7q35 Microdeletion and 15q13.3 and Xp22.33 Microduplications in a Patient with Severe Myoclonic Epilepsy, Microcephaly, Dysmorphisms, Severe Psychomotor Delay and Intellectual Disability
Abstract
1. Introduction
2. Materials and Methods
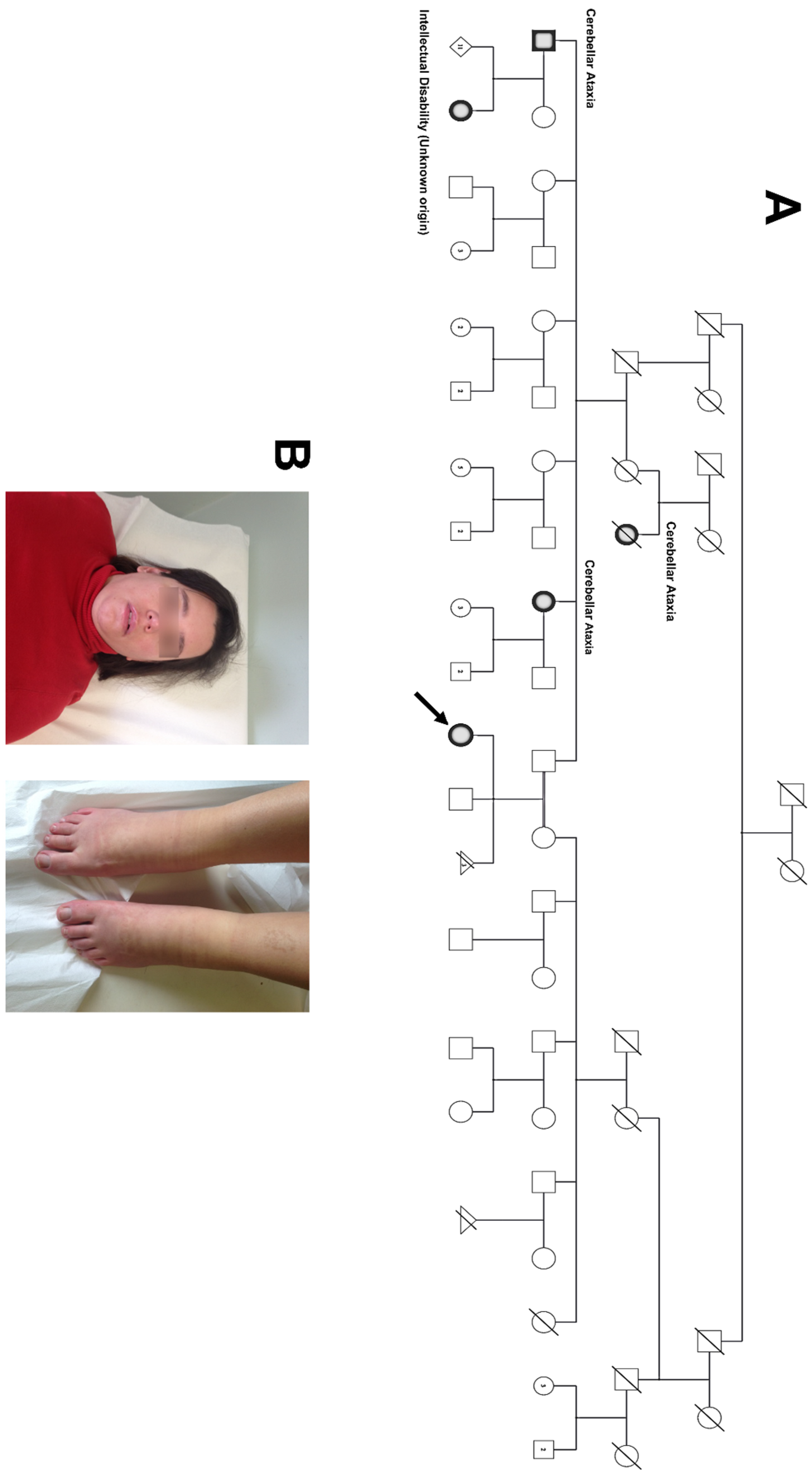
2.1. Proband and Family
2.2. Molecular Analysis
2.3. Statement of Ethics
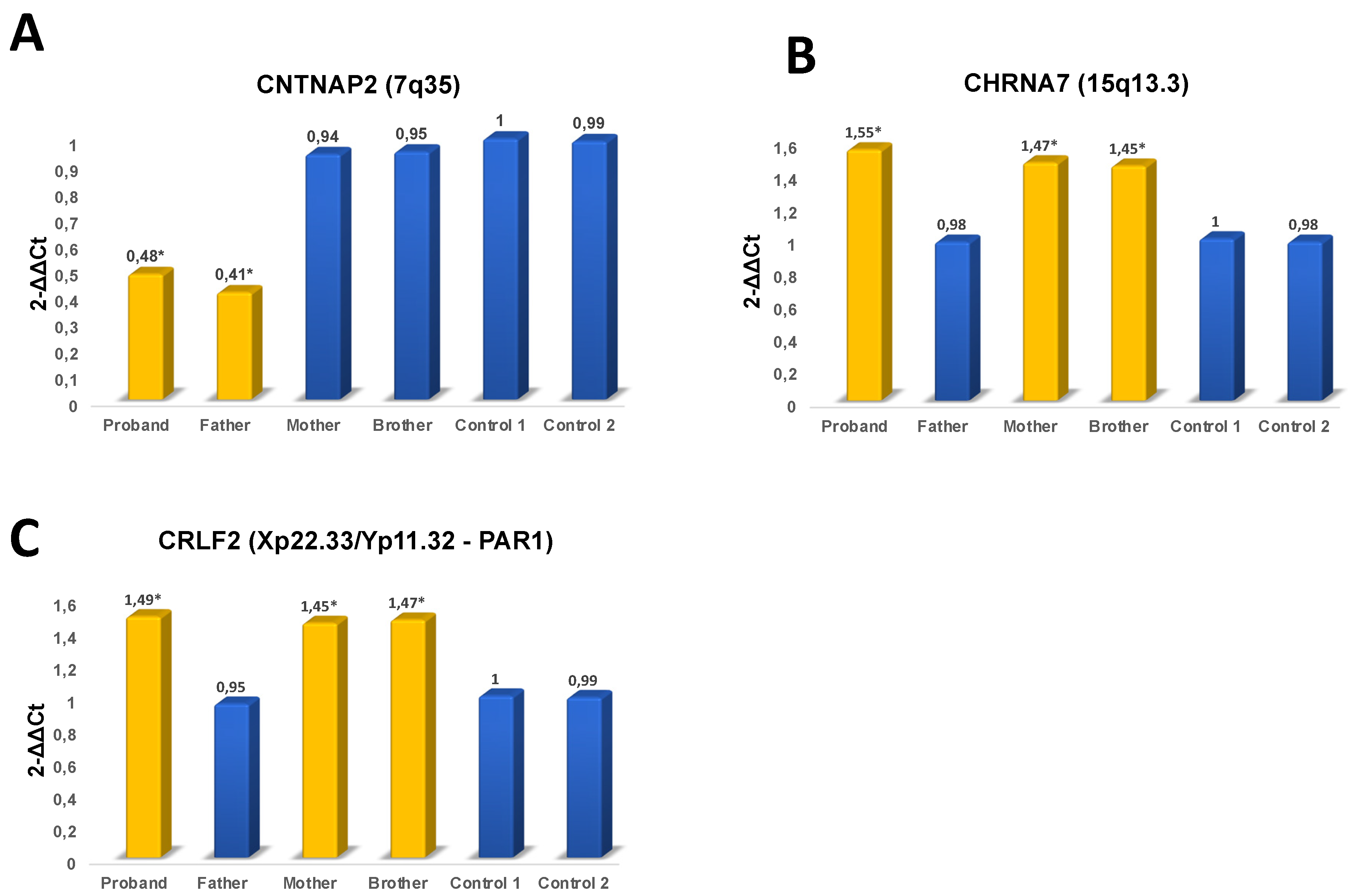
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takumi, T.; Tamada, K. CNV biology in neurodevelopmental disorders. Curr. Opin. Neurobiol. 2018, 48, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, A.K.; Corvin, A.P.; Gallagher, L. Copy-number variants in neurodevelopmental disorders: Promises and challenges. Trends Genet. 2009, 25, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Katsu, M.; Takigawa, I.; Yabe, I.; Matsushima, M.; Takahashi, I.; Katayama, T.; Utsumi, J.; Sasaki, H. Genomic copy number variation analysis in multiple system atrophy. Mol. Brain 2017, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Prunier, J.; Caron, S.; MacKay, J. CNVs into the wild: Screening the genomes of conifer trees (Picea spp.) reveals fewer gene copy number variations in hybrids and links to adaptation. BMC Genom. 2017, 18, 97. [Google Scholar] [CrossRef]
- Rosenfeld, J.A.; Coe, B.P.; Eichler, E.E.; Cuckle, H.; Shaffer, L.G. Estimates of penetrance for recurrent pathogenic copy-Number variations. Genet. Med. 2013, 15, 478–481. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, W.; Hurles, M.E.; Lupski, J.R. Copy number variation in human health, disease, and evolution. Annu. Rev.Genom. Human Genet. 2009, 10, 451–481. [Google Scholar] [CrossRef]
- Consortium, I.S. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 2008, 455, 237. [Google Scholar]
- Friedman, J.; Vrijenhoek, T.; Markx, S.; Janssen, I.; Van Der Vliet, W.; Faas, B.; Knoers, N.; Cahn, W.; Kahn, R.; Edelmann, L. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol. Psychiatry 2008, 13, 261. [Google Scholar] [CrossRef]
- Ghani, M.; Pinto, D.; Lee, J.H.; Grinberg, Y.; Sato, C.; Moreno, D.; Scherer, S.W.; Mayeux, R.; George-Hyslop, P.S.; Rogaeva, E. Genome-Wide survey of large rare copy number variants in Alzheimer’s disease among Caribbean hispanics. G3 Genes Genomes Genet. 2012, 2, 71–78. [Google Scholar] [CrossRef]
