Complex Analysis of Retroposed Genes’ Contribution to Human Genome, Proteome and Transcriptome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Re-Annotation of Retrocopies
2.2. Retrocopies Expression Analysis
2.3. Expression correlation
2.4. Conserved Domain Analysis
2.5. Gene Ontology Analysis
2.6. Mass Spectrometry-Based Proteomics Data Analysis
2.7. Identification of Ribosome-Associated Retrocopies
2.8. Identification of Retrocopies Overlapping With Other Genes, Trans-NATs, and Contributing Sequences to the Host Gene
2.9. Identification of miRNA Sponges
2.10. Identification of Fusion Transcript
2.11. Data Processing, Filtering and Visualization
3. Results and Discussion
3.1. Number of Retrocopies in the Human Genome and Their Localization
3.2. Patterns of Retrocopies Expression
3.3. Retrocopies Potential for Protein and Peptides Coding
3.3.1. Known Protein-Coding Retrogenes
3.3.2. Retrocopies Capability for Peptides Coding
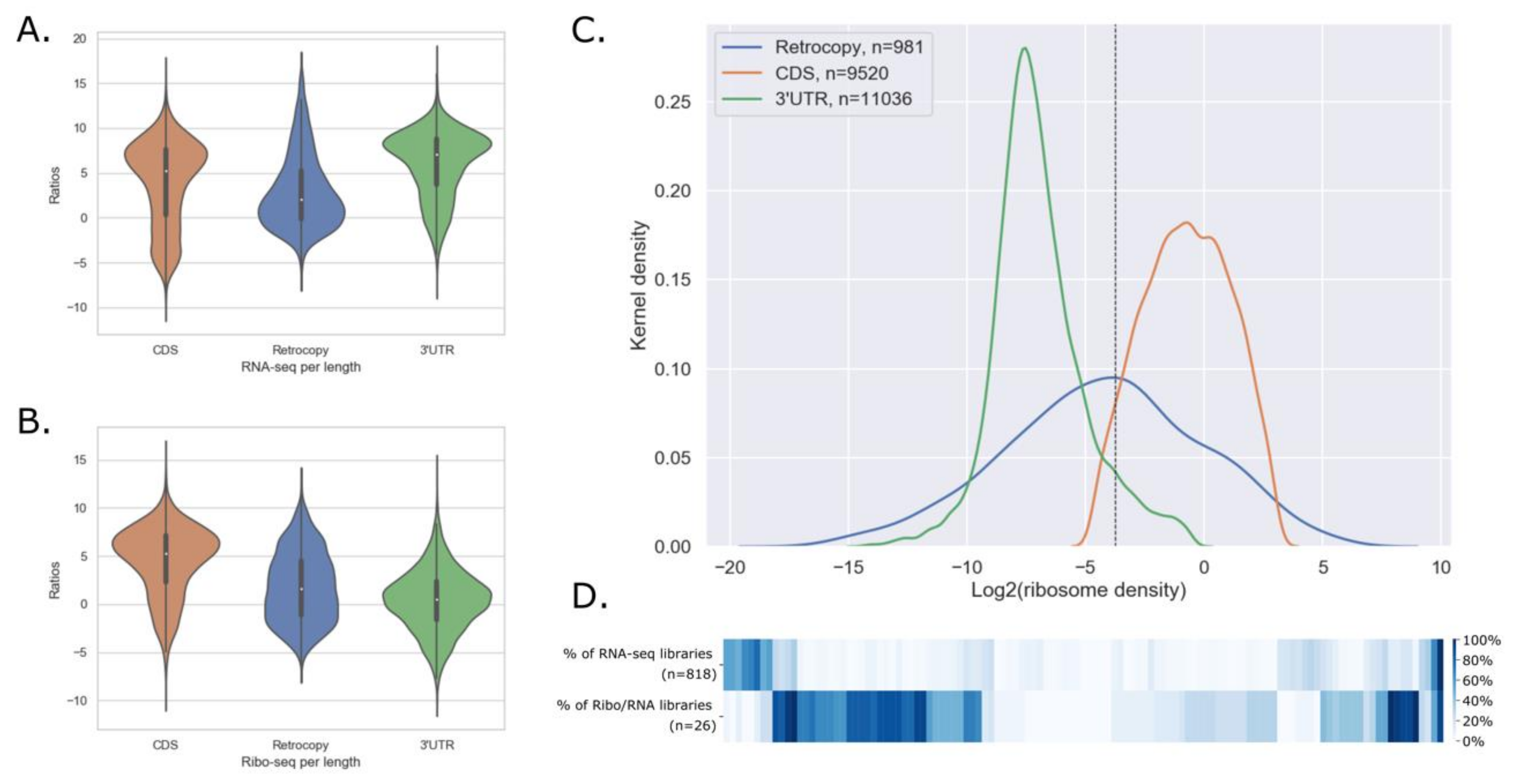
3.3.3. Ribosome Associated Retrocopies
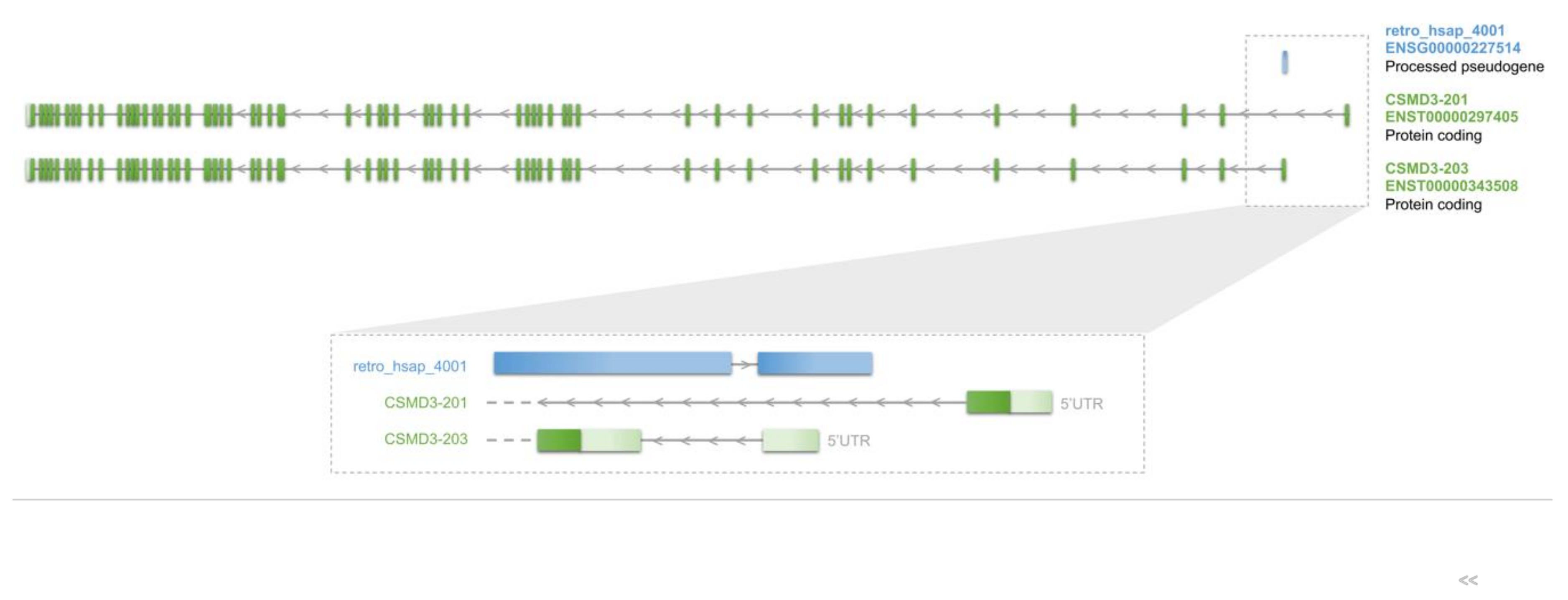
3.3.4. Novel Exons of Protein-Coding Genes
3.4. Retrocopies as Regulatory Elements
3.4.1. Competing Endogenous RNAs
3.4.2. Trans Natural Antisense Transcripts
3.4.3. Cis Antisense Transcripts
3.4.4. Splicing Regulation by Transcriptional Interference
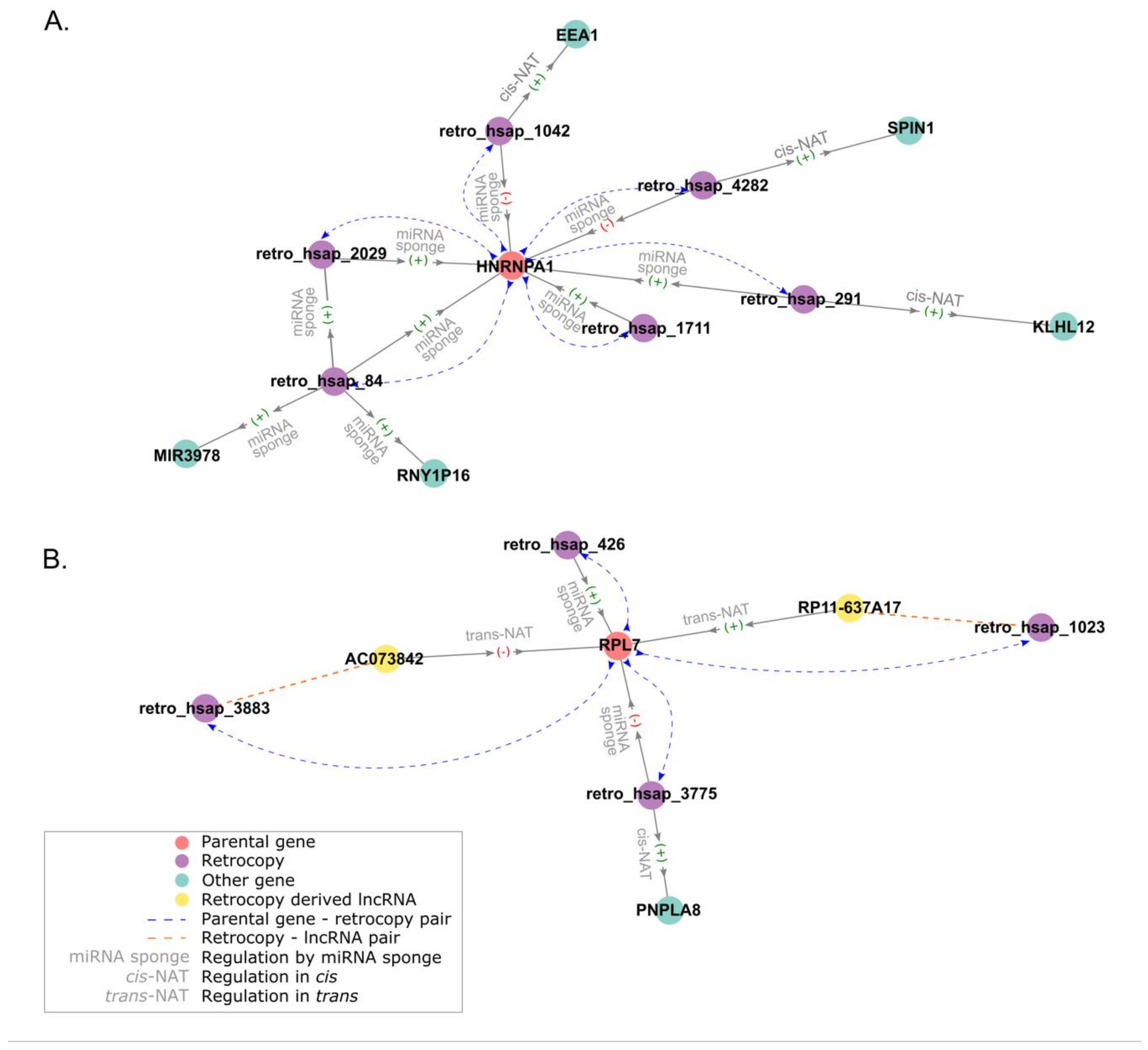
3.4.5. Regulatory Networks
3.4.6. Functional Evolution—A Case Study of retro_hsap_1589
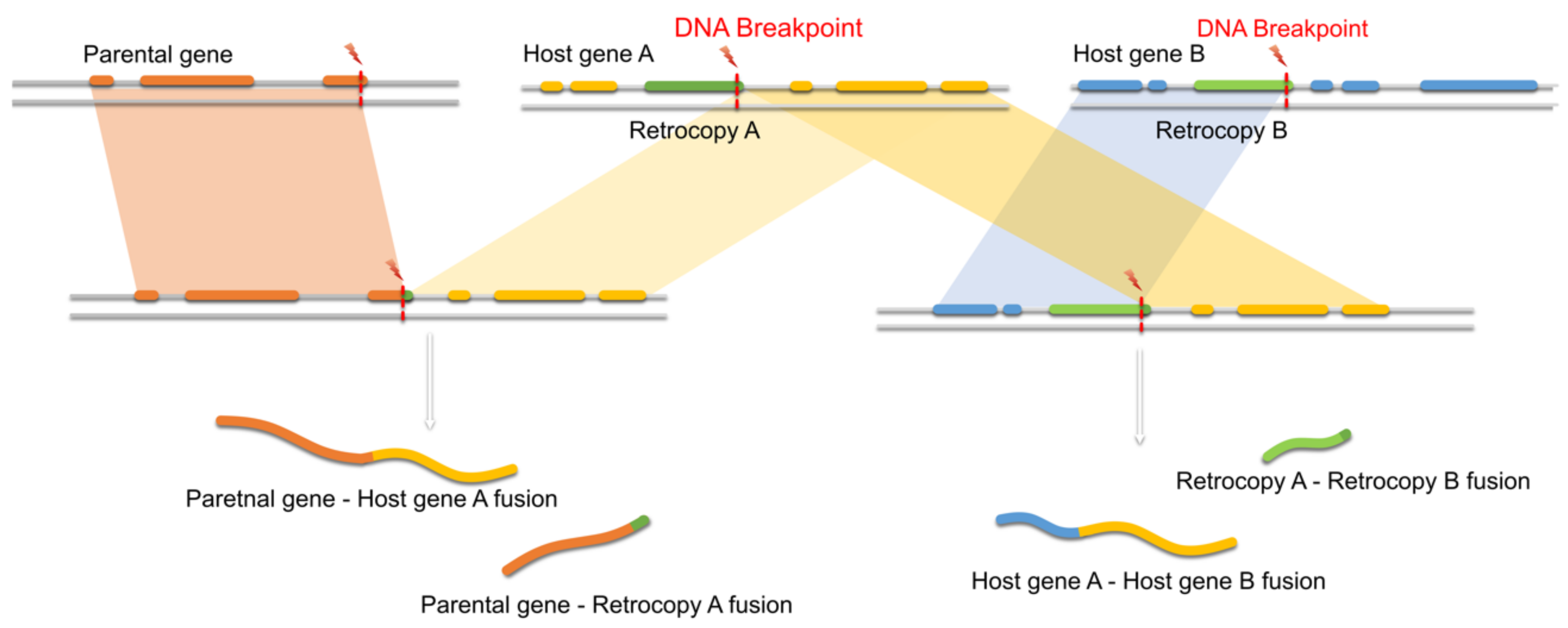
3.5. Retrocopies as Recombination Hot Spots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Koning, A.P.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [Green Version]
- Mighell, A.J.; Smith, N.R.; Robinson, P.A.; Markham, A.F. Vertebrate pseudogenes. FEBS Lett. 2000, 468, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Casola, C.; Betran, E. Evolutionary origin of regulatory regions of retrogenes in Drosophila. BMC Genom. 