Rolling-Circle Replication in Mitochondrial DNA Inheritance: Scientific Evidence and Significance from Yeast to Human Cells
Abstract
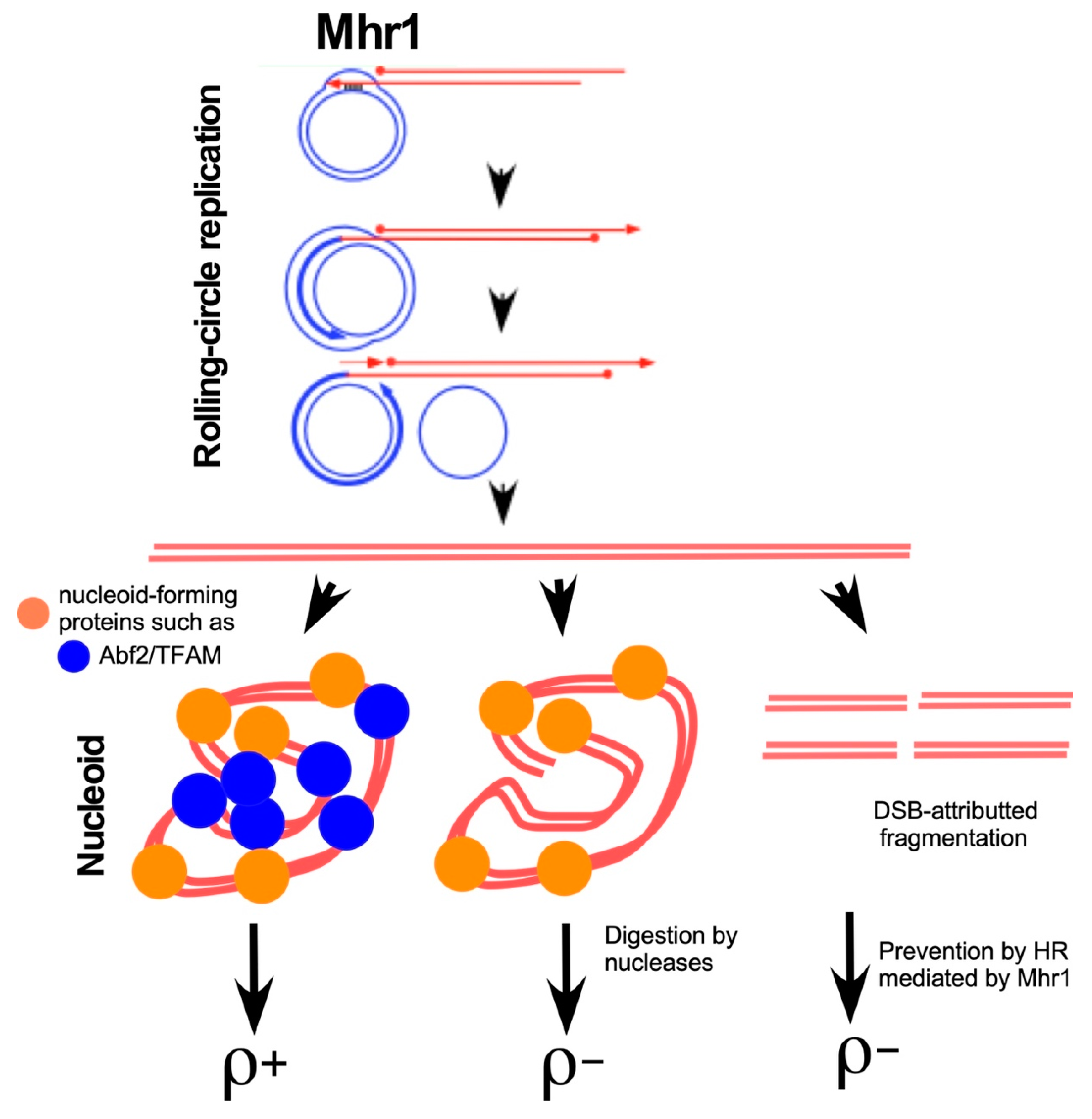
:1. Introduction
2. The Origin of θ-Type mtDNA Replication
3. The Main Problems of θ-Type Replication Mode for the Explanation of the Rapid Segregation of mt-Alleles towards Homoplasmy
4. Why Linear Double-Stranded mtDNA Is Undetectable
5. Evidence for Human mtDNA Recombination
6. The Rolling-Circle mtDNA Replication Mode is Universal
7. The Mhr1-Driven Mechanism of Rolling Circle mtDNA Replication in Yeast
8. Roles of RdRR in Mitochondrial Dynamics and Maintenance of mtDNA Integrity
9. Significance of the mtDNA Recombination-Driven Rolling-Circle mtDNA Replication
10. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species; |
| mtDNA | mitochondrial DNA |
| DSBs | double-stranded DNA breaks |
| S. cerevisiae | Saccharomyces cerevisiae; |
| MGME1 | mitochondrial genome maintenance exonuclease 1 |
| POLG | DNA polymerase subunit γ |
| PFGE | pulsed-field gel electrophoresis |
| 2D gel electrophoresis | two-dimensional gel electrophoresis |
References
- Ernster, L.; Schatz, G. Mitochondria: A historical review. J. Cell Biol. 1981, 91 Pt 2, 227s–255s. [Google Scholar] [CrossRef]
- Willis, E.J. The powerhouse of the cell. Ultrastruct. Pathol. 1992, 16, iii–vi. [Google Scholar] [CrossRef]
- Birnboim, H.C. DNA strand breaks in human leukocytes induced by superoxide anion, hydrogen peroxide and tumor promoters are repaired slowly compared to breaks induced by ionizing radiation. Carcinogenesis 1986, 7, 1511–1517. [Google Scholar] [CrossRef]
- Vawter, L.; Brown, W.M. Nuclear and mitochondrial DNA comparisons reveal extreme rate variation in the molecular clock. Science 1986, 234, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Neiman, M.; Taylor, D.R. The causes of mutation accumulation in mitochondrial genomes. Proc. Biol. Sci. 2009, 276, 1201–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Ma, H.; Juanes, R.C.; Tachibana, M.; Sparman, M.; Woodward, J.; Ramsey, C.; Xu, J.; Kang, E.J.; Amato, P.; et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012, 1, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauswirth, W.W.; Laipis, P.J. Mitochondrial DNA polymorphism in a maternal lineage of Holstein cows. Proc. Natl. Acad. Sci. USA 1982, 79, 4686–4690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, M.V.; Laipis, P.J.; Hauswirth, W.W. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res. 1989, 17, 7325–7331. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shitara, H.; Horii, T.; Nagao, Y.; Imai, H.; Abe, K.; Hara, T.; Hayashi, J.; Yonekawa, H. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat. Genet. 2007, 39, 386–390. [Google Scholar] [CrossRef]
- Cree, L.M.; Samuels, D.C.; de Sousa Lopes, S.C.; Rajasimha, H.K.; Wonnapinij, P.; Mann, J.R.; Dahl, H.H.; Chinnery, P.F. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet. 2008, 40, 249–254. [Google Scholar] [CrossRef]
- Khrapko, K. Two ways to make an mtDNA bottleneck. Nat. Genet. 2008, 40, 134–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wai, T.; Teoli, D.; Shoubridge, E.A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 2008, 40, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Robberson, D.L.; Kasamatsu, H.; Vinograd, J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc. Natl. Acad. Sci. USA 1972, 69, 737–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, D.A. Replication of animal mitochondrial DNA. Cell 1982, 28, 693–705. [Google Scholar] [CrossRef]
