Could the Heat Shock Proteins 70 Family Members Exacerbate the Immune Response in Multiple Sclerosis? An in Silico Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. In Silico Analysis
3. Results
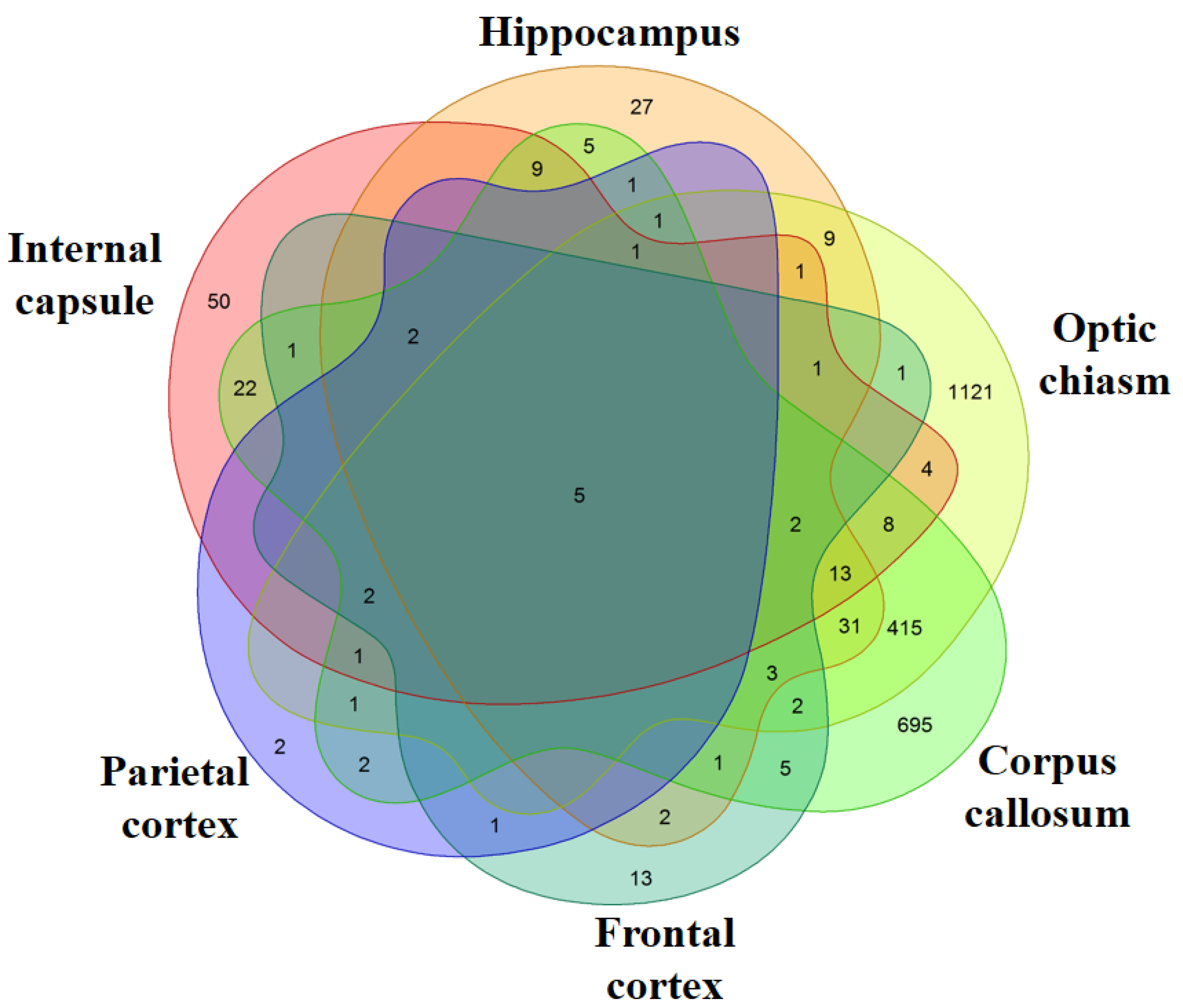
Transcriptomic Analysis of MS Patients and Healthy Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Paolicelli, D.; Manni, A.; Iaffaldano, A.; Trojano, M. Efficacy and safety of oral therapies for relapsing-remitting multiple sclerosis. CNS Drugs 2020, 34, 65–92. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Turturici, G.; Sconzo, G.; Geraci, F. Hsp70 and its molecular role in nervous system diseases. Biochem. Res. Int. 2011, 2011, 618127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raska, M.; Weigl, E. Heat shock proteins in autoimmune diseases. Biomed. Pap. Med. Fac. Univ. Palacky, Olomouc, Czechoslov. 2005, 149, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinar, O.; Ozden, Y.A.; Omur, E.; Muhtesem, G. Heat shock proteins in multiple sclerosis. Adv. Exp. Med. Biol. 2017, 958, 29–42. [Google Scholar]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Asea, A.; Savettieri, G.; Ragonese, P.; Geraci, F. Positive or negative involvement of heat shock proteins in multiple sclerosis pathogenesis: An overview. J. Neuropathol. Exp. Neurol. 2014, 73, 1092–1106. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. Ncbi geo: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [Green Version]
- Coordinators, N.R. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef] [Green Version]
- Voskuhl, R.R.; Itoh, N.; Tassoni, A.; Matsukawa, M.A.; Ren, E.; Tse, V.; Jang, E.; Suen, T.T.; Itoh, Y. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef] [Green Version]
- Leinonen, R.; Sugawara, H.; Shumway, M.; International Nucleotide Sequence Database Collaboration. The sequence read archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- University of California Santa Cruz (Ucsc). Available online: http://labshare.cshl.edu/shares/gingeraslab/www-data/dobin/STAR/STARgenomes/Old/ENSEMBL/homo_sapiens/ENSEMBL.homo_sapiens.release-75/ (accessed on 1 June 2019).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Star: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. Htseq--a python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Ramachandran, S.; Bell, R.B. Heat shock protein 70 gene polymorphisms and multiple sclerosis. Tissue Antigens 1995, 46, 140–141. [Google Scholar] [CrossRef]
- Boiocchi, C.; Monti, M.C.; Osera, C.; Mallucci, G.; Pistono, C.; Ferraro, O.E.; Nosari, G.; Romani, A.; Cuccia, M.; Govoni, S.; et al. Heat shock protein 70-hom gene polymorphism and protein expression in multiple sclerosis. J. Neuroimmunol. 2016, 298, 189–193. [Google Scholar] [CrossRef]
- Boiocchi, C.; Osera, C.; Monti, M.C.; Ferraro, O.E.; Govoni, S.; Cuccia, M.; Montomoli, C.; Pascale, A.; Bergamaschi, R. Are hsp70 protein expression and genetic polymorphism implicated in multiple sclerosis inflammation? J. Neuroimmunol. 2014, 268, 84–88. [Google Scholar] [CrossRef]
