Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. SSR Genotyping
2.3. SNP Genotyping
2.4. Microsatellite Database – Formation and Harmonization
2.5. Data Analysis
2.6. Genetic Structure
2.7. Parentage Analysis
3. Results
3.1. Genetic Characterization of Croatian Grapevine Germplasm Repositories
3.2. Descriptive Molecular Statistics for SSR And SNP Data
3.3. Comparison of SNP and SSR for Variety Discrimination
3.4. Chlorotype Distribution in Croatian Germplasm
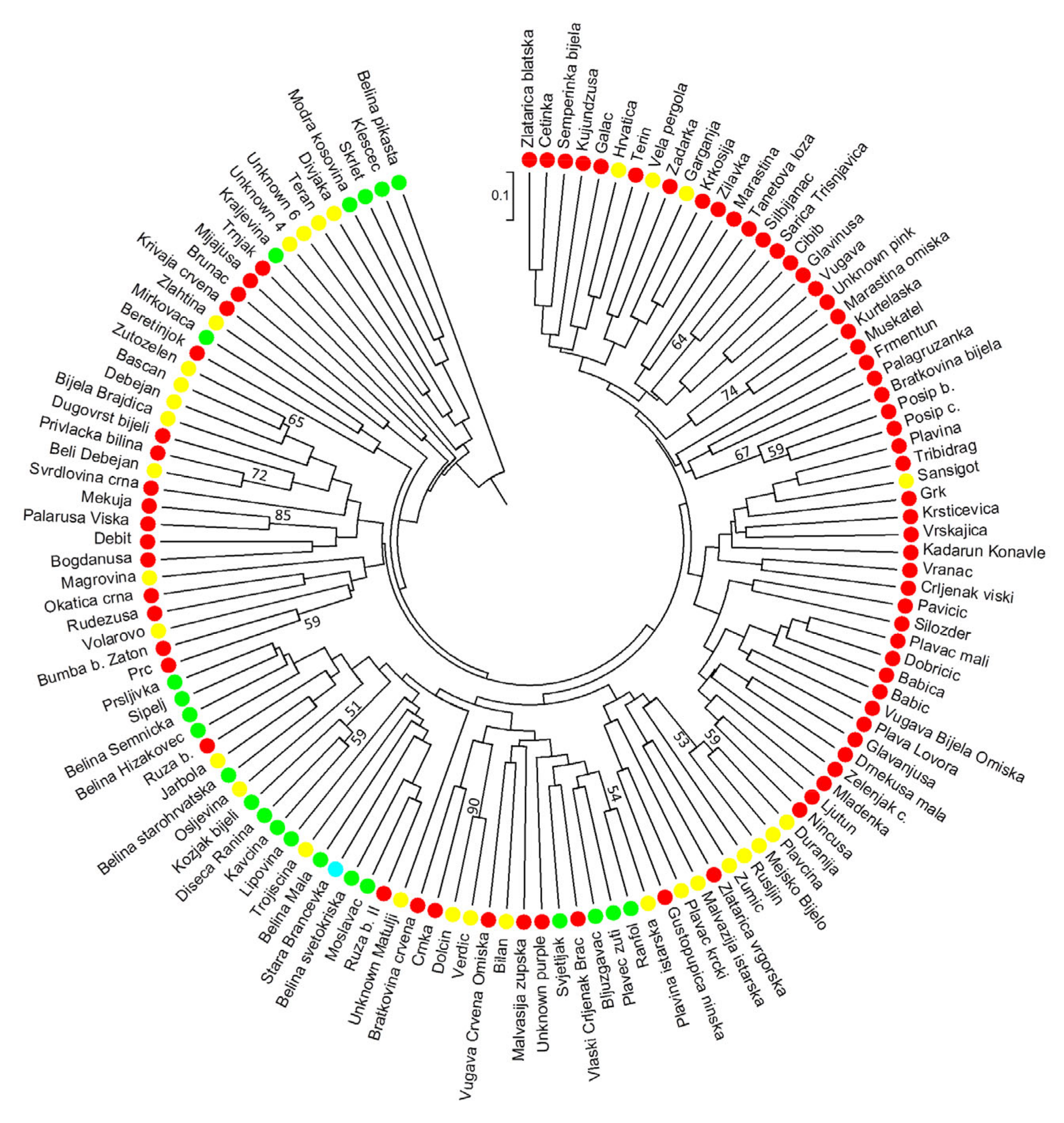
3.5. Cluster Analysis and Genetic Structure
3.6. Parentage Analysis
4. Discussion
4.1. Determined Status of Croatian Grapevine Collections
4.2. Choice of Markers for Routine Fingerprinting
4.3. Will SNP Markers Replace SSRs for Routine Identification of Grapevine Varieties?
4.4. Links to Other Countries through Synonyms
4.5. Chlorotype Variation and Geographical Distribution in Croatia
4.6. Genetic Diversity and Structure of Croatian Germplasm
4.7. Origin of Presumably Croatian Varieties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT (2019) Statistics Division of the Food and Agriculture Organization (FAO) of the United Nations, Rome. Available online: www.fao.org/faostat/en/#data/QC (accessed on 9 March 2020).
- Adam-Blondon, A.F.; Martinez-Zapater, J.M.; Kole, C. Genetics, Genomics, and Breeding of Grapes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–30. [Google Scholar]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- Anderson, K.; Aryal, N.R. Which Winegrape Varieties are Grown Where? University of Adelaide Press: Adelaide, South Australia, 2015; pp. 4–5. [Google Scholar]
- Hares, D.R. Selection and implementation of expanded CODIS core loci in the United States. Forensic Sci. Int. Genet. 2015, 17, 33–34. [Google Scholar] [CrossRef]
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Costantini, L.; Crespan, M.; Dangl, G.S.; Eisenheld, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for identification of grape varieties. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef]
- Maul, E.; Sudharma, K.N.; Kecke, S.; Marx, G.; Müller, C.; Audeguin, L.; Bosell, M.; Boursiquot, J.M.; Bucchetti, B.; Cabello, F.; et al. The European Vitis Database (www. eu-vitis. de)–a technical innovation through an uploading and interactive modification system. Vitis 2012, 51, 79–85. [Google Scholar]
- European Vitis Database. Available online: www.eu-vitis.de (accessed on 21 February 2012).