- Napoli, E.; Russo, S.; Casula, L.; Alesi, V.; Amendola, F.A.; Angioni, A.; Novelli, A.; Valeri, G.; Menghini, D.; Vicari, S. Array-CGH analysis in a cohort of phenotypically well-Characterized individuals with “essential” autism spectrum disorders. J. Autism Dev. Disord. 2018, 48, 442–449. [Google Scholar] [CrossRef]
- Wincent, J.; Kolbjer, S.; Martin, D.; Luthman, A.; Åmark, P.; Dahlin, M.; Anderlid, B.M. Copy number variations in children with brain malformations and refractory epilepsy. Am. J. Med Genet. Part A 2015, 167, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gochman, P.; Broadnax, D.D.; Rapoport, J.L.; Ahn, K. 15q13. 3 duplication in two patients with childhood-onset schizophrenia. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2016, 171, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Gillentine, M.A.; Schaaf, C.P. The human clinical phenotypes of altered CHRNA7 copy number. Biochem. Pharmacol. 2015, 97, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Van Bon, B.; Mefford, H.; Menten, B.; Koolen, D.; Sharp, A.; Nillesen, W.; Innis, J.; De Ravel, T.; Mercer, C.; Fichera, M. Further delineation of the 15q13 microdeletion and duplication syndromes: A clinical spectrum varying from non-pathogenic to a severe outcome. J. Med Genet. 2009, 46, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.C. Case report: Neuronal migration disorder associated with chromosome 15q13. 3 duplication in a boy with autism and seizures. J. Child Neurol. 2014, 29, NP186–NP188. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.M.; Franke, B.; Mick, E.; Anney, R.J.; Freitag, C.M.; Gill, M.; Thapar, A.; O’Donovan, M.C.; Owen, M.J.; Holmans, P. Genome-Wide analysis of copy number variants in attention deficit hyperactivity disorder: The role of rare variants and duplications at 15q13. 3. Am. J. Psychiatry 2012, 169, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Shen, Y.; Weiss, L.A.; Korn, J.; Anselm, I.; Bridgemohan, C.; Cox, G.F.; Dickinson, H.; Gentile, J.; Harris, D.J. Microdeletion/duplication at 15q13. 2q13. 3 among individuals with features of autism and other neuropsychiatric disorders. J. Med Genet. 2009, 46, 242–248. [Google Scholar] [CrossRef]
- Pettigrew, K.A.; Reeves, E.; Leavett, R.; Hayiou-Thomas, M.E.; Sharma, A.; Simpson, N.H.; Martinelli, A.; Thompson, P.; Hulme, C.; Snowling, M.J. Copy number variation screen identifies a rare de novo deletion at chromosome 15q13. 1-13.3 in a child with language impairment. PLoS ONE 2015, 10, e0134997. [Google Scholar] [CrossRef]