- Tarzjani, S.P.C.; Fazeli, S.A.H.S.; Sanati, M.H.; Nabavi, S.M. Heat shock protein 70 and the risk of multiple sclerosis in the iranian population. Cell J. 2019, 20, 599–603. [Google Scholar]
- Niino, M.; Kikuchi, S.; Fukazawa, T.; Yabe, I.; Sasaki, H.; Tashiro, K. Heat shock protein 70 gene polymorphism in japanese patients with multiple sclerosis. Tissue Antigens 2001, 58, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lechner, P.; Buck, D.; Sick, L.; Hemmer, B.; Multhoff, G. Serum heat shock protein 70 levels as a biomarker for inflammatory processes in multiple sclerosis. Mult. Scler. J.-Exp. Transl. Clin. 2018, 4, 2055217318767192. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.J.; Comabella, M.; Rio, J.; Castillo, J.; Castillo, M.; Martin, R.; Montalban, X.; Espejo, C. Up-regulation of inducible heat shock protein-70 expression in multiple sclerosis patients. Autoimmunity 2014, 47, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melief, J.; Orre, M.; Bossers, K.; van Eden, C.G.; Schuurman, K.G.; Mason, M.R.J.; Verhaagen, J.; Hamann, J.; Huitinga, I. Transcriptome analysis of normal-appearing white matter reveals cortisol- and disease-associated gene expression profiles in multiple sclerosis. Acta Neuropathol. Commun. 2019, 7, 60. [Google Scholar] [CrossRef]
- Beere, H.M. “The stress of dying”: The role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 2004, 117, 2641–2651. [Google Scholar] [CrossRef] [Green Version]
- Mansilla, M.J.; Costa, C.; Eixarch, H.; Tepavcevic, V.; Castillo, M.; Martin, R.; Lubetzki, C.; Aigrot, M.S.; Montalban, X.; Espejo, C. Hsp70 regulates immune response in experimental autoimmune encephalomyelitis. PLoS ONE 2014, 9, e105737. [Google Scholar] [CrossRef] [Green Version]
- Mansilla, M.J.; Montalban, X.; Espejo, C. Heat shock protein 70: Roles in multiple sclerosis. Mol. Med. 2012, 18, 1018–1028. [Google Scholar] [CrossRef]
- Parsian, A.J.; Sheren, J.E.; Tao, T.Y.; Goswami, P.C.; Malyapa, R.; Van Rheeden, R.; Watson, M.S.; Hunt, C.R. The human hsp70b gene at the hspa7 locus of chromosome 1 is transcribed but non-functional. Biochim. Et Biophys. Acta 2000, 1494, 201–205. [Google Scholar] [CrossRef]
- Brocchieri, L.; de Macario, E.C.; Macario, A.J. Hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 2008, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Becirovic, L.; Brown, I.R. Targeting of heat shock protein hspa6 (hsp70b’) to the periphery of nuclear speckles is disrupted by a transcription inhibitor following thermal stress in human neuronal cells. Neurochem. Res. 2017, 42, 406–414. [Google Scholar] [CrossRef]
- Shorbagi, S.; Brown, I.R. Dynamics of the association of heat shock protein hspa6 (hsp70b’) and hspa1a (hsp70-1) with stress-sensitive cytoplasmic and nuclear structures in differentiated human neuronal cells. Cell Stress Chaperones 2016, 21, 993–1003. [Google Scholar] [CrossRef] [Green Version]