- Maul, E.; Töpfer, R.; Carka, F.; Cornea, V.; Crespan, M.; Dallakyan, M.; de Andrés Domínguez, T.; de Lorenzis, G.; Dejeu, L.; Goryslavets, S.; et al. Identification and characterization of grapevine genetic resources maintained in Eastern European Collections. Vitis 2015, 54, 5–12. [Google Scholar]
- Bergamini, C.; Perniola, R.; Cardone, M.F.; Gasparro, M.; Pepe, R.; Caputo, A.R.; Antonacci, D. The molecular characterization by SSRs reveals a new South Italian kinship and the origin of the cultivar Uva di Troia. SpringerPlus. 2016, 5, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.; Raimondi, S.; Pirolo, C.S.; Torello Marinoni, D.; Ruffa, P.; Venerito, P.; La Notte, P. Genetic characterization of grape cultivars from Apulia (Southern Italy) and synonymies in other Mediterranean regions. Am. J. Enol. Viticult. 2014, 65, 244–249. [Google Scholar] [CrossRef]
- Lacombe, T.; Boursiquot, J.M.; Laucou, V.; Dechesne, F.; Varès, D.; This, P. Relationships and genetic diversity within the accessions related to Malvasia held in the Domaine de Vassal grape germplasm repository. Am. J. Enol. Viticult. 2007, 58, 124–131. [Google Scholar]
- Laucou, V.; Lacombe, T.; Dechesne, F.; Siret, R.; Bruno, J.P.; Dessup, M.; Dessup, T.; Ortigosa, P.; Parra, P.; Roux, C.; et al. High throughput analysis of grape genetic diversity as a tool for germplasm collection management. Theor. Appl. Genet. 2011, 122, 1233–1245. [Google Scholar] [CrossRef]
- Lacombe, T.; Boursiquot, J.M.; Laucou, V.; Di Vecchi-Staraz, M.; Péros, J.-P.; Patrice, T. Large-scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 2013, 126, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Bacilieri, R.; Lacombe, T.; Le Cunff, L.; Di Vecchi-Staraz, M.; Laucou, V.; Genna, B.; Péros, J.P.; This, P.; Boursiquot, J.M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant Biol. 2013, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, S.D.; Péros, J.P.; Lacombe, T.; Launay, A.; Le Paslier, M.C.; Bérard, A.; Mangin, B.; Valière, S.; Martins, F.; Le Cunff, L.; et al. Genetic diversity, linkage disequilibrium and power of a large grapevine (Vitis vinifera L.) diversity panel newly designed for association studies. BMC Plant Biol. 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.P.; Luis Santiago, J.; Pinto-Carnide, O.; Leal, F.; Del Carmen Martínez, M.; Ortiz, J.M. Determination of relationships among autochthonous grapevine varieties (Vitis vinifera L.) in the Northwest of the Iberian Peninsula by using microsatellite markers. Gen. Resour. Crop Evol. 2006, 53, 1255–1261. [Google Scholar] [CrossRef]
- Zarouri, B.; Vargas, A.M.; Gaforio, L.; Aller, M.; de Andrés, M.T.; Cabezas, J.A. Whole-genome genotyping of grape using a panel of microsatellite multiplex PCRs. Tree Genet. Genomes 2015, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Di Gaspero, G.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymys and parentages, and reveals a large admixture amongst varieties of different geographic origin. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Dangl, G.S.; Mendum, M.L.; Prins, B.H.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Simple sequence repeat analysis of a clonally propagated species: A tool for managing a grape germplasm collection. Genome 2001, 44, 432–438. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Dangl, G.S.; Prins, B.H.; Boursiquot, J.M.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Genetic structure and differentiation in cultivated grape Vitis vinifera L. Genet. Res. 2003, 81, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Sefc, K.M.; Regner, F.; Glössl, J.; Steinkellner, H. Genotyping of grapevine and rootstock cultivars using microsatellite markers. Vitis 1998, 37, 15–20. [Google Scholar]
- Lopes, M.S.; Sefc, K.M.; Eires Dias, E.; Steinkellner, H.; Machado, M.L.C.; Machado, A.C. The use of microsatellites for germplasm management in a Portuguese grapevine collection. Theor. Appl. Genet. 1999, 99, 733–739. [Google Scholar] [CrossRef]
- Lopes, M.S.; Dos Santos, M.R.; Dias, J.E.; Mendonça, D.; Da Câmara Machado, A. Discrimination of Portuguese grapevines based on microsatellite markers. J. Biotechnol. 2006, 127, 34–44. [Google Scholar] [CrossRef]
- Lefort, F.; Roubelakis-Angelakis, K.K. Genetic comparison of Greek cultivars of Vitis vinifera L. by nuclear microsatellite profiling. Am. J. Enol. Viticult. 2001, 52, 101–108. [Google Scholar]
- Halász, G.; Veres, A.; Kozma, P.; Kiss, E.; Balogh, A.; Galli, Z.; Szőke, A.; Hoffmann, S.; Heszky, L. Microsatellite fingerprinting of grapevine (Vitis vinifera L.) varieties of the Carpathian Basin. Vitis 2005, 44, 173. [Google Scholar]
- Vouillamoz, J.F.; McGovern, P.E.; Ergul, A.; Söylemezoğlu, G.; Tevzadze, G.; Meredith, C.P.; Grando, M.S. Genetic characterization and relationships of traditional grape cultivars from Transcaucasia and Anatolia. Plant. Genet. Resour. C 2006, 4, 144–158. [Google Scholar] [CrossRef]
- Štajner, N.; Korošek-Koruza, Z.; Rusjan, D.; Javornik, B. Microsatellite genotyping of old Slovenian grapevine varieties (Vitis vinifera L.) of the Primorje (coastal) winegrowing region. Vitis 2008, 47, 201–204. [Google Scholar]
- Štajner, N.; Rusjan, D.; Korošec-Koruza, Z.; Javornik, B. Genetic characterization of old Slovenian grapevine varieties of Vitis vinifera L. by microsatellite genotyping. Am. J. Enol. Vitic. 2011, 62, 250–255. [Google Scholar] [CrossRef]
- Dzhambazova, T.; Tsvetkov, I.; Atanassov, I.; Rusanov, K.; Martínez-Zapater, J.M.; Atanassov, A.; Hvarleva, T. Genetic diversity in native Bulgarian grapevine germplasm (Vitis vinifera L.) based on nuclear and chloroplast microsatellite polymorphisms. Vitis 2009, 48, 115–121. [Google Scholar]
- Bešlić, Z.; Todić, S.; Korać, N.; Lorenzi, S.; Emanuelli, F.; Grando, M.S. Genetic characterization and relationships of traditional grape cultivars from Serbia. Vitis 2012, 51, 183–189. [Google Scholar]
- Tomić, L.; Štajner, N.; Jovanović-Cvetković, T.; Cvetković, M.; Javornik, B. Identity and genetic relatedness of Bosnia and Herzegovina grapevine germplasm. Sci. Hortic. 2012, 143, 122–126. [Google Scholar] [CrossRef]
- Sefc, K.M.; Lopes, M.S.; Lefort, F.; Botta, R.; Roubelakis-Angelakis, K.A.; Ibáñez, J.; Pejić, I.; Wagner, H.W.; Glössl, J.; Steinkellner, H. Microsatellite variability in grapevine cultivars from different European regions and evaluation of assignment testing to assess the geographic origin of cultivars. Theor. Appl. Genet. 2000, 100, 498–505. [Google Scholar] [CrossRef]
- Martín, J.P.; Arranz, C.; Castro, I.D.; Yuste, J.; Rubio, J.A.; Pinto-Carnide, O.; Ortiz, J.M. Prospection and identification of grapevine varieties cultivated in north Portugal and northwest Spain. Vitis 2011, 50, 29–33. [Google Scholar]
- Žulj Mihaljević, M.; Šimon, S.; Pejić, I.; Carka, F.; Sevo, R.; Kojić, A.; Gaši, F.; Tomić, L.; Jovanović Cvetković, T.; Maletić, E.; et al. Molecular characterization of old local grapevine varieties from South East European countries. Vitis 2013, 52, 69–76. [Google Scholar]
- Štajner, N.; Tomić, L.; Ivanišević, D.; Korać, N.; Cvetković-Jovanović, T.; Beleski, K.; Angelova, E.; Maraš, V.; Javornik, B. Microsatellite inferred genetic diversity and structure of Western Balkan grapevines (Vitis vinifera L.). Tree Genet. Genomes 2014, 10, 127–140. [Google Scholar] [CrossRef]
- Cabezas, J.A.; Ibáñez, J.; Lijavetzky, D.; Vélez, D.; Bravo, G.; Rodríguez, V.; Carreño, I.; Jermakow, A.M.; Carreño, J.; Ruiz-García, L.; et al. A 48 SNP set for grapevine cultivar identification. BMC Plant Biol. 2011, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Zinelabidine, L.H.; Laiadi, Z.; Benmehaia, R.; Gago, P.; Boso, S.; Santiago, J.L.; Haddioui, A.; Ibáñez, J.; Martínez-Zapater, J.M.; Martínez, M.C. Comparative ampelographic and genetic analysis of grapevine cultivars from Algeria and Morocco. Aust. J. Grape Wine Res. 2014, 20, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.; Ibáñez, J.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Eiras-Dias, J.E. Characterisation of the Portuguese grapevine germplasm with 48 single-nucleotide polymorphisms. Aust. J. Grape Wine Res. 2016, 22, 504–516. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic structure and domestication history of the grape. PNAS 2011, 108, 3530–3535. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzis, G.; Chipashvili, R.; Failla, O.; Maghradze, D. Study of genetic variability in Vitis vinifera L. germplasm by high-throughput Vitis18kSNP array: The case of Georgian genetic resources. BMC Plant Biol. 2015, 15, 154. [Google Scholar] [CrossRef] [Green Version]
