.; Singh, B.D. Comparative phylogenetic analysis of genome-wide Mlo gene family members from Glycine max and Arabidopsis thaliana. Mol. Genet. Genom. 2014, 289, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.A.; Appiano, M.; Bijsterbosch, G.; Visser, R.G.F.; Schouten, H.J.; Bai, Y. Functional characterization of cucumber (Cucumis sativus L.) Clade V MLO genes. BMC Plant. Biol. 2017, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Y.; Hu, S.; Guo, Y. Genome-wide survey and phylogenetic analysis of MADS-box gene family in Brassica napus. Chin. Bull. Bot. 2017, 52, 699–712. [Google Scholar] [CrossRef]
- Yan, P.; Zhou, S.; Li, X.; Zhao, S.; Zhou, H.; Zhou, Y.H.; Xu, S.L.; Ke, T. Genome-wide comparative analysis of MLO related genes in Brassica lineage. Chin. J. Oil Crop. Sci. 2017, 39, 729–736. [Google Scholar] [CrossRef]
- Edwards, A.W.F. The genetical theory of natural selection. Genetics 2000, 154, 1419–1426. [Google Scholar]
- Zhai, J.; Jeong, D.-H.; De Paoli, E.; Park, S.; Rosen, B.D.; Li, Y.; González, A.J.; Yan, Z.; Kitto, S.L.; Grusak, M.A.; et al. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 2011, 25, 2540–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, N.; Zhang, M.Q. Functional genomics as applied to mapping transcription regulatory networks. Curr. Opin. Microbiol. 2002, 5, 313–317. [Google Scholar] [CrossRef]
- Andolfo, G.; Iovieno, P.; Ricciardi, L.; Lotti, C.; Filippone, E.; Pavan, S.; Ercolano, M.R. Evolutionary conservation of MLO gene promoter signatures. BMC Plant. Biol. 2019, 19, 150. [Google Scholar] [CrossRef] [Green Version]

| Species | Genome Size/Mb * | Lineage |
|---|---|---|
| Solanum lycopersicum L. | 792.04 | Dicot |
| Cucumis sativus L. | 323.99 | |
| Populus trichocarpa Torr. & Gary | 434.29 | |
| Arabidopsis thaliana (L.) Heynh | 119.67 | |
| Vitis vinifera L. | 427.19 | |
| Brachypodium distachyon (L.) Beauv. | 218.35 | Monocot |
| Oryza sativa L. | 383.72 | |
| Zea mays L. | 2171.65 | |
| Amborella trichopoda Baill. | 706.50 | Basalmost angiosperm |
| Picea sitchensis Engelm | 18225.20 | Gymnosperm |
| Selaginella moellendorffii Hieron. | 212.32 | Fern |
| Physcomitrella patens (Hedw.) Mitt. | 472.08 | Bryophyte |
| Volvox carteri F.Stein | 137.68 | Algae |
| Chlamydomonas reinhardtii P.A. Dangeard | 120.41 | |
| Chlorella variabilis | 46.16 | |
| Chlorella sorokiniana | 58.61 | |
| Gonium pectorale O.F. Müller | 148.81 | |
| Monoraphidium neglectum | 69.71 | |
| Auxenochlorella protothecoides (Krüger) T. Kalina & M. Puncochárová | 22.92 | |
| Micractinium conductrix | 61.02 | |
| Chlamydomonas eustigma | 66.63 | |
| Micromonas sp. RCC299 | 21.11 | |
| Micromonas pusilla CCMP1545 | 21.96 | |
| Ostreococcus tauri | 13.90 | |
| Ostreococcus lucimarinus | 13.20 | |
| Bathycoccus prasinos W. Eikrem & J. Throndsen | 15.07 | |
| Chara vulgaris L. | / | |
| Ectocarpus siliculosus (Dillw.) Lyngb. | 195.81 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| SlRPL2 | GTCATCCTTTCAGGTACAAGCA | CGTTACAAACAACAGCTCCTTC |
| SlMLO1 | GCAAACAGCAGACCAACCA | TTTCATTAGCCCACCCTTCA |