- Yasukawa, T.; Reyes, A.; Cluett, T.J.; Yang, M.Y.; Bowmaker, M.; Jacobs, H.T.; Holt, I.J. Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. EMBO J. 2006, 25, 5358–5371. [Google Scholar] [CrossRef]
- Zinovkina, L.A. DNA Replication in Human Mitochondria. Biochemistry (Moscow) 2019, 84, 884–895. [Google Scholar] [CrossRef]
- Yasukawa, T.; Kang, D. An overview of mammalian mitochondrial DNA replication mechanisms. J. Biochem. 2018, 164, 183–193. [Google Scholar] [CrossRef]
- Ling, F.; Niu, R.; Hatakeyama, H.; Goto, Y.; Shibata, T.; Yoshida, M. Reactive oxygen species stimulate mitochondrial allele segregation toward homoplasmy in human cells. Mol. Biol. Cell 2016, 27, 1684–1693. [Google Scholar] [CrossRef]
- Ono, T.; Isobe, K.; Nakada, K.; Hayashi, J.I. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat. Genet. 2001, 28, 272–275. [Google Scholar] [CrossRef]
- Kang, E.; Wang, X.; Tippner-Hedges, R.; Ma, H.; Folmes, C.D.; Gutierrez, N.M.; Lee, Y.; Van Dyken, C.; Ahmed, R.; Li, Y.; et al. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human iPSCs. Cell Stem Cell 2016, 18, 625–636. [Google Scholar] [CrossRef]
- Hamalainen, R.H. Mitochondrial DNA mutations in iPS cells: mtDNA integrity as standard iPSC selection criteria? EMBO J. 2016, 35, 1960–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Koch, M.; Spitzer, M.H.; Gravina, A.; Alawi, M.; Marishta, A.; Peters, B.; Kosaloglu-Yalcin, Z.; et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat. Biotechnol. 2019, 37, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Bacman, S.R.; Williams, S.L.; Pinto, M.; Peralta, S.; Moraes, C.T. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 2013, 19, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Bacman, S.R.; Kauppila, J.H.K.; Pereira, C.V.; Nissanka, N.; Miranda, M.; Pinto, M.; Williams, S.L.; Larsson, N.G.; Stewart, J.B.; Moraes, C.T. MitoTALEN reduces mutant mtDNA load and restores tRNA(Ala) levels in a mouse model of heteroplasmic mtDNA mutation. Nat. Med. 2018, 24, 1696–1700. [Google Scholar] [CrossRef]
- Bacman, S.R.; Gammage, P.A.; Minczuk, M.; Moraes, C.T. Manipulation of mitochondrial genes and mtDNA heteroplasmy. Methods Cell Biol. 2020, 155, 441–487. [Google Scholar] [PubMed]
- Bui, E.T.; Bradley, P.J.; Johnson, P.J. A common evolutionary origin for mitochondria and hydrogenosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9651–9656. [Google Scholar] [CrossRef] [Green Version]
- Asai, T.; Bates, D.B.; Kogoma, T. DNA replication triggered by double-stranded breaks in E. coli: Dependence on homologous recombination functions. Cell 1994, 78, 1051–1061. [Google Scholar] [CrossRef] [Green Version]
- Bendich, A.J. The end of the circle for yeast mitochondrial DNA. Mol. Cell 2010, 39, 831–832. [Google Scholar] [CrossRef]
- Lewis, S.C.; Joers, P.; Willcox, S.; Griffith, J.D.; Jacobs, H.T.; Hyman, B.C. A rolling circle replication mechanism produces multimeric lariats of mitochondrial DNA in Caenorhabditis elegans. PLoS Genet. 2015, 11, e1004985. [Google Scholar] [CrossRef] [Green Version]