2008, 9, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betran, E.; Wang, W.; Jin, L.; Long, M. Evolution of the phosphoglycerate mutase processed gene in human and chimpanzee revealing the origin of a new primate gene. Mol. Biol. Evol. 2002, 19, 654–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, A.C.; Dupanloup, I.; Vinckenbosch, N.; Reymond, A.; Kaessmann, H. Emergence of young human genes after a burst of retroposition in primates. PLoS Biol. 2005, 3, e357. [Google Scholar] [CrossRef] [Green Version]
- Sakai, H.; Koyanagi, K.O.; Imanishi, T.; Itoh, T.; Gojobori, T. Frequent emergence and functional resurrection of processed pseudogenes in the human and mouse genomes. Gene 2007, 389, 196–203. [Google Scholar] [CrossRef]
- Brosius, J. Retroposons—Seeds of evolution. Science 1991, 251, 753. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Zheng, D.; Liu, Y.J.; Fang, G.; Frankish, A.; Carriero, N.; Robilotto, R.; Cayting, P.; Gerstein, M. Comparative analysis of processed ribosomal protein pseudogenes in four mammalian genomes. Genome Biol. 2009, 10, R2. [Google Scholar] [CrossRef] [Green Version]
- Szczesniak, M.W.; Ciomborowska, J.; Nowak, W.; Rogozin, I.B.; Makalowska, I. Primate and rodent specific intron gains and the origin of retrogenes with splice variants. Mol Biol Evol 2011, 28, 33–37. [Google Scholar] [CrossRef]
- Parker, H.G.; VonHoldt, B.M.; Quignon, P.; Margulies, E.H.; Shao, S.; Mosher, D.S.; Spady, T.C.; Elkahloun, A.; Cargill, M.; Jones, P.G.; et al. An expressed fgf4 retrogene is associated with breed-defining chondrodysplasia in domestic dogs. Science 2009, 325, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.W.; Liu, S.; Zhang, X.; Li, W.B.; Chen, Y.; Huang, X.; Sun, L.; Luo, W.; Netzer, W.J.; Threadgill, R.; et al. A functional mouse retroposed gene Rps23r1 reduces Alzheimer’s beta-amyloid levels and tau phosphorylation. Neuron 2009, 64, 328–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulak, M.; Fong, L.; Mika, K.; Chigurupati, S.; Yon, L.; Mongan, N.P.; Emes, R.D.; Lynch, V.J. TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. Elife 2016, 5. [Google Scholar] [CrossRef]