- Szafranski, P.; Schaaf, C.P.; Person, R.E.; Gibson, I.B.; Xia, Z.; Mahadevan, S.; Wiszniewska, J.; Bacino, C.A.; Lalani, S.; Potocki, L. Structures and molecular mechanisms for common 15q13. 3 microduplications involving CHRNA7: Benign or pathological? Human Mutat. 2010, 31, 840–850. [Google Scholar] [CrossRef]
- Melchior, L.; Bertelsen, B.; Debes, N.M.; Groth, C.; Skov, L.; Mikkelsen, J.D.; Brøndum-Nielsen, K.; Tümer, Z. Microduplication of 15q13. 3 and Xq21. 31 in a family with Tourette syndrome and comorbidities. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2013, 162, 825–831. [Google Scholar] [CrossRef]
- Szatkiewicz, J.P.; O’Dushlaine, C.; Chen, G.; Chambert, K.; Moran, J.L.; Neale, B.M.; Fromer, M.; Ruderfer, D.; Akterin, S.; Bergen, S.E. Copy number variation in schizophrenia in Sweden. Mol. Psychiatry 2014, 19, 762. [Google Scholar] [CrossRef] [PubMed]
- Hassfurther, A.; Komini, E.; Fischer, J.; Leipoldt, M. Clinical and genetic heterogeneity of the 15q13. 3 microdeletion syndrome. Mol. Syndromol. 2015, 6, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Stobbe, G.; Liu, Y.; Wu, R.; Hudgings, L.H.; Thompson, O.; Hisama, F.M. Diagnostic yield of array comparative genomic hybridization in adults with autism spectrum disorders. Genet. Med. 2014, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tropeano, M.; Howley, D.; Gazzellone, M.J.; Wilson, C.E.; Ahn, J.W.; Stavropoulos, D.J.; Murphy, C.M.; Eis, P.S.; Hatchwell, E.; Dobson, R.J. Microduplications at the pseudoautosomal SHOX locus in autism spectrum disorders and related neurodevelopmental conditions. J. Med Genet. 2016, 53, 536–547. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-Time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rodenas-Cuadrado, P.; Ho, J.; Vernes, S.C. Shining a light on CNTNAP2: Complex functions to complex disorders. Eur. J. Human Genet. 2014, 22, 171. [Google Scholar] [CrossRef]
- Poot, M. Intragenic CNTNAP2 deletions: A bridge too far. Mol. Syndromol. 2017, 8, 118–130. [Google Scholar] [CrossRef]
- Toma, C.; Pierce, K.D.; Shaw, A.D.; Heath, A.; Mitchell, P.B.; Schofield, P.R.; Fullerton, J.M. Comprehensive cross-Disorder analyses of CNTNAP2 suggest it is unlikely to be a primary risk gene for psychiatric disorders. PLoS Genet. 2018, 14, e1007535. [Google Scholar] [CrossRef]