- Nass, M.M. The circularity of mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1966, 56, 1215–1222. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.H.; Fennell, D.J. Visualization of yeast mitochondrial DNA with the fluorescent stain “DAPI”. Methods Enzymol. 1979, 56, 728–733. [Google Scholar] [PubMed]
- Dujon, B. Mitochondrial genetics and function. In The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance; Strathern, J.N., Jones, E.W., Broach, J.R., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1981; pp. 505–635. [Google Scholar]
- Ling, F.; Shibata, T. Mhr1p-dependent concatemeric mitochondrial DNA formation for generating yeast mitochondrial homoplasmic cells. Mol. Biol. Cell 2004, 15, 310–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birky, C.W., Jr. Transmission genetics of mitochondria and chloroplasts. Annu. Rev. Genet. 1978, 12, 471–512. [Google Scholar] [CrossRef] [PubMed]
- Jenuth, J.P.; Peterson, A.C.; Fu, K.; Shoubridge, E.A. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat. Genet. 1996, 14, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Stampar, S.N.; Broe, M.B.; Macrander, J.; Reitzel, A.M.; Brugler, M.R.; Daly, M. Linear Mitochondrial Genome in Anthozoa (Cnidaria): A Case Study in Ceriantharia. Sci. Rep. 2019, 9, 6094. [Google Scholar] [CrossRef] [Green Version]
- Peeva, V.; Blei, D.; Trombly, G.; Corsi, S.; Szukszto, M.J.; Rebelo-Guiomar, P.; Gammage, P.A.; Kudin, A.P.; Becker, C.; Altmuller, J.; et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018, 9, 1727. [Google Scholar] [CrossRef] [Green Version]
- Matic, S.; Jiang, M.; Nicholls, T.J.; Uhler, J.P.; Dirksen-Schwanenland, C.; Polosa, P.L.; Simard, M.L.; Li, X.; Atanassov, I.; Rackham, O.; et al. Mice lacking the mitochondrial exonuclease MGME1 accumulate mtDNA deletions without developing progeria. Nat. Commun. 2018, 9, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleszka, R.; Skelly, P.J.; Clark-Walker, G.D. Rolling circle replication of DNA in yeast mitochondria. EMBO J. 1991, 10, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Bendich, A.J. Structural analysis of mitochondrial DNA molecules from fungi and plants using moving pictures and pulsed-field gel electrophoresis. J. Mol. Biol. 1996, 255, 564–588. [Google Scholar] [CrossRef]
- Enquist, L.W.; Skalka, A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J. Mol. Biol. 1973, 75, 185–212. [Google Scholar] [CrossRef]
- Silberstein, Z.; Maor, S.; Berger, I.; Cohen, A. Lambda Red-mediated synthesis of plasmid linear multimers in Escherichia coli K12. Mol. Gen. Genet. 1990, 223, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Sommer, S.; Bailone, A.; Kogoma, T. Homologous recombination-dependent initiation of DNA replication from DNA damage-inducible origins in Escherichia coli. EMBO J. 1993, 12, 3287–3295. [Google Scholar] [CrossRef] [PubMed]
- Kogoma, T.; Hong, X.; Cadwell, G.W.; Barnard, K.G.; Asai, T. Requirement of homologous recombination functions for viability of the Escherichia coli cell that lacks RNase HI and exonuclease V activities. Biochimie 1993, 75, 89–99. [Google Scholar] [CrossRef]