- Laucou, V.; Launay, A.; Bacilieri, R.; Lacombe, T.; Adam-Blondon, A.F.; Berard, A.; Chauveau, A.; de AndreÂs, M.T.; Hausmann, L.; Ibáñez, J.; et al. Extended diversity analysis of cultivated grapevine Vitis vinifera with 10K genome-wide SNPs. PLoS ONE 2018, 13, e0192540. [Google Scholar] [CrossRef]
- Sefc, K.M.; Steinkellner, H.; Lefort, F.; Botta, R.; Da Câmara Machado, A.; Borrego, J.; Maletić, E.; Glössl, J. Evaluation of the genetic contribution of local wild vines to EU grapevine cultivars. Am. J. Enol. Viticult. 2003, 54, 15–21. [Google Scholar]
- Zdunić, G.; Preece, J.E.; Aradhya, M.; Velasco, D.; Koehmstedt, A.; Dangl, G.S. Genetic diversity and differentiation within and between cultivated (Vitis vinifera L. ssp. sativa) and wild (Vitis vinifera L. ssp. sylvestris) grapes. Vitis 2013, 52, 29–32. [Google Scholar]
- Bowers, J.E.; Meredith, C.P. The parentage of a classic wine grape, Cabernet Sauvignon. Nat. Genet. 1997, 16, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Goryslavets, S.; Risovanna, V.; Bacilieri, R.; Hausman, J.F.; Heuertz, M. A parentage study of closely related Ukrainian wine grape varieties using microsatellite markers. Cytol. Genet. 2010, 44, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, S.; Hasnaoui, N.; Zinelabidine, L.H.; Ferchichi, A.; Martínez-Zapater, J.M.; Ibáñez, J. Genetic diversity and parentage of Tunisian wild and cultivated grapevines (Vitis vinifera L.) as revealed by single nucleotide polymorphism (SNP) markers. Tree Genet. Genomes 2014, 10, 1103–1112. [Google Scholar] [CrossRef]
- Mena, A.; Martínez, J.; Fernández-González, M. Recovery, identification and relationships by microsatellite analysis of ancient grapevine cultivars from Castilla-La Mancha: The largest wine growing region in the world. Gen. Resour. Crop Evol. 2014, 61, 625–637. [Google Scholar] [CrossRef]
- Carka, F.; Maul, E.; Sevo, R. Study and parentage analysis of old Albanian grapevine cultivars by ampelography and microsatellite markers. Vitis 2015, 54, 127–131. [Google Scholar]
- Štajner, N.; Tomić, L.; Progar, V.; Pokorn, T.; Lacombe, T.; Laucou, V.; Boursiquot, J.M.; Javornik, B.; Bacilieri, R. Genetic clustering and parentage analysis of Western Balkan grapevines (Vitis vinifera L.). Vitis 2015, 54, 67–72. [Google Scholar]
- Raimondi, S.; Ruffa, P.; Boccacci, P.; Abbà, S.; Schneider, A. A few main parents contributed to the traditional grapevine cultivar assortment in north-western Italy, as revealed by microsatellites and single-nucleotide polymorphisms. In Proceedings of the ISHS Acta Horticulturae 1248: XII International Conference on Grapevine Breeding and Genetics, Bordeaux, France, 15–20 July 2018; Delrot, S., Ollat, N., Gallusci, P., Eds.; Volume 1248, pp. 295–300. [Google Scholar] [CrossRef]
- Arroyo-García, R.; Ruiz-García, L.; Bolling, L.; Ocete, R.; López, M.A.; Arnold, C.; Ergul, A.; Söylemezoglu, G.; Uzun, H.I.; Cabello, F.; et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Mol. Ecol. 2006, 15, 3707–3714. [Google Scholar] [CrossRef] [Green Version]
- Zaninović, M. Iliri i vinova loza. Godišnjak Centra Balkanološka Ispitivanja 1976, 13, 261–272. [Google Scholar]
- Bulić, S. Dalmatinska ampelografija, 1st ed.; Poljoprivredni nakladni Zavod: Zagreb, Croatia, 1949; pp. 5–343. [Google Scholar]
- Pejić, I.; Maletić, E. Conservation, evaluation and revitalization of native grapevine varieties. Mitt. Klosterneuburg. 2010, 60, 363–368. [Google Scholar]
- Maletić, E.; Sefc, K.M.; Steinkellner, H.; Kontić, J.K.; Pejić, I. Genetic characterization of Croatian grapevine cultivars and the detection of synonymous cultivars in neighboring regions. Vitis 1999, 38, 79–83. [Google Scholar]
- Zdunić, G.; Pejić, I.; Karoglan Kontić, J.; Vukičević, D.; Vokurka, A.; Pezo, I.; Maletić, E. Comparison of genetic and morphological data for inferring similarity among native Dalmatian (Croatia) grapevine cultivars (Vitis vinifera L.). J. Food. Agric. Environ. 2008, 6, 333–336. [Google Scholar]
- Zdunić, G.; Preece, J.E.; Dangl, G.S.; Koehmstedt, A.; Mucalo, A.; Maletić, E.; Pejić, I. Genetic characterization of grapevine cultivars collected throughout the Dalmatian region. Am. J. Enol. Viticult. 2013, 64, 285–290. [Google Scholar] [CrossRef]
- Žulj Mihaljević, M.; Anhalt, U.C.M.; Rühl, E.; Tomić Mugoša, M.; Forneck, A.; Zdunić, G.; Preiner, D.; Pejić, I. Cultivar Identity, Intravarietal Variation, and Health Status of Native Grapevine Varieties in Croatia and Montenegro. Am. J. Enol. Viticult. 2015, 66, 531–541. [Google Scholar] [CrossRef]
- Maletić, E.; Pejić, I.; Kontić, J.K.; Zdunić, D.; Preiner, D.; Šimon, S.; Stupić, D.; Andabaka, Ž.; Žulj Mihaljević, M.; Bubola, M.; et al. Ampelographic and genetic characterization of Croatian grapevine varieties. Vitis 2015, 54, 93–98. [Google Scholar]
- Šimon, S.; Preiner, D.; Maletić, E.; Pejić, I. Genetic similarity among Croatian and Greek grapevine cultivars assessed by SSRs. In Proceedings of the 9th International Conference on Grape Genetics and Breeding, Udine, Italy, 2–06 July 2006; Peterlunger, E., Di Gaspero, G., Eds.; [Google Scholar]
- Piljac, J.; Maletić, E.; Karoglan Kontić, J.; Dangl, G.S.; Pejić, I.; Mirošević, N.; Meredith, C.P. The parentage of a major white wine cultivar of Croatia. Vitis 2002, 41, 83–87. [Google Scholar]
- Maletić, E.; Pejić, I.; Karoglan Kontić, J.; Piljac, J.; Dangl, G.S.; Vokurka, A.; Lacombe, T.; Mirošević, N.; Meredith, C.P. Zinfandel, Dobričić, and Plavac mali: The genetic relationship among three cultivars of the Dalmatian coast of Croatia. Am. J. Enol. Viticult. 2004, 55, 174–180. [Google Scholar]
- Maletić, E.; Karoglan Kontić, J.; Pejić, I. Vinova loza: Ampelografija, ekologija i oplemenjivanje, 1st ed.; Školska knjiga: Zagreb, Croatia, 2008; pp. 62–109. [Google Scholar]
- Thomas, M.R.; Scott, N.S. Microsatellite repeats in grapevine reveal DNA polymorphisms when analysed as sequence-tagged sites (STSs). Theor. Appl. Genet. 1993, 86, 985–990. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat in loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and characterization of additional microsatellite DNA markers for grape. Am. J. Enol. Viticult. 1999, 50, 243–246. [Google Scholar]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zyprian, E.; Topfer, R. Development of microsatellite-derived markers for grapevine genotyping and genetic mapping; NCBI, GeneBank: Bethesda, MD, USA, 2005. [Google Scholar]
- Di Gaspero, G.; Peterlunger, E.; Testolin, R.; Edwards, K.J.; Cipriani, G. Conservation of microsatellite loci within the genus Vitis. Theor. Appl. Genet. 2000, 101, 301–308. [Google Scholar] [CrossRef]
- Merdinoglu, D.; Butterlin, G.; Bevilacqua, L.; Chiquet, V.; Adam-Blondon, A.F.; Decroocq, S. Development and characterization of a large set of microsatellite markers in grapevine (Vitis vinifera L.) suitable for multiplex PCR. Mol. Breed. 2005, 15, 349–366. [Google Scholar] [CrossRef]
- Cipriani, G.; Marrazzo, M.T.; Di Gaspero, G.; Pfeiffer, A.; Morgante, M.; Testolin, R. A set of microsatellite markers with long core repeat optimized for grape (Vitis spp.) genotyping. BMC Plant Biol. 2008, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.M.; Staub, J.E. The development and evaluation of consensus chloroplast primer pairs that possess highly variable sequence regions in a diverse array of plant taxa. Theor. Appl. Genet. 2003, 107, 757–767. [Google Scholar] [CrossRef]
- Weising, K.; Gardner, R.C. A set of conserved PCR primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledoneous angiosperms. Genome 1999, 42, 9–19. [Google Scholar] [CrossRef]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef]
- Hunt, H.V.; Lawes, M.C.; Bower, M.A.; Haeger, J.W.; Howe, C.J. A banned variety was the mother of several major wine grapes. Biol. Lett. 2009, 6, 367–369. [Google Scholar] [CrossRef]
- Macro Coding Genres. Available online: http://www1.montpellier.inra.fr/grapegen06/technical/menu_activities_room_index/page_wpI/WP1-macro_coding_genres-25-07-08.xls. (accessed on 21 February 2012).