- Kaessmann, H.; Vinckenbosch, N.; Long, M. RNA-based gene duplication: Mechanistic and evolutionary insights. Nat. Rev. Genet. 2009, 10, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciomborowska, J.; Rosikiewicz, W.; Szklarczyk, D.; Makalowski, W.; Makalowska, I. “Orphan” retrogenes in the human genome. Mol. Biol. Evol. 2013, 30, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, M.R.; Makalowska, I. Protein-Coding Genes’ Retrocopies and Their Functions. Viruses 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Young, J.; Menetrey, J.; Goud, B. RAB6C is a retrogene that encodes a centrosomal protein involved in cell cycle progression. J. Mol. Biol. 2010, 397, 69–88. [Google Scholar] [CrossRef]
- Yano, Y.; Saito, R.; Yoshida, N.; Yoshiki, A.; Wynshaw-Boris, A.; Tomita, M.; Hirotsune, S. A new role for expressed pseudogenes as ncRNA: Regulation of mRNA stability of its homologous coding gene. J. Mol. Med. 2004, 82, 414–422. [Google Scholar] [CrossRef]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, Y.E.; Long, M. New genes in Drosophila quickly become essential. Science 2010, 330, 1682–1685. [Google Scholar] [CrossRef] [Green Version]
- Vinckenbosch, N.; Dupanloup, I.; Kaessmann, H. Evolutionary fate of retroposed gene copies in the human genome. Proc. Natl. Acad. Sci. USA 2006, 103, 3220–3225. [Google Scholar] [CrossRef] [Green Version]
- Baertsch, R.; Diekhans, M.; Kent, W.J.; Haussler, D.; Brosius, J. Retrocopy contributions to the evolution of the human genome. BMC Genom. 2008, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Devor, E.J. Primate microRNAs miR-220 and miR-492 lie within processed pseudogenes. J. Hered. 2006, 97, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Nozawa, M.; Aotsuka, T.; Tamura, K. A novel chimeric gene, siren, with retroposed promoter sequence in the Drosophila bipectinata complex. Genetics 2005, 171, 1719–1727. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, G.C. Actin’ up: RhoB in cancer and apoptosis. Nat. Rev. Cancer 2001, 1, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, M.; Kurahashi, H.; Tanaka, T.; Nishida, K.; Shimomura, Y.; Tano, Y.; Nakamura, Y. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat. Genet. 1999, 21, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Grander, D.; Johnsson, P. Pseudogene-Expressed RNAs: Emerging Roles in Gene Regulation and Disease. Curr. Top Microbiol. Immunol. 2016, 394, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, P.; Morris, K.V.; Grander, D. Pseudogenes: A novel source of trans-acting antisense RNAs. Methods Mol. Biol. 2014, 1167, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kalyana-Sundaram, S.; Kumar-Sinha, C.; Shankar, S.; Robinson, D.R.; Wu, Y.M.; Cao, X.; Asangani, I.A.; Kothari, V.; Prensner, J.R.; Lonigro, R.J.; et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell 2012, 149, 1622–1634. [Google Scholar] [CrossRef] [Green Version]
- Cheetham, S.W.; Faulkner, G.J.; Dinger, M.E. Overcoming challenges and dogmas to understand the functions of pseudogenes. Nat. Rev. Genet. 2020, 21, 191–201. [Google Scholar] [CrossRef]
- Esposito, F.; De Martino, M.; Forzati, F.; Fusco, A. HMGA1-pseudogene overexpression contributes to cancer progression. Cell Cycle 2014, 13, 3636–3639. [Google Scholar] [CrossRef] [Green Version]
- Mei, D.; Song, H.; Wang, K.; Lou, Y.; Sun, W.; Liu, Z.; Ding, X.; Guo, J. Up-regulation of SUMO1 pseudogene 3 (SUMO1P3) in gastric cancer and its clinical association. Med. Oncol. 2013, 30, 709. [Google Scholar] [CrossRef]
- Poliseno, L.; Marranci, A.; Pandolfi, P.P. Pseudogenes in Human Cancer. Front. Med. 2015, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Rosikiewicz, W.; Kabza, M.; Kosinski, J.G.; Ciomborowska-Basheer, J.; Kubiak, M.R.; Makalowska, I. RetrogeneDB-a database of plant and animal retrocopies. Database (Oxford) 2017, 2017. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Giron, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2018, 46, D754–D761. [Google Scholar] [CrossRef]
- BBDuk Guide. Available online: https://jgi.doe.gov/data-and-tools/bbtools/bb-tools-user-guide/bbduk-guide/ (accessed on 2 December 2018).