- Mosig, G. Recombination and recombination-dependent DNA replication in bacteriophage T4. Annu. Rev. Genet. 1998, 32, 379–413. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Hori, A.; Shibata, T. DNA recombination-initiation plays a role in the extremely biased inheritance of yeast [rho-] mitochondrial DNA that contains the replication origin ori5. Mol. Cell Biol. 2007, 27, 1133–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leadsham, J.E.; Sanders, G.; Giannaki, S.; Bastow, E.L.; Hutton, R.; Naeimi, W.R.; Breitenbach, M.; Gourlay, C.W. Loss of cytochrome c oxidase promotes RAS-dependent ROS production from the ER resident NADPH oxidase, Yno1p, in yeast. Cell Metab. 2013, 18, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Hori, A.; Yoshida, M.; Shibata, T.; Ling, F. Reactive oxygen species regulate DNA copy number in isolated yeast mitochondria by triggering recombination-mediated replication. Nucleic Acids Res. 2009, 37, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, M.E.; Layton, D.A. Conserved features of yeast and mammalian mitochondrial DNA replication. Curr. Opin. Genet. Dev. 1993, 3, 769–774. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Padua, R.A.; Campbell, C. Mammalian mitochondria possess homologous DNA recombination activity. J. Biol. Chem. 1996, 271, 27536–27543. [Google Scholar] [CrossRef] [Green Version]
- Kraytsberg, Y.; Schwartz, M.; Brown, T.A.; Ebralidse, K.; Kunz, W.S.; Clayton, D.A.; Vissing, J.; Khrapko, K. Recombination of human mitochondrial DNA. Science 2004, 304, 981. [Google Scholar] [CrossRef] [Green Version]
- Kajander, O.A.; Karhunen, P.J.; Holt, I.J.; Jacobs, H.T. Prominent mitochondrial DNA recombination intermediates in human heart muscle. EMBO Rep. 2001, 2, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohjoismaki, J.L.; Goffart, S.; Tyynismaa, H.; Willcox, S.; Ide, T.; Kang, D.; Suomalainen, A.; Karhunen, P.J.; Griffith, J.D.; Holt, I.J.; et al. Human heart mitochondrial DNA is organized in complex catenated networks containing abundant four-way junctions and replication forks. J. Biol. Chem. 2009, 284, 21446–21457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldenburg, D.J.; Bendich, A.J. Size and Structure of Replicating Mitochondrial DNA in Cultured Tobacco Cells. Plant Cell 1996, 8, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.Y.; Wilkie, D. Recombination of mitochondrial drug-resistance factors in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1968, 30, 368–372. [Google Scholar] [CrossRef]
- Honda, H.; Hirai, A. The gene for the alpha-subunit of ATPase: A site of homologous recombination in plant mitochondrial DNA also functions in somatic hybrid cells. Theor. Appl. Genet. 1992, 84, 33–38. [Google Scholar] [CrossRef]
- Ling, F.; Makishima, F.; Morishima, N.; Shibata, T. A nuclear mutation defective in mitochondrial recombination in yeast. EMBO J 1995, 14, 4090–4101. [Google Scholar] [CrossRef]
- Prasai, K.; Robinson, L.C.; Scott, R.S.; Tatchell, K.; Harrison, L. Evidence for double-strand break mediated mitochondrial DNA replication in Saccharomyces cerevisiae. Nucleic Acids Res. 2017, 45, 7760–7773. [Google Scholar] [CrossRef] [Green Version]
- Backert, S.; Dorfel, P.; Lurz, R.; Borner, T. Rolling-circle replication of mitochondrial DNA in the higher plant Chenopodium album (L.). Mol. Cell Biol. 1996, 16, 6285–6294. [Google Scholar] [CrossRef] [Green Version]