- Pastore, C.; Fontana, M.; Raimondi, S.; Ruffa, P.; Filippetti, I.; Schneider, A. Genetic Characterization of Grapevine Varieties from Emilia-Romagna (Northern Italy) Discloses Unexplored Genetic Resources. Am. J. Enol. Viticult 2020, (in press). [Google Scholar] [CrossRef]
- Bodor, P.; Szoke, A.; Toth-Lencses, K.; Veres, A.; Deak, T.; Kozma, P.; Denes Bisztraya, G.; Kiss, E. Differentiation of grapevine (Vitis vinifera L.) conculta members based on molecular tools. Biotechnol. Biotechnol. Equip. 2014, 28, 14–20. [Google Scholar] [CrossRef]
- Costantini, L.; Monaco, A.; Vouillamoz, J.F.; Forlani, M.; Grando, M.S. Genetic relationships among local Vitis vinifera cultivars from Campania (Italy). Vitis 2005, 44, 25–34. [Google Scholar]
- Crespan, M.; Calò, A.; Giannetto, S.; Sparacio, A.; Storchi, P.; Costacurta, A. ‘Sangiovese’ and ‘Garganega’ are two key varieties of the Italian grapevine assortment evolution. Vitis 2008, 47, 97–104. [Google Scholar]
- Crespan, M.; Fabbro, A.; Giannetto, S.; Meneghetti, S.; Petrussi, C.; Del Zan, F.; Sivilotti, P. Recognition and genotyping of minor germplasm of Friuli Venezia Giulia revealed high diversity. Vitis 2011, 50, 21–28. [Google Scholar]
- González, M.F.; Gascueña, J.M.; Morales, A.M. Identification and relationships of grapevine cultivars authorized for cultivation in Castilla La Mancha (Spain). Am. J. Enol. Viticult. 2012, 63, 564–567. [Google Scholar] [CrossRef]
- Pellerone, F.I.; Edwards, K.J.; Thomas, M.R. Grapevine microsatellite repeats: Isolation, characterisation and use for genotyping of grape germplasm from Southern Italy. Vitis 2001, 40, 179–186. [Google Scholar]
- Ruffa, P.; Raimondi, S.; Boccacci, P.; Abbà, S.; Schneider, A. The key role of “Moscato bianco” and “Malvasia aromatica di Parma” in the parentage of traditional aromatic grape varieties. Tree Genet. Genomes 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Santana, J.C.; Hidalgo, E.; De Lucas, A.I.; Recio, P.; Ortiz, J.M.; Martín, J.P.; Yuste, J.; Arranz, C.; Rubio, J.A. Identification and relationships of accessions grown in the grapevine (Vitis vinifera L.) Germplasm Bank of Castilla y Léon (Spain) and the varieties authorized in the VQPRD areas of the region by SSR-marker analysis. Gen. Resour. Crop Evol. 2008, 55, 573–583. [Google Scholar] [CrossRef]
- Maul, E.; Eibach, R.; Zyprian, E.; Töpfer, R. The prolific grape variety (Vitis vinifera L.) ‘Heunisch Weiss’ (=‘Gouais blanc’): Bud mutants, “colored” homonyms and further offspring. Vitis 2015, 54, 79–86. [Google Scholar]
- De Lorenzis, G.; Imazio, S.; Rusjan, D.; Vouillamoz, J.F.; Nikolaou, N.; Failla, O.; Scienza, A. Genetic investigation of grapevine varieties ‘Ribolla Gialla’(Italy), ‘Rebula’(Slovenia) and ‘Robola’ (Ionian Islands). Sci. Hortic. 2013, 150, 425–431. [Google Scholar] [CrossRef]
- Crespan, M.; Crespan, G.; Giannetto, S.; Meneghetti, S.; Costacurta, A. Vitouska is the progeny of Prosecco tondo & Malvasia b. lunga. Vitis 2007, 46, 192–194. [Google Scholar]
- Šimon, S.; Maletić, E.; Kontić, J.K.; Crespan, M.; Schneider, A.; Pejić, I.C. Maraština a new member of Malvasia group. In II Int. Symp. "Mediterranean Malvasias", Salina (ME), 33. 2007. Available online: https://www.bib.irb.hr/350268 (accessed on 22 April 2013).
- Calò, A.; Costacurta, A.; Maraš, V.; Meneghetti, S.; Crespan, M. Molecular correlation of Zinfandel (Primitivo) with Austrian, Croatian, and Hungarian cultivars and Kratošija, an additional synonym. Am. J. Enol. Viticult. 2008, 59, 205–209. [Google Scholar]
- Rusjan, D.; Bubola, M.; Janjanin, D.; Užila, Z.; Radeka, S.; Poljuha, D.; Pelengić, R.; Javornik, B.; Štajner, N. Ampelographic characterization of grapevine accessions denominated ‘Refošk’, ‘Refosco’, ‘Teran’ and ‘Terrano’ (Vitis vinifera L.) from Slovenia, Croatia and Italy. Vitis 2015, 54, 77–80. [Google Scholar]
- Crespan, M.; Cabello, F.; Giannetto, S.; Ibáñez, J.; Kontic, J.K.; Maletic, E.; Pejić, I.; Rodriguez-Torres, I.; Antonacci, D. Malvasia delle Lipari, Malvasia di Sardegna, Greco di Gerace, Malvasia de Sitges and Malvasia dubrovacka-synonyms of an old and famous grape cultivar. Vitis 2006, 45, 69–73. [Google Scholar]
- Park, S.D.E. Excel Microsatellite Toolkit. Computer Program and Documentation Distributed by the Author. 2008. Available online: http://animalgenomics.ucd.ie/sdepark/ms-toolkit/ (accessed on 20 March 2012).
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314. [Google Scholar] [PubMed]
- Summers, K.; Amos, W. Behavioral, ecological and molecular genetic analyses of reproductive strategies in the Amazonian dart-poison frog, Dendrobates ventrimaculatus. Behav. Ecol. 1997, 8, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Waits, L.P.; Luikart, G.; Taberlet, P. Estimating the probability of identity among genotypes in natural populations: Cautions and guidelines. Mol. Ecol. 2001, 10, 249–256. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Goudet, J. FSTAT, A Program to Estimate and Test Gene Diversities and Fixation Indices Version 2.9.3.2. 2001. Available online: http://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 20 May 2016).