- Poot, M.; Beyer, V.; Schwaab, I.; Damatova, N.; van’t Slot, R.; Prothero, J.; Holder, S.E.; Haaf, T. Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics 2010, 11, 81–89. [Google Scholar] [CrossRef]
- Mikhail, F.M.; Lose, E.J.; Robin, N.H.; Descartes, M.D.; Rutledge, K.D.; Rutledge, S.L.; Korf, B.R.; Carroll, A.J. Clinically relevant single gene or intragenic deletions encompassing critical neurodevelopmental genes in patients with developmental delay, mental retardation, and/or autism spectrum disorders. Am. J. Med Genet. Part A 2011, 155, 2386–2396. [Google Scholar] [CrossRef]
- Lowther, C.; Costain, G.; Stavropoulos, D.; Melvin, R.; Silversides, C.; Andrade, D. Genetics in Medicine: Official Journal of the American College of Medical Genetics; Nature Publishing Group: New York, NY, USA, 2014. [Google Scholar]
- Cooper, G.; Coe, B.; Girirajan, S.; Rosenfeld, J.A.; Vu, T.H.; Baker, C.; Williams, C.; Stalker, H.; Hamid, R.; Hannig, V.; et al. A copy number variation morbidity map of developmental delay. Nat. Genet. Nat. Publ. Group 2011, 43, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Roll, J.D.; Reuther, G.W. CRLF2 and JAK2 in B-progenitor acute lymphoblastic leukemia: A novel association in oncogenesis. Cancer Res. 2010, 70, 7347–7352. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Sharma, J.; Raju, R.; Palapetta, S.M.; Prasad, T.; Huang, T.-C.; Yoda, A.; Tyner, J.W.; Van Bodegom, D.; Weinstock, D.M. TSLP signaling pathway map: A platform for analysis of TSLP-mediated signaling. Database 2014, 2014. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Soumelis, V.; Watanabe, N.; Ito, T.; Wang, Y.-H.; de Waal Malefyt, R.; Omori, M.; Zhou, B.; Ziegler, S.F. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007, 25, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Kitic, M.; Wimmer, I.; Adzemovic, M.; Kögl, N.; Rudel, A.; Lassmann, H.; Bradl, M. Thymic stromal lymphopoietin is expressed in the intact central nervous system and upregulated in the myelin-degenerative central nervous system. Glia 2014, 62, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.R.; Kim, B.S. The itch–scratch cycle: A neuroimmune perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.C.; Mullighan, C.G.; Chen, I.-M.; Wharton, W.; Mikhail, F.M.; Carroll, A.J.; Kang, H.; Liu, W.; Dobbin, K.K.; Smith, M.A. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2010, 115, 5312–5321. [Google Scholar] [CrossRef]