- Dragovic, Z.; Broadley, S.A.; Shomura, Y.; Bracher, A.; Hartl, F.U. Molecular chaperones of the hsp110 family act as nucleotide exchange factors of hsp70s. EMBO J. 2006, 25, 2519–2528. [Google Scholar] [CrossRef] [Green Version]
- Deane, C.A.S.; Brown, I.R. Intracellular targeting of heat shock proteins in differentiated human neuronal cells following proteotoxic stress. J. Alzheimer’s Dis. Jad 2018, 66, 1295–1308. [Google Scholar] [CrossRef]
- Yamagishi, N.; Ishihara, K.; Hatayama, T. Hsp105alpha suppresses hsc70 chaperone activity by inhibiting hsc70 atpase activity. J. Biol. Chem. 2004, 279, 41727–41733. [Google Scholar] [CrossRef] [Green Version]
- Kern, F.; Stanika, R.I.; Sarg, B.; Offterdinger, M.; Hess, D.; Obermair, G.J.; Lindner, H.; Bandtlow, C.E.; Hengst, L.; Schweigreiter, R. Nogo-a couples with apg-1 through interaction and co-ordinate expression under hypoxic and oxidative stress. Biochem. J. 2013, 455, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Scieglinska, D.; Piglowski, W.; Chekan, M.; Mazurek, A.; Krawczyk, Z. Differential expression of hspa1 and hspa2 proteins in human tissues; tissue microarray-based immunohistochemical study. Histochem. Cell Biol. 2011, 135, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Mahanta, S. Association of heat-shock proteins in various neurodegenerative disorders: Is it a master key to open the therapeutic door? Mol. Cell Biochem 2014, 386, 45–61. [Google Scholar] [CrossRef]
- Mycko, M.P.; Cwiklinska, H.; Szymanski, J.; Szymanska, B.; Kudla, G.; Kilianek, L.; Odyniec, A.; Brosnan, C.F.; Selmaj, K.W. Inducible heat shock protein 70 promotes myelin autoantigen presentation by the hla class ii. J. Immunol. 2004, 172, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Luckey, D.; Bastakoty, D.; Mangalam, A.K. Role of hla class ii genes in susceptibility and resistance to multiple sclerosis: Studies using hla transgenic mice. J. Autoimmun. 2011, 37, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Greer, J.M. The role of hla in ms susceptibility and phenotype. Curr. Top. Behav. Neurosci. 2015, 26, 1–27. [Google Scholar]
- Apperson, M.L.; Tian, Y.; Stamova, B.; Ander, B.P.; Jickling, G.C.; Agius, M.A.; Sharp, F.R. Genome wide differences of gene expression associated with hla-drb1 genotype in multiple sclerosis: A pilot study. J. Neuroimmunol. 2013, 257, 90–96. [Google Scholar] [CrossRef]
- Bever, C.T., Jr.; Garver, D.W. Increased cathepsin b activity in multiple sclerosis brain. J. Neurol. Sci. 1995, 131, 71–73. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Richter, K.; Rufer, A.C.; Muller, M.; Burger, D.; Casagrande, F.; Grossenbacher, T.; Huber, S.; Hug, M.N.; Koldewey, P.; D’Osualdo, A.; et al. Small molecule ax-024 reduces t cell proliferation independently of cd3ε-nck1 interaction, which is governed by a domain-swap in the nck1-sh3.1 domain. J. Biol. Chem. 2020. [Google Scholar] [CrossRef] [Green Version]
- Hatami, M.; Salmani, T.; Arsang-Jang, S.; Omrani, M.D.; Mazdeh, M.; Ghafouri-Fard, S.; Sayad, A.; Taheri, M. Stat5a and stat6 gene expression levels in multiple sclerosis patients. Cytokine 2018, 106, 108–113. [Google Scholar] [CrossRef]