- El Mousadik, A.; Petit, R.J. High level of genetic differentiation for allelic richness among populations of the argan tree (Argania spinosa (L.) Skeels) endemic to Morocco. Theor. Appl. Genet. 1996, 92, 832–839. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Ruiz-Linares, A.; Tomfohrde, J.; Minch, E.; Kidd, J.R.; Cavalli-Sforza, L.L. High resolution of human evolutionary trees with polymorphic microsatellites. Nature 1994, 368, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Minch, E.; Ruiz-Linares, A.; Goldstein, D.; Feldman, M.; Cavalli-Sforza, L.L. Microsat (Version 1.4 d): A Computer Program for Calculating Various Statistics on Microsatellite Allele Data; University of Stanford: Stanford, CA, USA, 1995. [Google Scholar]
- Kumar, S.; Banks, T.W.; Cloutier, S. SNP discovery through next-generation sequencing and its applications. Int. J. Plant Genom. 2012, 2012, 831460. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Earl, D.A. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramasamy, R.K.; Ramasamy, S.; Bindroo, B.B.; Naik, V.G. STRUCTURE PLOT: A program for drawing elegant STRUCTURE bar plots in user friendly interface. Springer Plus 2014, 3, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žulj Mihaljević, M.; Maletić, E.; Zdunić, G.; Pejić, I. The key role of Plavac mali in the ancestry of Dalmatian grape cultivars and deconstruction of its parentage. (unpublished; manuscript in preparation).
- Peršurić, Đ.; Ilak Peršurić, A.S.; Godena, S.; Sinčić, M.; Petrušić, D.; Užila, Z. Ampelographic Description and Sanitary Analysis of Four Istrian Grapevine Varieties (Vitis vinifera L.). Agric. Conspec. Sci. 2012, 77, 113–117. [Google Scholar]
- Van Hintum, T.J.; Visser, D.L. Duplication within and between germplasm collections. Gen. Resour. Crop Evol. 1995, 42, 135–145. [Google Scholar] [CrossRef]
- Fresnedo-Ramírez, J.; Yang, S.; Sun, Q.; Cote, L.M.; Schweitzer, P.A.; Reisch, B.I.; Ledbetter, C.A.; Luby, J.J.; Clark, M.D.; Londo, J.P.; et al. An integrative AmpSeq platform for highly multiplexed marker-assisted pyramiding of grapevine powdery mildew resistance loci. Mol. Breed. 2017, 37, 145. [Google Scholar] [CrossRef]
- Flutre, T.; Bacilieri, R.; Bécavin, I.; Berger, G.; Bertrand, Y.; Boursiquot, J.M.; Fodor, A.; Lacombe, T.; Laucou, V.; Launay, A.; et al. Genome-wide association study of a diverse grapevine panel: Example of berry weight. In Proceedings of the ISHS Acta Horticulturae 1248: XII International Conference on Grapevine Breeding and Genetics, Bordeaux, France, 15–20 July 2018; Delrot, S., Ollat, N., Gallusci, P., Eds.; pp. 227–234. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Mercati, F.; Bergamini, C.; Cardone, M.F.; Lupini, A.; Mauceri, A.; Caputo, A.R.; Abbate, L.; Barbagallo, M.G.; Antonacci, D.; et al. SNP genotyping elucidates the genetic diversity of Magna Graecia grapevine germplasm and its historical origin and dissemination. BMC Plant Biol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Cabezas, J.A.; Ibáñez, A.; Rodríguez, V.; Martínez-Zapater, J.M. High throughput SNP discovery and genotyping in grapevine (Vitis vinifera L.) by combining a re-sequencing approach and SNPlex technology. BMC Genom. 2007, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluhić, D.; Peršurić, Đ.; Bubola, M.; Peršurić, A.S.I. Ampelographic characteristics of autochthonous population muškat momjanski bijeli. In Proceedings of the Drugi Hrvatski Oplemenjivački i Sjemenarski kongres, Poreč, Croatia, Sjemenarstvo, 23–27 May 2006; Kolak, I., Ed.; Hrvatsko Agronomsko Društvo: Zagreb, Croatia, 2006; Volume 23 Suppl. 1, pp. 62–63. [Google Scholar]
- Salmaso, M.; Vannozzi, A.; Lucchin, M. Chloroplast microsatellite markers to assess genetic diversity and origin of an endangered Italian grapevine collection. Am. J. Enol. Viticult. 2010, 61, 551–556. [Google Scholar] [CrossRef]
- Negrul, A.M. Evolucija kuljturnyx form vinograda. Dokl. Akad. Nauk. SSSR 1938, 8, 585. [Google Scholar]



| No. | Accession 1 | Accession 2 | Accession 3 | Accession 4 | Matches |
|---|---|---|---|---|---|
| 1 | Plavina istarska (5IPT) | Surina (18IPT) | Maločrn, Plavina Maločrn [30]; Tükörszolo (P14#992), EuVDB *; Piccola nera [14]; Gnjet [37] | ||
| 2 | Garganja (6IPT) | Garganja (2-H) | Lipolist (B&H) [36] | ||
| 3 | Vela pergola (24IPT) | Popovo oko (20IPT) | Malvazija crvena (21IPT) | Unknown 5 (7IPT) | Prošip Bijela (B&H) [37] |
| 4 | Plavinica (8IPT) | Plavina (4-C) | Lun (3-K) | Plavka (B&H) [36] | |
| 5 | Duranija (9IPT) | Draganela [57] | Duranja [30] | ||
| 6 | Jarbola (10IPT) | Jarbola (11-A) | |||
| 7 | Borgonja (11IPT) | Frankovka (CZE041-24V0100221), EuVDB; Tamjanika crna [37] | |||
| 8 | Muškat ruža porečki (13IPT) | Rosenmuskateller (DEU098-2000-021), EuVDB; Cipro [30] | |||
| 9 | Hrvatica (14 IPT) | Kamenina (KRK02, KRK14) | Corredera (DEU098-1993-001), EuVDB | ||
| 10 | Teran (15IPT) | Teran (2-B) | Terrano [14]; Lambrusco picol ross [79]; Refošk [93] | ||
| 11 | Malvazija istarska (16IPT) | Malvazija istarska (9-D) | Malvazija istarska (12-A) | ||
| 12 | Muškat momjanski (17IPT) | Muscat a petit grains (REF09); Muscat d’Alsace rose (FRA139-559Mtp8), EuVDB Moscatel Galego Branco ** [40] | |||