| SlMLO2 | CGCGTGCTTGAAGCTGAT | GACCAAAGGGAACAAATGCTA |
| SlMLO4 | CAAGGTCCTCTGTGGGTTCA | GCACGGATTATCGGTGTAGTT |
| SlMLO6 | TGAATGTTAGCGGGTGGC | AAGGCAAAATGAATGAGGTGA |
| SlMLO14 | GTGGGGATTTGTGGTGGG | AAGTTCGTCTCGTGGTTTTAGC |
| SlMLO16 | TGGCTTCATTACGGCACAT | CTCCAACTTAGTCCCAATCACC |
| Species | S | % S | SP | PIP | Eta | h | Hd | Pi | K |
|---|---|---|---|---|---|---|---|---|---|
| Solanum lycopersicum | 863 | 82.66 | 94 | 769 | 1818 | 14 | 1.000 ± 0.027 | 0.45364 | 473.604 |
| Cucumis sativus | 747 | 80.06 | 98 | 649 | 1513 | 12 | 1.000 ± 0.034 | 0.44781 | 417.803 |
| Populus trichocarpa | 882 | 85.22 | 84 | 798 | 1878 | 24 | 1.000 ± 0.012 | 0.42523 | 440.112 |
| Arabidopsis thaliana | 863 | 81.49 | 73 | 790 | 1799 | 15 | 1.000 ± 0.024 | 0.44542 | 471.705 |
| Vitis vinifera | 942 | 81.35 | 79 | 863 | 2013 | 17 | 1.000 ± 0.020 | 0.43146 | 499.625 |
| Brachypodium distachyon | 885 | 79.73 | 137 | 748 | 1794 | 12 | 1.000 ± 0.034 | 0.43561 | 483.530 |
| Oryza sativa | 859 | 77.18 | 241 | 618 | 1502 | 7 | 1.000 ± 0.076 | 0.45463 | 506.000 |
| Zea mays | 745 | 78.59 | 110 | 635 | 1479 | 11 | 1.000 ± 0.039 | 0.43750 | 414.745 |
| Amborella trichopoda | 1106 | 77.61 | 257 | 849 | 2018 | 7 | 1.000 ± 0.076 | 0.47549 | 677.571 |
| Selaginella moellendorffii | 631 | 72.03 | 114 | 517 | 1103 | 8 | 1.000 ± 0.063 | 0.41553 | 364.000 |
| Physcomitrella patens | 870 | 78.80 | 112 | 758 | 1762 | 11 | 1.000 ± 0.039 | 0.45469 | 501.982 |
| Volvox carteri | 1750 | 53.42 | 1750 | 0 | 1750 | 2 | 1.000 ± 0.500 | 0.53419 | 1750.000 |
| Chlamydomonas reinhardtii | 2591 | 58.47 | 2591 | 0 | 2591 | 2 | 1.000 ± 0.500 | 0.58474 | 2591.000 |
| Species | D | D * | F * | Rm | SS | NSS | Ka/Ks |
|---|---|---|---|---|---|---|---|
| Solanum lycopersicum | −0.77822 | 0.26621 | −0.02567 | 102 | 237.08 | 806.92 | 0.2144 |
| Cucumis sativus | −0.78581 | 0.27567 | −0.00579 | 90 | 213.24 | 719.76 | 0.1617 |
| Populus trichocarpa | −0.50659 | 0.58373 | 0.27683 | 97 | 237.70 | 797.30 | 0.1830 |
| Arabidopsis thaliana | −0.65747 | 0.43878 | 0.15009 | 118 | 239.48 | 819.52 | 0.1896 |
| Vitis vinifera | −0.69747 | 0.44997 | 0.13406 | 122 | 265.47 | 892.53 | 0.1714 |
| Brachypodium distachyon | −0.88048 | 0.08421 | −0.19513 | 115 | 257.26 | 852.74 | 0.2636 |
| Oryza sativa | −1.02985 | −0.14537 | −0.38216 | 106 | 256.12 | 856.88 | 0.2535 |
| Zea mays | −0.86823 | 0.05308 | −0.20977 | 100 | 215.59 | 732.41 | 0.2635 |
| Amborella trichopoda | −1.04622 | −0.06242 | −0.31490 | 156 | 321.55 | 1103.45 | 0.1958 |
| Selaginella moellendorffii | −0.79390 | 0.09768 | −0.12459 | 88 | 203.06 | 672.94 | 0.1530 |
| Physcomitrella patens | −0.80473 | 0.12445 | −0.13098 | 98 | 259.36 | 844.64 | 0.2215 |
| Volvox carteri | / | / | / | 0 | 854.17 | 2421.83 | 0.4734 |
| Chlamydomonas reinhardtii | / | / | / | 0 | 1230.42 | 3200.58 | 0.9794 |
| Gene Name | Gene ID | miRNA ID | Accession no. | Mature Sequence |
|---|---|---|---|---|
| SlMLO2 | Solyc08g015870 | sly-miR396a-5p | MIMAT0035455 | UUCCACAGCUUUCUUGAACUG |
| sly-miR396b | MIMAT0035481 | UUCCACAGCUUUCUUGAACUU | ||