- Ghalamfarsa, G.; Mahmoudi, M.; Mohammadnia-Afrouzi, M.; Yazdani, Y.; Anvari, E.; Hadinia, A.; Ghanbari, A.; Setayesh, M.; Yousefi, M.; Jadidi-Niaragh, F. Il-21 and il-21 receptor in the immunopathogenesis of multiple sclerosis. J. Immunotoxicol. 2016, 13, 274–285. [Google Scholar] [CrossRef]
- Sha, Y.; Markovic-Plese, S. A role of il-1r1 signaling in the differentiation of th17 cells and the development of autoimmune diseases. Self/Nonself 2011, 2, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Sha, Y.; Markovic-Plese, S. Activated il-1ri signaling pathway induces th17 cell differentiation via interferon regulatory factor 4 signaling in patients with relapsing-remitting multiple sclerosis. Front. Immunol. 2016, 7, 543. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.D.; Pan, H.F.; Ye, D.Q.; Xu, Y. Targeting irf4 in autoimmune diseases. Autoimmun. Rev. 2012, 11, 918–924. [Google Scholar] [CrossRef]
- Hardy, I.R.; Anceriz, N.; Rousseau, F.; Seefeldt, M.B.; Hatterer, E.; Irla, M.; Buatois, V.; Chatel, L.E.; Getahun, A.; Fletcher, A.; et al. Anti-cd79 antibody induces b cell anergy that protects against autoimmunity. J. Immunol. 2014, 192, 1641–1650. [Google Scholar] [CrossRef]
- Inabe, K.; Ishiai, M.; Scharenberg, A.M.; Freshney, N.; Downward, J.; Kurosaki, T. Vav3 modulates b cell receptor responses by regulating phosphoinositide 3-kinase activation. J. Exp. Med. 2002, 195, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gehring, T.; Seeholzer, T.; Krappmann, D. Bcl10 - bridging cards to immune activation. Front. Immunol. 2018, 9, 1539. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics, C. Network-based multiple sclerosis pathway analysis with gwas data from 15,000 cases and 30,000 controls. Am. J. Hum. Genet. 2013, 92, 854–865. [Google Scholar]
- Anderson, K.J.; Allen, R.L. Regulation of t-cell immunity by leucocyte immunoglobulin-like receptors: Innate immune receptors for self on antigen-presenting cells. Immunology 2009, 127, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Regis, G.; Conti, L.; Boselli, D.; Novelli, F. Ifngammar2 trafficking tunes ifngamma-stat1 signaling in t lymphocytes. Trends Immunol. 2006, 27, 96–101. [Google Scholar] [CrossRef]
- Conze, D.; Krahl, T.; Kennedy, N.; Weiss, L.; Lumsden, J.; Hess, P.; Flavell, R.A.; Le Gros, G.; Davis, R.J.; Rincon, M. C-jun nh(2)-terminal kinase (jnk)1 and jnk2 have distinct roles in cd8(+) t cell activation. J. Exp. Med. 2002, 195, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Wang, J.; Zeng, Y.; Rao, L.; Chen, H.; Li, W.; Li, Y.; Li, H.; Cui, C.; Xiao, L. Generating hescs with reduced immunogenicity by disrupting tap1 or tapbp. Biosci. Biotechnol. Biochem. 2016, 80, 1484–1491. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, O.; Alvarez-Cermeno, J.C.; Arroyo-Gonzalez, R.; Brieva, L.; Calles-Hernandez, M.C.; Casanova-Estruch, B.; Comabella, M.; de las Heras, V.; Garcia-Merino, J.A.; Hernandez-Perez, M.A.; et al. Review of the novelties presented at the 27th congress of the european committee for treatment and research in multiple sclerosis (ectrims) (i). Rev. De Neurol. 2012, 54, 677–691. [Google Scholar]