| 13 | Cimaroša (22IPT) | Velteliner rouge (FRA139-284Mtp4), EuVDB | |||
| 14 | Dolcin (23IPT) | Dolčin (2-D) | Vitouska (ITA368#SANOSVALDO/VC_10), EuVDB, [83,90] Vitovska [30] | ||
| 15 | Unknown 7 (25IPT) | Refosco peduncolo rosso (ITA362-1662), EuVDB | |||
| 16 | Pagadebit istarski (26IPT) | Kuč (3-C) | Maraština omiška (26-SK) | Mostosa = Empibotte bianco, [14]; Uva vacca [79] | |
| 17 | Bašćan (KRK01) | Stara brajda (3-L) | Susac (10-E) | Crna Brajda (8-SK) | Bascina bijela (DEU098-1990-130), EuVDB |
| 18 | Plavčina (KRK04) | Plavčina (10-B) | |||
| 19 | Ošljevina (KRK03) | Zelenika [29]; Rožica [30] | |||
| 20 | Troiščina (KRK08) | Trojiščina (11-B) | |||
| 21 | Žumić (KRK) | Žumić (5-G) | |||
| 22 | Brajdica (KRK) | Brajdica (2-J) | |||
| 23 | Debejan (KRK) | Debejan (10-C) | |||
| 24 | Gnjatonja(12-SK) | Dugovrst (5-B/2013) | |||
| 25 | Svrdlovina (17-SK) | Svrdlovina (27-A) | Gustopupica bibinjska (4-i_novi ) | ||
| 26 | Okatica crna(19-SK) | Okatica vrgorska (VRG08) | |||
| 27 | Palaruša bijela (22-SK) | Bumba bijela (8-K) | Medna bijela (internal acc.) | Žlozder [37] | |
| 28 | Okatica bijela (23-SK) | Palaruša hvarska (4-D) | Palaruša viška (5-D) | ||
| 29 | Silbijanac (25-SK) | Silbijanac (3-G) | |||
| 30 | Divljak (ZAG01) | Ranfol (ZAG05) | Ranfol (ZAG27) | Ranfol (9-E) | |
| 31 | Dišeća ranina (ZAG08) | Dišeća ranina (9-K) | |||
| 32 | Kavčina (ZAG10) | Kövidinka (P14#520), EuVDB | |||
| 33 | Belina Mala (ZAG11) | Biancghera [30] | |||
| 34 | Belina starohrvatska (ZAG16) | Gouais blanc (FRA139-211Mtp1), EuVDB; Heunisch weiss [88]; Branco Valente **[40] | |||
| 35 | Sokol (ZAG21) | Sokol (12-B) | Luglienga bianca (ITA360-501), EuVDB Caramela ** [40] | ||
| 36 | Volovina (ZAG26) | Moscato violetto [14]; Muscat rouge de Madère (ITA025-CO018), EuVDB | |||
| 37 | Modra kosovina (ZAG30) | Zimmettraube weiss (DEU098-1980-381), EuVDB | |||
| 38 | Moslavac (ZAG32) | Moslavac (9-L) | Furmint [14] Šipon [37] | ||
| 39 | Kozjak (ZAG42) | Coarna alba (DEU098-1991-188), EuVDB; Uva picciona [79] | |||
| 40 | Smudna belina (ZAG48) | Svjetljak (12-G) | |||
| 41 | Beli Debejan (10-D) | Gegić (3-F) | |||
| 42 | Verdić (11-F) | Teran bijeli [57]; Glera, Prosecco [29]; Beli Teran [30]; Prosecco tondo [89] | |||
| 43 | Petovka (3-E) | Cetinka (6-F) | Plavina bijela ninska (12-C) | ||
| 44 | Malvasia dubrovačka (2-A) | Malvasia delle Lipari [94] | |||
| 45 | Drnekuša vela (3-D) | Glavanjuša (5-A) | |||
| 46 | Cipar (3-J) | Grec rouge [14]; Rabigato Franco ** [40] | |||
| 47 | Maraština (4-A) | Malvasia del Chianti [14]; Pavlos [91] | |||
| 48 | Babić (4-B) | Babica plosnata (8-L) | |||
| 49 | Lasina (4-E) | Vlaški crljenak Brač (5-J) | |||
| 50 | Ninska crvena (4-K) | Šemperinka (8-G) | Vranac (7-G) | Vranac (15-F) | Vranac [14] |
| 51 | Pavicić (5-E) | Oskorušica (8-E) | |||
| 52 | Bak (5-F) | Siložder (6-L) | |||
| 53 | Bilan (5-H) | Racuk [29] | |||
| 54 | Beretinjok (5-I) | Topol (3-H_novi) | Bianco d’Alessano [11] | ||
| 55 | Muškat ruža omiški (5-K) | Muscat de Hambourg [14] | |||
| 56 | Muškat bijeli omiški (5-L) | Muscat d’Alexandrie [14] | |||
| 57 | Zlatarica vrgorska (6-C) | Kadarun IV, Surac IV [33]; Kadarun (B&H) [36]; Francavidda [11] | |||
| 58 | Pošip crni (6-D) | Šljiva (26-A) | Razaklija (28-B) | ||
| 59 | Marinkovića grozje (6-I) | Dattier de Beyrouth = Afuz Ali [14]; Radovača [37] | |||
| 60 | Plavac mali (7-A) | Plavac mali sivi (7-C) | |||
| 61 | Mijajuša (7-K) | Assouad karech [14]; Xerichi kokkino, EuVDB | |||
| 62 | Tribidrag (8-A) | Primitivo = Zinfandel [14] Kratošija [92] | |||
| 63 | Lelekuša (9-J) | Bratkovina crvena (30-A) | |||
| 64 | Frmentum (6-H) | Santa Teresa [11] | |||
| 65 | Bratkovina bijela (29-A) | Pošipica (33-B/2013) | Stradunska [36] | Maruggio (Maresco)= Uva del Monaco [11]; Popetre [30] | |
| 66 | Bljuzgavac (27-B_novi) | Blank blauer [14]; | |||
| 67 | Lipovina (24-A/2013) | Harslevelu [14] | |||
| 68 | VRG10 | Lisičina [60] | |||
| 69 | Kadarka | Olasz Kadarka [14] | |||
| 70 | Portugizac | Portugieser blau (REF12) | Portugais bleu [14] |
| Locus | Allele Range (bp) | N | MD | Na | Ne | Nar | He | HO | F | HW | F null | PIC | P(ID)unrelated | P(ID)sib |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VVS2 | 123–153 | 127 | 0 | 12 | 4.65 | 10.51 | 0.79 | 0.78 | 0.007 | NS | 0.0063 | 0.758 | 0.073 | 0.376 |
| VVMD7 | 231–261 | 127 | 0 | 10 | 3.66 | 8.70 | 0.73 | 0.80 | −0.095 | NS | −0.0436 | 0.685 | 0.116 | 0.416 |
| VVMD27 | 171–190 | 127 | 0 | 8 | 3.49 | 6.94 | 0.71 | 0.80 | −0.115 | NS | −0.0583 | 0.67 | 0.126 | 0.425 |
| ssrVrZAG62 | 179–203 | 127 | 0 | 10 | 5.34 | 8.97 | 0.81 | 0.89 | −0.095 | NS | −0.0484 | 0.789 | 0.058 | 0.358 |
| ssrVrZAG79 | 234–258 | 127 | 0 | 11 | 5.76 | 10.45 | 0.83 | 0.86 | −0.039 | NS | −0.0205 | 0.804 | 0.053 | 0.35 |
| VVMD5 | 218–242 | 127 | 0 | 10 | 5.08 | 9.36 | 0.80 | 0.84 | −0.049 | NS | −0.0258 | 0.78 | 0.062 | 0.364 |
| VVMD25 | 237–265 | 127 | 0 | 10 | 3.77 | 8.07 | 0.74 | 0.74 | −0.007 | NS | −0.0024 | 0.691 | 0.115 | 0.411 |
| VVMD28 | 216–276 | 127 | 0 | 12 | 8.20 | 11.09 | 0.88 | 0.94 | −0.067 | ND | −0.0329 | 0.866 | 0.027 | 0.318 |