- Backert, S. R-loop-dependent rolling-circle replication and a new model for DNA concatemer resolution by mitochondrial plasmid mp1. EMBO J. 2002, 21, 3128–3136. [Google Scholar] [CrossRef] [Green Version]
- Shadel, G.S. Yeast as a model for human mtDNA replication. Am. J. Hum. Genet. 1999, 65, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Shibata, T. Recombination-dependent mtDNA partitioning: In vivo role of Mhr1p to promote pairing of homologous DNA. EMBO J. 2002, 21, 4730–4740. [Google Scholar] [CrossRef] [PubMed]
- MacAlpine, D.M.; Perlman, P.S.; Butow, R.A. The numbers of individual mitochondrial DNA molecules and mitochondrial DNA nucleoids in yeast are co-regulated by the general amino acid control pathway. EMBO J. 2000, 19, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleff, S.; Kemper, B.; Sternglanz, R. Identification and characterization of yeast mutants and the gene for a cruciform cutting endonuclease. EMBO J. 1992, 11, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Yoshida, M.; Shibata, T. Heteroduplex joint formation free of net topological change by Mhr1, a mitochondrial recombinase. J. Biol. Chem. 2009, 284, 9341–9353. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Morioka, H.; Ohtsuka, E.; Shibata, T. A role for MHR1, a gene required for mitochondrial genetic recombination, in the repair of damage spontaneously introduced in yeast mtDNA. Nucleic Acids Res. 2000, 28, 4956–4963. [Google Scholar] [CrossRef] [Green Version]
- Ling, F.; Hori, A.; Yoshitani, A.; Niu, R.; Yoshida, M.; Shibata, T. Din7 and Mhr1 expression levels regulate double-strand-break-induced replication and recombination of mtDNA at ori5 in yeast. Nucleic Acids Res. 2013, 41, 5799–5816. [Google Scholar] [CrossRef] [Green Version]
- Prasai, K.; Robinson, L.C.; Tatchell, K.; Harrison, L. Saccharomyces cerevisiae Mhr1 can bind Xho I-induced mitochondrial DNA double-strand breaks in vivo. Mitochondrion 2018, 42, 23–32. [Google Scholar] [CrossRef]
- Chen, X.J.; Guan, M.X.; Clark-Walker, G.D. MGM101, a nuclear gene involved in maintenance of the mitochondrial genome in Saccharomyces cerevisiae. Nucleic Acids Res. 1993, 21, 3473–3477. [Google Scholar] [CrossRef] [Green Version]
- Mbantenkhu, M.; Wang, X.; Nardozzi, J.D.; Wilkens, S.; Hoffman, E.; Patel, A.; Cosgrove, M.S.; Chen, X.J. Mgm101 is a Rad52-related protein required for mitochondrial DNA recombination. J. Biol. Chem. 2011, 286, 42360–42370. [Google Scholar] [CrossRef] [Green Version]
- Mbantenkhu, M.; Wierzbicki, S.; Wang, X.; Guo, S.; Wilkens, S.; Chen, X.J. A short carboxyl-terminal tail is required for single-stranded DNA binding, higher-order structural organization, and stability of the mitochondrial single-stranded annealing protein Mgm101. Mol. Biol. Cell 2013, 24, 1507–1518. [Google Scholar] [CrossRef]
- Nardozzi, J.D.; Wang, X.; Mbantenkhu, M.; Wilkens, S.; Chen, X.J. A properly configured ring structure is critical for the function of the mitochondrial DNA recombination protein, Mgm101. J. Biol. Chem. 2012, 287, 37259–37268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Gorlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaelin, W.G., Jr. ROS: Really involved in oxygen sensing. Cell Metab. 2005, 1, 357–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, E.A.; Shadel, G.S. Alternative mitochondrial fuel extends life span. Cell Metab. 