| Gene | Name | Healthy Expression | Patient Expression | Fold Change | q-Value |
|---|---|---|---|---|---|
| Corpus callosum | |||||
| HSPA7 | heat shock protein family A (Hsp70) member 7 (pseudogene) | 71.61 | 335.48 | 2.23 | 1.03 × 10−2 |
| HSPA6 | heat shock protein family A (Hsp70) member 6 | 135.30 | 3854.91 | 4.83 | 3.51 × 10−5 |
| HSPA4L | heat shock protein family A (Hsp70) member 4 like | 927.82 | 3203.44 | 1.79 | 3.24 × 10−3 |
| HSPA1B | heat shock protein family A (Hsp70) member 1B | 8385.32 | 36140.05 | 2.11 | 2.75 × 10−2 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A | 9503.44 | 40458.48 | 2.09 | 3.73 × 10−2 |
| HSPH1 | heat shock protein family H (Hsp110) member 1 | 4420.35 | 19934.90 | 2.17 | 3.57 × 10−3 |
| Hippocampus | |||||
| HSPA1B | heat shock protein family A (Hsp70) member 1B | 2504.61 | 20344.57 | 3.02 | 3.19 × 10−3 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A | 2741.64 | 21590.48 | 2.98 | 7.50 × 10−3 |
| Optic chiasm | |||||
| HSPA2 | heat shock protein family A (Hsp70) member 2 | 17822.56 | 4332.32 | −2.04 | 1.27 × 10−10 |
| HSPA7 | heat shock protein family A (Hsp70) member 7 (pseudogene) | 174.04 | 687.19 | 1.98 | 3.87 × 10−2 |
| HSPH1 | heat shock protein family H (Hsp110) member 1 | 5894.37 | 22661.93 | 1.94 | 4.65 × 10−3 |
| HSPA1B | heat shock protein family A (Hsp70) member 1B | 11058.84 | 40687.72 | 1.88 | 1.61 × 10−2 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A | 10798.31 | 33538.69 | 1.64 | 3.82 × 10−2 |
| HSPA6 | heat shock protein family A (Hsp70) member 6 | 524.15 | 7134.02 | 3.77 | 8.86 × 10−4 |
| Internal capsule | |||||
| HSPA1B | heat shock protein family A (Hsp70) member 1B | 1847.82 | 22464.49 | 3.60 | 1.60 × 10−4 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A | 2756.75 | 27201.29 | 3.30 | 5.84 × 10−5 |
| HSPA7 | heat shock protein family A (Hsp70) member 7 (pseudogene) | 27.79 | 286.33 | 3.33 | 3.05 × 10−3 |
| Frontal Cortex | |||||
| HSPA1B | heat shock protein family A (Hsp70) member 1B | 1490.03 | 13373.55 | 3.17 | 6.63 × 10−3 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A | 1670.50 | 14461.95 | 3.11 | 1.24 × 10−2 |
| HSPA7 | heat shock protein family A (Hsp70) member 7 (pseudogene) | 17.54 | 178.84 | 3.37 | 3.31 × 10−2 |
| Parietal Cortex | |||||
| HSPA1B | heat shock protein family A (Hsp70) member 1B | 1727.28 | 18950.37 | 3.46 | 3.64 × 10−3 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A | 2061.80 | 23408.21 | 3.51 | 5.67 × 10−3 |
| HSPA7 | heat shock protein family A (Hsp70) member 7 (pseudogene) | 12.57 | 190.93 | 3.93 | 3.64 × 10−3 |
| HSPA6 | heat shock protein family A (Hsp70) member 6 | 36.82 | 1185.20 | 5.02 | 6.19 × 10−3 |
| Gene | Name | Healthy Expression | Patient Expression | Fold Change | q-Value |
|---|---|---|---|---|---|
| Corpus callosum | |||||
| TAPBP | TAP binding protein | 2254.66 | 3431.10 | 0.61 | 1.54 × 10−2 |