| VVMD32 | 240–274 | 127 | 0 | 9 | 5.07 | 8.47 | 0.80 | 0.91 | −0.128 | NS | −0.0627 | 0.778 | 0.064 | 0.365 |
| VViq52 | 101–111 | 126 | 0.79 | 5 | 2.78 | 4.71 | 0.64 | 0.71 | −0.103 | NS | −0.0544 | 0.57 | 0.2 | 0.48 |
| VVIp31 | 192–216 | 127 | 0 | 12 | 7.18 | 11.18 | 0.86 | 0.95 | −0.098 | ND | −0.0476 | 0.845 | 0.035 | 0.328 |
| VVip60 | 321–348 | 124 | 2.36 | 12 | 4.74 | 10.61 | 0.79 | 0.82 | −0.032 | NS | −0.0184 | 0.76 | 0.074 | 0.374 |
| VMC1b11 | 185–215 | 125 | 1.57 | 10 | 4.88 | 9.62 | 0.80 | 0.85 | −0.066 | NS | −0.0362 | 0.774 | 0.063 | 0.368 |
| VMC4f3 | 185–246 | 127 | 0 | 15 | 4.27 | 12.38 | 0.77 | 0.76 | 0.013 | NS | 0.0066 | 0.741 | 0.079 | 0.387 |
| VVih54 | 166–205 | 126 | 0.79 | 12 | 4.11 | 10.15 | 0.76 | 0.81 | −0.07 | NS | −0.0341 | 0.732 | 0.084 | 0.393 |
| VViv67 | 354–406 | 126 | 0.79 | 12 | 5.25 | 10.28 | 0.81 | 0.83 | −0.03 | NS | −0.0152 | 0.785 | 0.061 | 0.361 |
| VVib01 | 307–325 | 127 | 0 | 5 | 2.79 | 5.00 | 0.64 | 0.67 | −0.044 | NS | −0.0205 | 0.58 | 0.19 | 0.477 |
| VVMD24 | 224–235 | 127 | 0 | 6 | 2.55 | 5.92 | 0.61 | 0.61 | 0.003 | NS | 0.0046 | 0.559 | 0.202 | 0.496 |
| VVMD21 | 244–281 | 126 | 0.79 | 7 | 2.60 | 5.92 | 0.62 | 0.71 | −0.148 | NS | −0.0712 | 0.57 | 0.193 | 0.491 |
| VVIn16 | 167–177 | 127 | 0 | 5 | 2.45 | 4.98 | 0.59 | 0.58 | 0.03 | NS | 0.0116 | 0.523 | 0.235 | 0.513 |
| VVIn73 | 272–285 | 127 | 0 | 7 | 1.47 | 5.14 | 0.32 | 0.32 | 0.011 | ND | 0.0095 | 0.287 | 0.496 | 0.715 |
| VViv37 | 153–183 | 106 | 16.54 | 13 | 3.93 | 11.33 | 0.75 | 0.79 | −0.063 | NS | −0.0422 | 0.721 | 0.09 | 0.399 |
| Vchr8b | 115–169 | 125 | 1.57 | 15 | 7.41 | 13.59 | 0.87 | 0.69 | 0.205 | ** | 0.1177 | 0.852 | 0.031 | 0.325 |
| Vchr10b | 145–154 | 126 | 0.79 | 3 | 2.45 | 3.00 | 0.59 | 0.66 | −0.112 | NS | −0.0525 | 0.505 | 0.254 | 0.517 |
| Vchr14b | 188–239 | 125 | 1.57 | 13 | 4.09 | 11.63 | 0.76 | 0.32 | 0.576 | *** | 0.4073 | 0.728 | 0.087 | 0.394 |
| Vchr4a | 200–224 | 115 | 9.45 | 6 | 2.67 | 5.08 | 0.63 | 0.64 | −0.03 | NS | −0.0106 | 0.552 | 0.214 | 0.491 |
| Vchr9a | 108–142 | 126 | 0.79 | 8 | 4.26 | 7.36 | 0.77 | 0.83 | −0.079 | NS | −0.0355 | 0.73 | 0.09 | 0.39 |
| Vchr16a | 118–186 | 127 | 0 | 8 | 1.98 | 7.32 | 0.50 | 0.49 | 0.014 | NS | 0.012 | 0.457 | 0.293 | 0.576 |
| Vchr19a | 143–170 | 118 | 7.09 | 8 | 3.13 | 7.27 | 0.68 | 0.71 | −0.047 | NS | −0.0305 | 0.639 | 0.144 | 0.446 |
| Vchr17a | 197–205 | 119 | 6.3 | 2 | 1.83 | 2.00 | 0.45 | 0.38 | 0.168 | NS | 0.0914 | 0.351 | 0.401 | 0.623 |
| Vchr18a | 170–210 | 122 | 3.94 | 10 | 4.72 | 8.28 | 0.79 | 0.75 | 0.043 | NS | 0.0211 | 0.761 | 0.072 | 0.374 |
| Vchr1b | 112–128 | 122 | 3.94 | 4 | 2.85 | 3.97 | 0.65 | 0.61 | 0.065 | NS | 0.0277 | 0.578 | 0.194 | 0.474 |
| Vchr7b | 189–205 | 121 | 4.72 | 4 | 3.74 | 4.00 | 0.73 | 0.80 | −0.094 | NS | −0.0447 | 0.683 | 0.122 | 0.414 |
| Vchr11b | 169–181 | 109 | 14.17 | 6 | 4.05 | 5.38 | 0.75 | 0.78 | −0.036 | NS | −0.0188 | 0.708 | 0.106 | 0.4 |
| Vchr12a | 141–162 | 121 | 4.72 | 6 | 2.98 | 5.40 | 0.67 | 0.72 | −0.082 | NS | −0.0417 | 0.605 | 0.172 | 0.461 |
| Vchr2b | 130–139 | 62 | 51.18 | 4 | 1.64 | 4.00 | 0.39 | 0.37 | 0.047 | ND | 0.0171 | 0.348 | 0.414 | 0.659 |
| Mean | 122 | 3.718 | 9 | 3.9 | 7.72 | 0.70 | 0.71 | −0.015 | −0.0038 | 0.66 | ||||
| Over loci | 1.19 × 10−34 | 5.29 × 10−14 |
| SNP Locus * | N | MD | Na | Ne | Nar | Ho | He | F | HW | MAF | PIC | P(ID) unrelated | P(ID) sib | F null | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP1003_336 | 124 | 0 | 2 | 1.78 | 2.00 | 0.42 | 0.44 | 0.04 | NS | A: | 0.323 | 0.342 | 0.412 | 0.635 | 0.0207 |
| SNP1015_67 | 124 | 0 | 2 | 1.46 | 2.00 | 0.35 | 0.32 | −0.094 | ND | C: | 0.476 | 0.267 | 0.517 | 0.721 | −0.0447 |
| SNP1027_69 | 124 | 0 | 2 | 1.96 | 2.00 | 0.51 | 0.49 | −0.036 | NS | G: | 0.266 | 0.37 | 0.38 | 0.6 | −0.0175 |
| SNP1035_226 | 124 | 0 | 2 | 1.39 | 2.00 | 0.31 | 0.28 | −0.089 | ND | T: | 0.48 | 0.242 | 0.556 | 0.748 | −0.0427 |
| SNP1079_58 | 124 | 0 | 2 | 1.96 | 2.00 | 0.60 | 0.49 | −0.219 | NS | A: | 0.246 | 0.37 | 0.38 | 0.6 | −0.0988 |
| SNP1119_176 | 124 | 0 | 2 | 1.98 | 2.00 | 0.48 | 0.49 | 0.02 | NS | C: | 0.375 | 0.372 | 0.378 | 0.598 | 0.01 |
| SNP1127_70 | 124 | 0 | 2 | 1.64 | 2.00 | 0.44 | 0.39 | −0.115 | NS | A: | 0.198 | 0.314 | 0.448 | 0.667 | −0.0543 |
| SNP1157_64 | 124 | 0 | 2 | 1.08 | 2.00 | 0.07 | 0.07 | −0.038 | ND | G: | 0.266 | 0.068 | 0.867 | 0.932 | −0.0097 |
| SNP1215_138 | 124 | 0 | 2 | 2.00 | 2.00 | 0.53 | 0.50 | −0.067 | NS | C: | 0.117 | 0.374 | 0.376 | 0.594 | −0.0324 |
| SNP1229_219 | 124 | 0 | 2 | 1.64 | 2.00 | 0.47 | 0.39 | −0.197 | NS | C: | 0.016 | 0.314 | 0.448 | 0.667 | −0.0899 |
| SNP1323_155 | 124 | 0 | 2 | 1.69 | 2.00 | 0.48 | 0.41 | −0.164 | NS | G: | 0.44 | 0.325 | 0.433 | 0.654 | −0.0759 |
| SNP1347_100 | 124 | 0 | 2 | 1.87 | 2.00 | 0.56 | 0.47 | −0.198 | NS | A: | 0.351 | 0.357 | 0.395 | 0.616 | −0.09 |
| SNP1349_174 | 124 | 0 | 2 | 1.99 | 2.00 | 0.56 | 0.50 | −0.116 | NS | T: | 0.431 | 0.374 | 0.376 | 0.595 | −0.055 |
| SNP1399_81 | 124 | 0 | 2 | 1.06 | 2.00 | 0.06 | 0.06 | −0.029 | ND | A: | 0.286 | 0.053 | 0.895 | 0.946 | −0.0062 |
| SNP1411_565 | 124 | 0 | 2 | 1.32 | 2.00 | 0.27 | 0.24 | −0.098 | ND | C: | 0.351 | 0.213 | 0.603 | 0.78 | −0.0466 |