2012, 15, 417–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Schroeder, E.A.; Ocampo, A.; Barrientos, A.; Shadel, G.S. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011, 13, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Jedrychowski, M.P.; Lu, G.Z.; Erickson, B.K.; Szpyt, J.; Pierce, K.A.; Laznik-Bogoslavski, D.; Vetrivelan, R.; Clish, C.B.; et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016, 532, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.J.; Butow, R.A. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 2005, 6, 815–825. [Google Scholar] [CrossRef]
- Lee, S.R.; Han, J. Mitochondrial Nucleoid: Shield and Switch of the Mitochondrial Genome. Oxid. Med. Cell Longev. 2017, 2017, 8060949. [Google Scholar] [CrossRef]
- Diffley, J.F.; Stillman, B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J. Biol. Chem. 1992, 267, 3368–3374. [Google Scholar] [PubMed]
- Newman, S.M.; Zelenaya-Troitskaya, O.; Perlman, P.S.; Butow, R.A. Analysis of mitochondrial DNA nucleoids in wild-type and a mutant strain of Saccharomyces cerevisiae that lacks the mitochondrial HMG box protein Abf2p. Nucleic Acids Res. 1996, 24, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.R.; Friddle, R.; Noy, A.; Baldwin, E.; Martin, S.S.; Corzett, M.; Balhorn, R.; Baskin, R.J. Packaging of single DNA molecules by the yeast mitochondrial protein Abf2p. Biophys. J. 2003, 85, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.E.; Marinov, G.K.; Wold, B.J.; Chan, D.C. Genome-Wide Analysis Reveals Coating of the Mitochondrial Genome by TFAM. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [Green Version]
- Clayton, D.A. Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 1992, 141, 217–232. [Google Scholar]
- Van Dyck, E.; Clayton, D.A. Transcription-dependent DNA transactions in the mitochondrial genome of a yeast hypersuppressive petite mutant. Mol. Cell Biol. 1998, 18, 2976–2985. [Google Scholar] [CrossRef] [Green Version]
- Kukat, C.; Larsson, N.G. mtDNA makes a U-turn for the mitochondrial nucleoid. Trends Cell Biol. 2013, 23, 457–463. [Google Scholar] [CrossRef]
- MacAlpine, D.M.; Perlman, P.S.; Butow, R.A. The high mobility group protein Abf2p influences the level of yeast mitochondrial DNA recombination intermediates in vivo. Proc. Natl. Acad. Sci. USA 1998, 95, 6739–6743. [Google Scholar] [CrossRef] [Green Version]
- Zelenaya-Troitskaya, O.; Newman, S.M.; Okamoto, K.; Perlman, P.S.; Butow, R.A. Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics 1998, 148, 1763–1776. [Google Scholar]
- Ling, F.; Bradshaw, E.; Yoshida, M. Prevention of mitochondrial genomic instability in yeast by the mitochondrial recombinase Mhr1. Sci. Rep. 2019, 9, 5433. [Google Scholar] [CrossRef] [Green Version]
- Niu, R.; Yoshida, M.; Ling, F. Increases in mitochondrial DNA content and 4977-bp deletion upon ATM/Chk2 checkpoint activation in HeLa cells. PLoS ONE 2012, 7, e40572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, A.; Yoshida, M.; Ling, F. Mitochondrial fusion increases the mitochondrial DNA copy number in budding yeast. Genes Cells 2011, 16, 527–544. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, E.S.; Chabbert, C.D.; Klaus, B.; Steinmetz, L.M. A genome-wide map of mitochondrial DNA recombination in yeast. Genetics 2014, 198, 755–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnane, A.W.; Marzuki, S.; Ozawa, T.; Tanaka, M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1989, 1, 642–645. [Google Scholar] [CrossRef]