| IRF4 | interferon regulatory factor 4 | 17.70 | 64.86 | 1.88 | 3.12 × 10−3 |
| CTSB | cathepsin B | 8640.73 | 17124.86 | 0.99 | 3.08 × 10−2 |
| IFI30 | IFI30 lysosomal thiol reductase | 13.63 | 37.46 | 1.48 | 4.26 × 10−2 |
| HSP90AB1 | heat shock protein 90 α family class B member 1 | 23769.44 | 45751.07 | 0.94 | 4.21 × 10−2 |
| HLA-DQB1 | major histocompatibility complex, class II, DQ β 1 | 249.73 | 1016.29 | 2.03 | 1.39 × 10−2 |
| CD79A | CD79a molecule | 6.88 | 83.66 | 3.59 | 3.33 × 10−6 |
| Hippocampus | |||||
| IL21R | interleukin 21 receptor | 10.40 | 112.12 | 3.42 | 1.92 × 10−2 |
| HLA-DQB1 | major histocompatibility complex, class II, DQ β 1 | 143.49 | 643.50 | 2.17 | 4.96 × 10−2 |
| Optic chiasm | |||||
| IRF4 | interferon regulatory factor 4 | 35.94 | 138.01 | 1.95 | 2.12 × 10−3 |
| VAV3 | vav guanine nucleotide exchange factor 3 | 90.27 | 174.52 | 0.96 | 2.62 × 10−2 |
| CTSB | cathepsin B | 13960.93 | 26669.15 | 0.93 | 4.79 × 10−3 |
| NCK1 | NCK adaptor protein 1 | 488.05 | 708.92 | 0.54 | 2.37 × 10−2 |
| STAT6 | signal transducer and activator of transcription 6 | 2661.12 | 4805.78 | 0.85 | 1.76 × 10−2 |
| HLA-DRB5 | major histocompatibility complex, class II, DR β 5 | 1051.38 | 3271.14 | 1.64 | 1.34 × 10−2 |
| HSP90AB1 | heat shock protein 90 α family class B member 1 | 26497.02 | 45815.97 | 0.79 | 1.77 × 10−2 |
| HLA-DQB1 | major histocompatibility complex, class II, DQ β 1 | 577.34 | 1919.98 | 1.73 | 2.19 × 10−2 |
| IFNGR2 | interferon γ receptor 2 | 1096.46 | 1814.94 | 0.73 | 7.04 × 10−3 |
| TAPBP | TAP binding protein | 3067.78 | 4365.48 | 0.51 | 3.09 × 10−2 |
| BCL10 | BCL10 immune signaling adaptor | 233.04 | 378.67 | 0.71 | 4.26 × 10−2 |
| CD79A | CD79a molecule | 9.29 | 210.76 | 4.48 | 1.31 × 10−11 |
| Internal capsule | |||||
| HLA-DQB1 | major histocompatibility complex, class II, DQ β 1 | 100.94 | 610.62 | 2.60 | 3.47 × 10−2 |
| LILRA6 | leukocyte immunoglobulin like receptor A6 | 6.43 | 40.55 | 2.60 | 2.79 × 10−2 |
| MAPK9 | mitogen-activated protein kinase 9 | 2429.45 | 1365.84 | −0.83 | 4.39 × 10−2 |
| IL1R1 | interleukin 1 receptor type 1 | 345.25 | 1046.19 | 1.60 | 4.00 × 10−2 |
| Frontal Cortex | |||||
| No gene. | |||||
| Parietal Cortex | |||||
| No gene. | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiricosta, L.; Gugliandolo, A.; Bramanti, P.; Mazzon, E. Could the Heat Shock Proteins 70 Family Members Exacerbate the Immune Response in Multiple Sclerosis? An in Silico Study. Genes 2020, 11, 615. https://doi.org/10.3390/genes11060615
Chiricosta L, Gugliandolo A, Bramanti P, Mazzon E. Could the Heat Shock Proteins 70 Family Members Exacerbate the Immune Response in Multiple Sclerosis? An in Silico Study. Genes. 2020; 11(6):615. https://doi.org/10.3390/genes11060615
Chicago/Turabian StyleChiricosta, Luigi, Agnese Gugliandolo, Placido Bramanti, and Emanuela Mazzon. 2020. "Could the Heat Shock Proteins 70 Family Members Exacerbate the Immune Response in Multiple Sclerosis? An in Silico Study" Genes 11, no. 6: 615. https://doi.org/10.3390/genes11060615