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Marchler-Bauer, A.; Lu, S.; Anderson, J.B.; Chitsaz, F.; Derbyshire, M.K.; DeWeese-Scott, C.; Fong, J.H.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; et al. CDD: A Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011, 39, D225–D229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, H.; Muruganujan, A.; Thomas, P.D. PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013, 41, D377–D386. [Google Scholar] [CrossRef] [Green Version]
- Vizcaino, J.A.; Cote, R.G.; Csordas, A.; Dianes, J.A.; Fabregat, A.; Foster, J.M.; Griss, J.; Alpi, E.; Birim, M.; Contell, J.; et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: Status in 2013. Nucleic Acids Res. 2013, 41, D1063–D1069. [Google Scholar] [CrossRef]
- Michel, A.M.; Kiniry, S.J.; O’Connor, P.B.F.; Mullan, J.P.; Baranov, P.V. GWIPS-viz: 2018 update. Nucleic Acids Res. 2018, 46, D823–D830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, W.J.; Zweig, A.S.; Barber, G.; Hinrichs, A.S.; Karolchik, D. BigWig and BigBed: Enabling browsing of large distributed datasets. Bioinformatics. 2010, 26, D2204–D2207. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Fukunaga, T.; Hamada, M. Identification and analysis of ribosome-associated lncRNAs using ribosome profiling data. BMC Genom. 2018, 19, 414. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human MicroRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Aken, B.L.; Achuthan, P.; Akanni, W.; Amode, M.R.; Bernsdorff, F.; Bhai, J.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; et al. Ensembl 2017. Nucleic Acids Res. 2017, 45, D635–D642. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014, 42, D92–D97. [Google Scholar] [CrossRef] [Green Version]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and statistical modeling with python. In Proceedings of the 9th Python in Science Conference, Austin, TX, US, 28 June–3 July 2010; van der Walt, S., Millman, J., Eds.; SciPy: Austin, TX, USA, 2010; pp. D92–D96. [Google Scholar] [CrossRef] [Green Version]
- Gorohovski, A.; Tagore, S.; Palande, V.; Malka, A.; Raviv-Shay, D.; Frenkel-Morgenstern, M. ChiTaRS-3.1-the enhanced chimeric transcripts and RNA-seq database matched with protein-protein interactions. Nucleic Acids Res. 2017, 45, D790–D795. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Q.; Tang, M.; Barthel, F.; Amin, S.; Yoshihara, K.; Lang, F.M.; Martinez-Ledesma, E.; Lee, S.H.; Zheng, S.; et al. TumorFusions: An integrative resource for cancer-associated transcript fusions. Nucleic Acids Res. 2018, 46, D1144–D1149. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Liang, W.W.; Foltz, S.M.; Mutharasu, G.; Jayasinghe, R.G.; Cao, S.; Liao, W.W.; Reynolds, S.M.; Wyczalkowski, M.A.; Yao, L.; et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018, 23, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.; Zhou, X. FusionGDB: Fusion gene annotation DataBase. Nucleic Acids Res. 2019, 47, D994–D1004. [Google Scholar] [CrossRef]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, US, 28 June–3 July 2010; van der Walt, S., Millman, J., Eds.; SciPy: Austin, TX, USA, 2010; pp. D56–D61. [Google Scholar] [CrossRef] [Green Version]
- mwaskom/seaborn, version 0.10.0; Zenodo: Meyrin, Switzerland, 2020. [CrossRef]
- Flicek, P.; Amode, M.R.; Barrell, D.; Beal, K.; Billis, K.; Brent, S.; Carvalho-Silva, D.; Clapham, P.; Coates, G.; Fitzgerald, S.; et al. Ensembl 2014. Nucleic Acids Res. 2014, 42, D749–D755. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.A.; Hitz, B.C.; Sloan, C.A.; Chan, E.T.; Davidson, J.M.; Gabdank, I.; Hilton, J.A.; Jain, K.; Baymuradov, U.K.; Narayanan, A.K.; et al. The Encyclopedia of DNA elements (ENCODE): Data portal update. Nucleic Acids Res. 2018, 46, D794–D801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Chang, Z.; Wu, C.; Zhu, Y.; Li, K.; Xu, Y. Identification of potential cancer-related pseudogenes in lung adenocarcinoma based on ceRNA hypothesis. Oncotarget 2017, 8, 59036–59047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, J.D.; Baran-Gale, J.; Perou, C.M.; Sethupathy, P.; Prins, J.F. Pseudogenes transcribed in breast invasive carcinoma show subtype-specific expression and ceRNA potential. BMC Genom. 2015, 16, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Orera, J.; Messeguer, X.; Subirana, J.A.; Alba, M.M. Long non-coding RNAs as a source of new peptides. Elife 2014, 3, e03523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khavinson, V.K.; Lin’kova, N.S.; Tarnovskaya, S.I. Short Peptides Regulate Gene Expression. Bull. Exp. Biol. Med. 2016, 162, 288–292. [Google Scholar] [CrossRef]
- Hanai, A.; Ohgi, M.; Yagi, C.; Ueda, T.; Shin, H.W.; Nakayama, K. Class I Arfs (Arf1 and Arf3) and Arf6 are localized to the Flemming body and play important roles in cytokinesis. J. Biochem. 2016, 159, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Welsh, C.F.; Moss, J.; Vaughan, M. ADP-ribosylation factors: A family of approximately 20-kDa guanine nucleotide-binding proteins that activate cholera toxin. Mol. Cell Biochem. 1994, 138, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Taatjes, D.J.; Roth, J. In focus in HCB. Histochem. Cell Biol. 2017, 148, 575–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wonderlich, E.R.; Leonard, J.A.; Kulpa, D.A.; Leopold, K.E.; Norman, J.M.; Collins, K.L. ADP ribosylation factor 1 activity is required to recruit AP-1 to the major histocompatibility complex class I (MHC-I) cytoplasmic tail and disrupt MHC-I trafficking in HIV-1-infected primary T cells. J. Virol. 2011, 85, 12216–12226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milev, M.P.; Ravichandran, M.; Khan, M.F.; Schriemer, D.C.; Mouland, A.J. Characterization of staufen1 ribonucleoproteins by mass spectrometry and biochemical analyses reveal the presence of diverse host proteins associated with human immunodeficiency virus type 1. Front. Microbiol. 2012, 3, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, H.; Negishi, M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 2003, 424, 461–464. [Google Scholar] [CrossRef]