- Balciuniene, J.; Balciunas, D. A Nuclear mtDNA Concatemer (Mega-NUMT) Could Mimic Paternal Inheritance of Mitochondrial Genome. Front. Genet. 2019, 10, 518. [Google Scholar] [CrossRef]
- Holt, I.J.; Cooper, J.M.; Morgan-Hughes, J.A.; Harding, A.E. Deletions of muscle mitochondrial DNA. Lancet 1988, 1, 1462. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988, 331, 717–719. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA sequence variation in human evolution and disease. Proc. Natl. Acad. Sci. USA 1994, 91, 8739–8746. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev 1997, 77, 425–464. [Google Scholar] [CrossRef]
- Smeitink, J.A.; Zeviani, M.; Turnbull, D.M.; Jacobs, H.T. Mitochondrial medicine: A metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006, 3, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Zhang, Y.; Yan, J.; Chu, C.; Gao, F.; Jiang, Z.; Zhang, X.; Chen, Y.; Wei, X.; Feng, Y.; et al. Mitochondrial DNA mutation m.3243A>G is associated with altered mitochondrial function in peripheral blood mononuclear cells, with heteroplasmy levels and with clinical phenotypes. Diabet. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, S.K.; Hance, N.; El Meziane, A.; Juhola, M.K.; Juhola, K.M.; Karhu, R.; Spelbrink, J.N.; Holt, I.J.; Jacobs, H.T. Genotypic stability, segregation and selection in heteroplasmic human cell lines containing np 3243 mutant mtDNA. Genetics 2000, 154, 363–380. [Google Scholar] [PubMed]
- Raap, A.K.; Jahangir Tafrechi, R.S.; van de Rijke, F.M.; Pyle, A.; Wahlby, C.; Szuhai, K.; Ravelli, R.B.; de Coo, R.F.; Rajasimha, H.K.; Nilsson, M.; et al. Non-random mtDNA segregation patterns indicate a metastable heteroplasmic segregation unit in m.3243A>G cybrid cells. PLoS ONE 2012, 7, e52080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Nonaka, I.; Horai, S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 1990, 348, 651–653. [Google Scholar] [CrossRef]
- Reardon, W.; Ross, R.J.; Sweeney, M.G.; Luxon, L.M.; Pembrey, M.E.; Harding, A.E.; Trembath, R.C. Diabetes mellitus associated with a pathogenic point mutation in mitochondrial DNA. Lancet 1992, 340, 1376–1379. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, F.; Yoshida, M. Rolling-Circle Replication in Mitochondrial DNA Inheritance: Scientific Evidence and Significance from Yeast to Human Cells. Genes 2020, 11, 514. https://doi.org/10.3390/genes11050514
Ling F, Yoshida M. Rolling-Circle Replication in Mitochondrial DNA Inheritance: Scientific Evidence and Significance from Yeast to Human Cells. Genes. 2020; 11(5):514. https://doi.org/10.3390/genes11050514
Chicago/Turabian StyleLing, Feng, and Minoru Yoshida. 2020. "Rolling-Circle Replication in Mitochondrial DNA Inheritance: Scientific Evidence and Significance from Yeast to Human Cells" Genes 11, no. 5: 514. https://doi.org/10.3390/genes11050514
APA StyleLing, F., & Yoshida, M. (2020). Rolling-Circle Replication in Mitochondrial DNA Inheritance: Scientific Evidence and Significance from Yeast to Human Cells. Genes, 11(5), 514. https://doi.org/10.3390/genes11050514