- Girirajan, S.; Eichler, E.E. Phenotypic variability and genetic susceptibility to genomic disorders. Human Mol. Genet. 2010, 19, R176–R187. [Google Scholar] [CrossRef]
- Gau, S.S.F.; Liao, H.M.; Hong, C.C.; Chien, W.H.; Chen, C.H. Identification of two inherited copy number variants in a male with autism supports two-hit and compound heterozygosity models of autism. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2012, 159, 710–717. [Google Scholar] [CrossRef]
- Girirajan, S.; Rosenfeld, J.A.; Cooper, G.M.; Antonacci, F.; Siswara, P.; Itsara, A.; Vives, L.; Walsh, T.; McCarthy, S.E.; Baker, C. A recurrent 16p12. 1 microdeletion supports a two-hit model for severe developmental delay. Nat. Genet. 2010, 42, 203. [Google Scholar] [CrossRef]
- Rodenas-Cuadrado, P.; Pietrafusa, N.; Francavilla, T.; La Neve, A.; Striano, P.; Vernes, S.C. Characterisation of CASPR2 deficiency disorder-a syndrome involving autism, epilepsy and language impairment. BMC Med Genet. 2016, 17, 8. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paduano, F.; Colao, E.; Loddo, S.; Orlando, V.; Trapasso, F.; Novelli, A.; Perrotti, N.; Iuliano, R. 7q35 Microdeletion and 15q13.3 and Xp22.33 Microduplications in a Patient with Severe Myoclonic Epilepsy, Microcephaly, Dysmorphisms, Severe Psychomotor Delay and Intellectual Disability. Genes 2020, 11, 525. https://doi.org/10.3390/genes11050525
Paduano F, Colao E, Loddo S, Orlando V, Trapasso F, Novelli A, Perrotti N, Iuliano R. 7q35 Microdeletion and 15q13.3 and Xp22.33 Microduplications in a Patient with Severe Myoclonic Epilepsy, Microcephaly, Dysmorphisms, Severe Psychomotor Delay and Intellectual Disability. Genes. 2020; 11(5):525. https://doi.org/10.3390/genes11050525
Chicago/Turabian StylePaduano, Francesco, Emma Colao, Sara Loddo, Valeria Orlando, Francesco Trapasso, Antonio Novelli, Nicola Perrotti, and Rodolfo Iuliano. 2020. "7q35 Microdeletion and 15q13.3 and Xp22.33 Microduplications in a Patient with Severe Myoclonic Epilepsy, Microcephaly, Dysmorphisms, Severe Psychomotor Delay and Intellectual Disability" Genes 11, no. 5: 525. https://doi.org/10.3390/genes11050525
APA StylePaduano, F., Colao, E., Loddo, S., Orlando, V., Trapasso, F., Novelli, A., Perrotti, N., & Iuliano, R. (2020). 7q35 Microdeletion and 15q13.3 and Xp22.33 Microduplications in a Patient with Severe Myoclonic Epilepsy, Microcephaly, Dysmorphisms, Severe Psychomotor Delay and Intellectual Disability. Genes, 11(5), 525. https://doi.org/10.3390/genes11050525