| sly-miR6027-5p | MIMAT0032133 | AUGGGUAGCACAAGGAUUAAUG | ||
| sly-miR167a | MIMAT0007917 | UGAAGCUGCCAGCAUGAUCUA | ||
| sly-miR167b-5p | MIMAT0035457 | UAAAGCUGCCAGCAUGAUCUGG | ||
| sly-miR1917 | MIMAT0007909 | AUUAAUAAAGAGUGCUAAAGU | ||
| SlMLO3 | Solyc06g010030 | sly-miR6027-5p | MIMAT0032133 | AUGGGUAGCACAAGGAUUAAUG |
| SlMLO4 | Solyc00g007200 | sly-miR156a | MIMAT0009138 | UUGACAGAAGAUAGAGAGCAC |
| sly-miR156b | MIMAT0009139 | UUGACAGAAGAUAGAGAGCAC | ||
| sly-miR156c | MIMAT0009140 | UUGACAGAAGAUAGAGAGCAC | ||
| sly-miR482c | MIMAT0023603 | UCUUGCCAAUACCGCCCAUUCC | ||
| SlMLO5 | Solyc03g095650 | sly-miR6027-5p | MIMAT0032133 | AUGGGUAGCACAAGGAUUAAUG |
| sly-miR9469-3p | MIMAT0035436 | AUUCGGUCUUCUUAUGUGGAC | ||
| SlMLO7 | Solyc09g018830 | sly-miR172a | MIMAT0009143 | AGAAUCUUGAUGAUGCUGCAU |
| sly-miR172b | MIMAT0009144 | AGAAUCUUGAUGAUGCUGCAU | ||
| sly-miR1918 | MIMAT0007910 | UGUUGGUGAGAGUUCGAUUCUC | ||
| SlMLO8 | Solyc11g069220 | sly-miR6027-5p | MIMAT0032133 | AUGGGUAGCACAAGGAUUAAUG |
| sly-miR9470-3p | MIMAT0035440 | UUUGGCUCAUGGAUUUUAGC | ||
| sly-miR9478-3p | MIMAT0035474 | UUCGAUGACAUAUUUGAGCCU | ||
| SlMLO10 | Solyc02g083720 | sly-miR6022 | MIMAT0023590 | UGGAAGGGAGAAUAUCCAGGA |
| sly-miR9474-5p | MIMAT0035463 | UGUAGAAGUCAUGAAUAAAAUG | ||
| SlMLO12 | Solyc08g067760 | sly-miR482e-3p | MIMAT0032124 | UCUUUCCUACUCCUCCCAUACC |
| sly-miR482d-5p | MIMAT0035459 | GGAGUGGGUGGGAUGGAAAAA | ||
| sly-miR156a | MIMAT0009138 | UUGACAGAAGAUAGAGAGCAC | ||
| sly-miR156b | MIMAT0009139 | UUGACAGAAGAUAGAGAGCAC | ||
| sly-miR156c | MIMAT0009140 | UUGACAGAAGAUAGAGAGCAC | ||
| sly-miR172a | MIMAT0009143 | AGAAUCUUGAUGAUGCUGCAU | ||
| sly-miR172b | MIMAT0009144 | AGAAUCUUGAUGAUGCUGCAU | ||
| sly-miR6024 | MIMAT0023594 | UUUUAGCAAGAGUUGUUUUACC | ||
| SlMLO13 | Solyc10g044510 | sly-miR6027-3p | MIMAT0023611 | UGAAUCCUUCGGCUAUCCAUAA |
| sly-miR156e-5p | MIMAT0035453 | UGAUAGAAGAGAGUGAGCAC | ||
| sly-miR9472-3p | MIMAT0035450 | UUCACAAUCUCUGCUGAAAAA | ||
| SlMLO14 | Solyc07g063260 | sly-miR9469-3p | MIMAT0035436 | AUUCGGUCUUCUUAUGUGGAC |
| sly-miR1916 | MIMAT0007908 | AUUUCACUUAGACACCUCAA | ||
| SlMLO15 | Solyc02g077570 | sly-miR6022 | MIMAT0023590 | UGGAAGGGAGAAUAUCCAGGA |
| SlMLO16 | Solyc06g010010 | sly-miR6025 | MIMAT0042023 | UACCAAUAAUUGAGAUAACAUC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Wan, H.; Zai, W.; Xiong, Z.; Wu, W. Phylogenetic Relationship of Plant MLO Genes and Transcriptional Response of MLO Genes to Ralstonia solanacearum in Tomato. Genes 2020, 11, 487. https://doi.org/10.3390/genes11050487
Shi J, Wan H, Zai W, Xiong Z, Wu W. Phylogenetic Relationship of Plant MLO Genes and Transcriptional Response of MLO Genes to Ralstonia solanacearum in Tomato. Genes. 2020; 11(5):487. https://doi.org/10.3390/genes11050487
Chicago/Turabian StyleShi, Jianlei, Hongjian Wan, Wenshan Zai, Zili Xiong, and Weiren Wu. 2020. "Phylogenetic Relationship of Plant MLO Genes and Transcriptional Response of MLO Genes to Ralstonia solanacearum in Tomato" Genes 11, no. 5: 487. https://doi.org/10.3390/genes11050487