- Peotter, J.L.; Phillips, J.; Tong, T.; Dimeo, K.; Gonzalez, J.M., Jr.; Peters, D.M. Involvement of Tiam1, RhoG and ELMO2/ILK in Rac1-mediated phagocytosis in human trabecular meshwork cells. Exp. Cell Res. 2016, 347, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.M.; Yamauchi, A.; Uchida, S.; Preston, A.S.; Garcia-Perez, A.; Burg, M.B.; Handler, J.S. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J. Biol. Chem. 1992, 267, 6297–6301. [Google Scholar]
- Wright, E.M.; Loo, D.D.; Panayotova-Heiermann, M.; Lostao, M.P.; Hirayama, B.H.; Mackenzie, B.; Boorer, K.; Zampighi, G. “Active” sugar transport in eukaryotes. J. Exp. Biol. 1994, 196, 197–212. [Google Scholar]
- Han, X.; Poon, R.Y. Critical differences between isoforms of securin reveal mechanisms of separase regulation. Mol. Cell Biol. 2013, 33, 3400–3415. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.B.; Li, F.; Li, Y.Q.; Yang, F. Pituitary tumor transforming gene PTTG2 induces psoriasis by regulating vimentin and E-cadherin expression. Int. J. Clin. Exp. Pathol. 2015, 8, 10887–10893. [Google Scholar]
- Casola, C.; Betran, E. The Genomic Impact of Gene Retrocopies: What Have We Learned from Comparative Genomics, Population Genomics, and Transcriptomic Analyses? Genome Biol. Evol. 2017, 9, 1351–1373. [Google Scholar] [CrossRef]
- Chen, B.; Wang, C.; Zhang, J.; Zhou, Y.; Hu, W.; Guo, T. New insights into long noncoding RNAs and pseudogenes in prognosis of renal cell carcinoma. Cancer Cell Int. 2018, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Fontanil, T.; Cal, S.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Chacon-Duque, J.C.; Al-Saadi, F.; Johansson, J.A.; Quinto-Sanchez, M.; Acuna-Alonzo, V.; et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat. Commun. 2016, 7, 10815. [Google Scholar] [CrossRef] [Green Version]
- Pickrell, J.K.; Berisa, T.; Liu, J.Z.; Segurel, L.; Tung, J.Y.; Hinds, D.A. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 2016, 48, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Osaka, M.; Ito, D.; Suzuki, N. Disturbance of proteasomal and autophagic protein degradation pathways by amyotrophic lateral sclerosis-linked mutations in ubiquilin 2. Biochem. Biophys. Res. Commun. 2016, 472, 324–331. [Google Scholar] [CrossRef]
- Kim, M.S.; Pinto, S.M.; Getnet, D.; Nirujogi, R.S.; Manda, S.S.; Chaerkady, R.; Madugundu, A.K.; Kelkar, D.S.; Isserlin, R.; Jain, S.; et al. A draft map of the human proteome. Nature 2014, 509, 575–581. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, J. Are Human Translated Pseudogenes Functional? Mol. Biol. Evol. 2016, 33, 755–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Z.; Song, R.; Regev, A.; Struhl, K. Many lncRNAs, 5′UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife 2015, 4, e08890. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Russell, P.; Ingolia, N.T.; Weissman, J.S.; Lander, E.S. Ribosome profiling provides evidence that large noncoding RNAs do not encode proteins. Cell 2013, 154, 240–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abegglen, L.M.; Caulin, A.F.; Chan, A.; Lee, K.; Robinson, R.; Campbell, M.S.; Kiso, W.K.; Schmitt, D.L.; Waddell, P.J.; Bhaskara, S.; et al. Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans. JAMA 2015, 314, 1850–1860. [Google Scholar] [CrossRef]
- Carlevaro-Fita, J.; Rahim, A.; Guigo, R.; Vardy, L.A.; Johnson, R. Cytoplasmic long noncoding RNAs are frequently bound to and degraded at ribosomes in human cells. RNA 2016, 22, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, A.; Asakawa, S.; Sasaki, T.; Yamazaki, S.; Yamagata, H.; Kudoh, J.; Minoshima, S.; Kondo, I.; Shimizu, N. A novel giant gene CSMD3 encoding a protein with CUB and sushi multiple domains: A candidate gene for benign adult familial myoclonic epilepsy on human chromosome 8q23.3-q24.1. Biochem. Biophys. Res. Commun. 2003, 309, 143–154. [Google Scholar] [CrossRef]
- Chiefari, E.; Iiritano, S.; Paonessa, F.; Le Pera, I.; Arcidiacono, B.; Filocamo, M.; Foti, D.; Liebhaber, S.A.; Brunetti, A. Pseudogene-mediated posttranscriptional silencing of HMGA1 can result in insulin resistance and type 2 diabetes. Nat. Commun. 2010, 1, 40. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; De Martino, M.; D’Angelo, D.; Mussnich, P.; Raverot, G.; Jaffrain-Rea, M.L.; Fraggetta, F.; Trouillas, J.; Fusco, A. HMGA1-pseudogene expression is induced in human pituitary tumors. Cell Cycle 2015, 14, 1471–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryzghalov, O.; Szczesniak, M.W.; Makalowska, I. Retroposition as a source of antisense long non-coding RNAs with possible regulatory functions. Acta Biochim. Pol. 2016, 63, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Bergman, O.; Karry, R.; Milhem, J.; Ben-Shachar, D. NDUFV2 pseudogene (NDUFV2P1) contributes to mitochondrial complex I deficits in schizophrenia. Mol. Psychiatry 2018. [Google Scholar] [CrossRef]
- Johnsson, P.; Ackley, A.; Vidarsdottir, L.; Lui, W.O.; Corcoran, M.; Grander, D.; Morris, K.V. A pseudogene long-noncoding-RNA network regulates PTEN transcription and translation in human cells. Nat. Struct. Mol. Biol. 2013, 20, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Groen, J.N.; Capraro, D.; Morris, K.V. The emerging role of pseudogene expressed non-coding RNAs in cellular functions. Int. J. Biochem. Cell. Biol. 2014, 54, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Lizio, M.; Harshbarger, J.; Shimoji, H.; Severin, J.; Kasukawa, T.; Sahin, S.; Abugessaisa, I.; Fukuda, S.; Hori, F.; Ishikawa-Kato, S.; et al. Gateways to the FANTOM5 promoter level mammalian expression atlas. Genome Biol. 2015, 16, 22. [Google Scholar] [CrossRef] [Green Version]
- Muro, E.M.; Andrade-Navarro, M.A. Pseudogenes as an alternative source of natural antisense transcripts. BMC Evol. Biol. 2010, 10, 338. [Google Scholar] [CrossRef] [Green Version]
- Wight, M.; Werner, A. The functions of natural antisense transcripts. Essays Biochem. 2013, 54, 91–101. [Google Scholar] [CrossRef]
- Rosikiewicz, W.; Makalowska, I. Biological functions of natural antisense transcripts. Acta Biochim. Pol. 2016, 63, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, P.G.; Morris, K.V. Transcriptional regulation of Oct4 by a long non-coding RNA antisense to Oct4-pseudogene 5. Transcription 2010, 1, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Werner, A.; Swan, D. What are natural antisense transcripts good for? Biochem. Soc. Trans. 2010, 38, 1144–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Yang, P.; Wei, J.; Li, W.; Zhong, J.; Chen, H.; Cao, J. Overexpression of flavin-containing monooxygenase 5 predicts poor prognosis in patients with colorectal cancer. Oncol. Lett. 2018, 15, 3923–3927. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, L.; Huang, Y.; He, T.; Zhang, L.; Zhao, X.; Zhou, X.; Zhou, D.; Yan, Y.; Zhou, J.; et al. Pseudogene PDIA3P1 promotes cell proliferation, migration and invasion, and suppresses apoptosis in hepatocellular carcinoma by regulating the p53 pathway. Cancer Lett. 2017, 407, 76–83. [Google Scholar] [CrossRef]
- Shashi, V.; Xie, P.; Schoch, K.; Goldstein, D.B.; Howard, T.D.; Berry, M.N.; Schwartz, C.E.; Cronin, K.; Sliwa, S.; Allen, A.; et al. The RBMX gene as a candidate for the Shashi X-linked intellectual disability syndrome. Clin. Genet. 2015, 88, 386–390. [Google Scholar] [CrossRef]
- Adamson, B.; Smogorzewska, A.; Sigoillot, F.D.; King, R.W.; Elledge, S.J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat. Cell Biol. 2012, 14, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Song, Y.; Mu, L.; Li, Q.; Tang, J.; Yang, Z.; Meng, W. Long noncoding RNA TPT1-AS1 downregulates the microRNA-770-5p expression to inhibit glioma cell autophagy and promote proliferation through STMN1 upregulation. J. Cell Physiol. 2019. [Google Scholar] [CrossRef]
- Barman, P.; Reddy, D.; Bhaumik, S.R. Mechanisms of Antisense Transcription Initiation with Implications in Gene Expression, Genomic Integrity and Disease Pathogenesis. Noncoding RNA 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Kaer, K.; Branovets, J.; Hallikma, A.; Nigumann, P.; Speek, M. Intronic L1 retrotransposons and nested genes cause transcriptional interference by inducing intron retention, exonization and cryptic polyadenylation. PLoS ONE 2011, 6, e26099. [Google Scholar] [CrossRef]
- Shearwin, K.E.; Callen, B.P.; Egan, J.B. Transcriptional interference—A crash course. Trends Genet. 2005, 21, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigemasa, K.; Underwood, L.J.; Beard, J.; Tanimoto, H.; Ohama, K.; Parmley, T.H.; O’Brien, T.J. Overexpression of testisin, a serine protease expressed by testicular germ cells, in epithelial ovarian tumor cells. J. Soc. Gynecol. Investig. 2000, 7, 358–362. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhang, B.; Bie, Q.; Qian, H.; Xu, W. Transcriptome Analysis Reveals Key Genes and Pathways Associated with Metastasis in Breast Cancer. Onco Targets Ther. 2020, 13, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alazami, A.M.; Adly, N.; Al Dhalaan, H.; Alkuraya, F.S. A nullimorphic ERLIN2 mutation defines a complicated hereditary spastic paraplegia locus (SPG18). Neurogenetics 2011, 12, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Al-Saif, A.; Bohlega, S.; Al-Mohanna, F. Loss of ERLIN2 function leads to juvenile primary lateral sclerosis. Ann. Neurol. 2012, 72, 510–516. [Google Scholar] [CrossRef]
- Yildirim, Y.; Orhan, E.K.; Iseri, S.A.; Serdaroglu-Oflazer, P.; Kara, B.; Solakoglu, S.; Tolun, A. A frameshift mutation of ERLIN2 in recessive intellectual disability, motor dysfunction and multiple joint contractures. Hum. Mol. Genet. 2011, 20, 1886–1892. [Google Scholar] [CrossRef] [Green Version]
- Verkerk, A.; Zeidler, S.; Breedveld, G.; Overbeek, L.; Huigh, D.; Koster, L.; van der Linde, H.; de Esch, C.; Severijnen, L.A.; de Vries, B.B.A.; et al. CXorf56, a dendritic neuronal protein, identified as a new candidate gene for X-linked intellectual disability. Eur. J. Hum. Genet. 2018, 26, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Browman, D.T.; Resek, M.E.; Zajchowski, L.D.; Robbins, S.M. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J. Cell Sci. 2006, 119, 3149–3160. [Google Scholar] [CrossRef] [Green Version]
- Pollock, T.B.; Mack, J.M.; Day, R.J.; Isho, N.F.; Brown, R.J.; Oxford, A.E.; Morrison, B.E.; Hayden, E.J.; Rohn, T.T. A Fragment of Apolipoprotein E4 Leads to the Downregulation of a CXorf56 Homologue, a Novel ER-Associated Protein, and Activation of BV2 Microglial Cells. Oxid. Med. Cell Longev. 2019, 2019, 5123565. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Jiang, M.; Ou-Yang, Z.; Wu, H.; Dong, S.; Hei, M. High mobility group box 1 protein (HMGB1) as biomarker in hypoxia-induced persistent pulmonary hypertension of the newborn: A clinical and in vivo pilot study. Int. J. Med. Sci. 2019, 16, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Fujita, M.; Kato, A. Significance of Elevated HMGB1 Expression in Pituitary Apoplexy. Anticancer Res. 2019, 39, 4491–4494. [Google Scholar] [CrossRef]
- Zhen, C.; Wang, Y.; Li, D.; Zhang, W.; Zhang, H.; Yu, X.; Wang, X. Relationship of High-mobility group box 1 levels and multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2019, 31, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Benlier, N.; Erdogan, M.B.; Kecioglu, S.; Orhan, N.; Cicek, H. Association of high mobility group box 1 protein with coronary artery disease. Asian Cardiovasc. Thorac. Ann. 2019, 27, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, Q.; Xu, G.; Mao, F.; Qin, T.; Teng, H.; Cai, W.; Yu, P.; Cai, T.; et al. Comparative RNA-seq analysis reveals potential mechanisms mediating the conversion to androgen independence in an LNCaP progression cell model. Cancer Lett. 2014, 342, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Sen, S.K.; Wang, J.; Callinan, P.A.; Lee, J.; Cordaux, R.; Liang, P.; Batzer, M.A. Genomic rearrangements by LINE-1 insertion-mediated deletion in the human and chimpanzee lineages. Nucleic Acids Res. 2005, 33, 4040–4052. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Han, K.; Meyer, T.J.; Kim, H.S.; Batzer, M.A. Chromosomal inversions between human and chimpanzee lineages caused by retrotransposons. PLoS ONE 2008, 3, e4047. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.E.; Ayarpadikannan, S.; Kim, H.S. Role of transposable elements in genomic rearrangement, evolution, gene regulation and epigenetics in primates. Genes Genet. Syst. 2015, 90, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.J.; Gitton, Y. The untold stories of the speech gene, the FOXP2 cancer gene. Genes Cancer 2018, 9, 11–38. [Google Scholar] [CrossRef] [Green Version]
- Carelli, F.N.; Hayakawa, T.; Go, Y.; Imai, H.; Warnefors, M.; Kaessmann, H. The life history of retrocopies illuminates the evolution of new mammalian genes. Genome Res. 2016, 26, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.M.; Zheng, D.; Zhang, Z.; Carriero, N.; Gerstein, M. Transcribed processed pseudogenes in the human genome: An intermediate form of expressed retrosequence lacking protein-coding ability. Nucleic Acids Res. 2005, 33, 2374–2383. [Google Scholar] [CrossRef]
- Frith, M.C.; Wilming, L.G.; Forrest, A.; Kawaji, H.; Tan, S.L.; Wahlestedt, C.; Bajic, V.B.; Kai, C.; Kawai, J.; Carninci, P.; et al. Pseudo-messenger RNA: phantoms of the transcriptome. PLoS Genet. 2006, 2, e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemesh, R.; Novik, A.; Edelheit, S.; Sorek, R. Genomic fossils as a snapshot of the human transcriptome. Proc. Natl. Acad. Sci. USA 2006, 103, 1364–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Type of Expression | Number of Retrocopies | Identifiers from RetrogeneDB |
|---|---|---|
| Ubiquitous | 14 | retro_hsap_2, retro_hsap_4, retro_hsap_36, retro_hsap_57, retro_hsap_64, retro_hsap_75, retro_hsap_100, retro_hsap_105, retro_hsap_108, retro_hsap_217, retro_hsap_774, retro_hsap_901, retro_hsap_1605, retro_hsap_3990 |
| All cancer cell lines but not normal tissue | 3 | retro_hsap_1725, retro_hsap_1817, retro_hsap_2646 |
| Restricted to a specific tissue type | ||
| Fetal brain | 5 | retro_hsap_912, retro_hsap_913, retro_hsap_1813, retro_hsap_1883, retro_hsap_2045 |
| Heart and aorta | 2 | retro_hsap_316, retro_hsap_3488 |
| Liver | 2 | retro_hsap_623, retro_hsap_4127 |
| Lung | 2 | retro_hsap_3266, retro_hsap_4877 |
| Omental fat pad | 1 | retro_hsap_2759 |
| Peyer’s patch | 1 | retro_hsap_25 |
| Prostate gland | 5 | retro_hsap_101, retro_hsap_743, retro_hsap_770, retro_hsap_2122, retro_hsap_4833 |
| Skin | 8 | retro_hsap_178, retro_hsap_734, retro_hsap_1483, retro_hsap_1713, retro_hsap_2147, retro_hsap_2266, retro_hsap_3080, retro_hsap_3112 |
| Spleen | 14 | retro_hsap_241, retro_hsap_396, retro_hsap_671, retro_hsap_877, retro_hsap_1801, retro_hsap_2073, retro_hsap_2092, retro_hsap_2576, retro_hsap_2666, retro_hsap_2799, retro_hsap_3524, retro_hsap_3613, retro_hsap_3678, retro_hsap_3917 |
| Tibial nerve | 1 | retro_hsap_4800 |
| Transverse colon | 2 | retro_hsap_2044, retro_hsap_4063 |
| Uterus | 1 | retro_hsap_4139 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, M.R.; Szcześniak, M.W.; Makałowska, I. Complex Analysis of Retroposed Genes’ Contribution to Human Genome, Proteome and Transcriptome. Genes 2020, 11, 542. https://doi.org/10.3390/genes11050542
Kubiak MR, Szcześniak MW, Makałowska I. Complex Analysis of Retroposed Genes’ Contribution to Human Genome, Proteome and Transcriptome. Genes. 2020; 11(5):542. https://doi.org/10.3390/genes11050542
Chicago/Turabian StyleKubiak, Magdalena Regina, Michał Wojciech Szcześniak, and Izabela Makałowska. 2020. "Complex Analysis of Retroposed Genes’ Contribution to Human Genome, Proteome and Transcriptome" Genes 11, no. 5: 542. https://doi.org/10.3390/genes11050542
APA StyleKubiak, M. R., Szcześniak, M. W., & Makałowska, I. (2020). Complex Analysis of Retroposed Genes’ Contribution to Human Genome, Proteome and Transcriptome. Genes, 11(5), 542. https://doi.org/10.3390/genes11050542