| SNP1453_40 | 124 | 0 | 2 | 1.64 | 2.00 | 0.44 | 0.39 | −0.115 | NS | T: | 0.379 | 0.314 | 0.448 | 0.667 | −0.0543 |
| SNP1471_179 | 124 | 0 | 2 | 1.26 | 2.00 | 0.23 | 0.21 | −0.132 | ND | C: | 0.395 | 0.185 | 0.651 | 0.809 | −0.0569 |
| SNP1513_153 | 124 | 0 | 2 | 1.84 | 2.00 | 0.56 | 0.46 | −0.222 | NS | T: | 0.302 | 0.352 | 0.4 | 0.622 | −0.0998 |
| SNP191_100 | 124 | 0 | 2 | 1.07 | 2.00 | 0.07 | 0.06 | −0.033 | ND | C: | 0.169 | 0.06 | 0.881 | 0.939 | −0.0079 |
| SNP197_82 | 124 | 0 | 2 | 2.00 | 2.00 | 0.51 | 0.50 | −0.016 | NS | A: | 0.367 | 0.375 | 0.375 | 0.594 | −0.008 |
| SNP227_191 | 124 | 0 | 2 | 1.85 | 2.00 | 0.48 | 0.46 | −0.034 | NS | T: | 0.032 | 0.354 | 0.397 | 0.619 | −0.0167 |
| SNP259_199 | 123 | 0.81 | 2 | 1.76 | 2.00 | 0.46 | 0.43 | −0.051 | NS | G: | 0.488 | 0.339 | 0.415 | 0.637 | −0.025 |
| SNP269_308 | 124 | 0 | 2 | 1.92 | 2.00 | 0.45 | 0.48 | 0.055 | NS | C: | 0.302 | 0.364 | 0.387 | 0.608 | 0.0284 |
| SNP325_65 | 124 | 0 | 2 | 2.00 | 2.00 | 0.40 | 0.50 | 0.208 | NS | A: | 0.427 | 0.375 | 0.375 | 0.594 | 0.1163 |
| SNP425_205 | 124 | 0 | 2 | 1.03 | 2.00 | 0.03 | 0.03 | −0.016 | ND | G: | 0.472 | 0.031 | 0.938 | 0.969 | −0.0023 |
| SNP447_244 | 124 | 0 | 2 | 1.89 | 2.00 | 0.45 | 0.47 | 0.041 | NS | A: | 0.496 | 0.36 | 0.391 | 0.612 | 0.0207 |
| SNP581_114 | 124 | 0 | 2 | 1.97 | 2.00 | 0.70 | 0.49 | −0.424 | *** | A: | 0.44 | 0.371 | 0.379 | 0.598 | −0.1749 |
| SNP593_149 | 124 | 0 | 2 | 1.84 | 2.00 | 0.51 | 0.46 | −0.115 | NS | T: | 0.379 | 0.352 | 0.4 | 0.622 | −0.0546 |
| SNP613_315 | 124 | 0 | 2 | 1.34 | 2.00 | 0.27 | 0.25 | −0.048 | ND | A: | 0.306 | 0.222 | 0.589 | 0.77 | −0.0236 |
| SNP697_296 | 124 | 0 | 2 | 1.30 | 2.00 | 0.25 | 0.23 | −0.084 | ND | A: | 0.444 | 0.204 | 0.618 | 0.789 | −0.0401 |
| SNP819_210 | 124 | 0 | 2 | 1.59 | 2.00 | 0.44 | 0.37 | −0.196 | NS | G: | 0.028 | 0.302 | 0.465 | 0.681 | −0.0892 |
| SNP829_281 | 124 | 0 | 2 | 1.97 | 2.00 | 0.57 | 0.49 | −0.162 | NS | C: | 0.359 | 0.371 | 0.379 | 0.598 | −0.075 |
| SNP873_244 | 124 | 0 | 2 | 1.92 | 2.00 | 0.50 | 0.48 | −0.046 | NS | C: | 0.351 | 0.364 | 0.387 | 0.608 | −0.0225 |
| SNP879_308 | 124 | 0 | 2 | 2.00 | 2.00 | 0.57 | 0.50 | −0.146 | NS | A: | 0.488 | 0.375 | 0.375 | 0.594 | −0.068 |
| SNP895_382 | 124 | 0 | 2 | 1.89 | 2.00 | 0.52 | 0.47 | −0.096 | NS | A: | 0.113 | 0.36 | 0.391 | 0.612 | −0.046 |
| SNP945_88 | 124 | 0 | 2 | 2.00 | 2.00 | 0.52 | 0.50 | −0.049 | NS | T: | 0.266 | 0.375 | 0.375 | 0.594 | −0.0239 |
| SNP947_288 | 124 | 0 | 2 | 1.99 | 2.00 | 0.63 | 0.50 | −0.263 | NS | A: | 0.141 | 0.374 | 0.376 | 0.595 | −0.1163 |
| VVI_10113 | 124 | 0 | 2 | 1.89 | 2.00 | 0.40 | 0.47 | 0.143 | NS | A: | 0.317 | 0.36 | 0.391 | 0.612 | 0.0772 |
| VVI_10329 | 124 | 0 | 2 | 1.88 | 2.00 | 0.27 | 0.47 | 0.432 | *** | T: | 0.149 | 0.359 | 0.392 | 0.614 | 0.2757 |
| VVI_10353 | 124 | 0 | 2 | 1.84 | 2.00 | 0.49 | 0.46 | −0.08 | NS | A: | 0.468 | 0.352 | 0.4 | 0.622 | −0.0385 |
| VVI_10992 | 124 | 0 | 2 | 1.73 | 2.00 | 0.41 | 0.42 | 0.025 | NS | G: | 0.387 | 0.333 | 0.423 | 0.645 | 0.0128 |
| VVI_12882 | 124 | 0 | 2 | 1.73 | 2.00 | 0.35 | 0.42 | 0.178 | NS | A: | 0.036 | 0.333 | 0.423 | 0.645 | 0.0978 |
| VVI_1617 | 124 | 0 | 2 | 1.74 | 2.00 | 0.52 | 0.43 | −0.214 | NS | G: | 0.395 | 0.335 | 0.421 | 0.643 | −0.0967 |
| VVI_9227 | 124 | 0 | 2 | 1.25 | 2.00 | 0.19 | 0.20 | 0.034 | ND | G: | 0.133 | 0.18 | 0.66 | 0.815 | 0.0172 |
| VVI_9920 | 124 | 0 | 2 | 1.90 | 2.00 | 0.48 | 0.48 | −0.02 | NS | G: | 0.379 | 0.362 | 0.389 | 0.61 | −0.0098 |
| Marker Type | N loci | N | MD | A | Na | Ho | He | PIC | cum P(ID)unrelated | cum P(ID)sib |
|---|---|---|---|---|---|---|---|---|---|---|
| SSR | 9 * | 127 | 0 | 92 | 10.22 | 0.84 | 0.79 | 0.76 | 4.07 × 10−11 | 1.44 × 10−04 |
| SNP | 45 | 124 | 0.02% | 90 | 2 | 0.416 | 0.39 | 0.31 | 4.57 × 10−16 | 1.00 × 10−08 |
| Offspring | First Candidate Parent | Second Candidate Parent | SSR Total/ Mismatch | LOD | add. SSR Total/ Mismatch | SNP | Previously Reported |
|---|---|---|---|---|---|---|---|
| Sokol (n.d.) * | Bermestia bianca | Madeleine Salomon = Agostenga blanc | 20/0 | 46.01 | [14] | ||
| Dolcin (D) | Verdić (D) | Maraština (D) | 20/0 | 40.19 | 14/0 | 45/45 | [19,90] |
| Ljutun (D) | Plavac mali (D) | Bombino bianco (D) | 20/0 | 40.04 | 14/0 | ||
| Krstičevica (n.d.) | Plavina (C) | Bombino bianco (D) | 20/0 | 39.62 | 13/0 | ||
| Plavina (C) | Tribidrag (D) | Verdeca = Lagorthi | 20/0 | 37.72 | [12,14] | ||
| Kurtelaška (D) | Kuč (A) | ♀ Bombino bianco (D) | 20/0 | 37.56 | 14/0 | [14] | |
| Belina svetokriška (C) | Alba imputotato | Belina starohrvatska (C) | 19/0 | 36.38 | |||
| Debit (D) | Lasina (C) | ♀ Bombino bianco (D) | 20/0 | 36.34 | 8/0 | [14] | |
| Kozjak b. (C) | Bulanyi | Csomorika | 18/0 | 36.19 | |||
| Ninčuša (D) | Plavac mali (D) | Bombino bianco (D) | 20/0 | 35.70 | 14/0 | [14] | |
| Beli Debejan (D) | Privlačka bilina (n.d.) | ♀ Bombino bianco (D) | 19/0 | 35.35 | 15/0 | ||
| Muškatel (n.d.) | Dattier de Beyrouth = Afuz Ali | Perle de Csaba ) | 20/0 | 34.97 | |||
| Mejsko bijelo (A) | Duranija (A) | Žumić (A) | 20/0 | 34.77 | 8/1 | 45/1 | |
| Lipovina (n.d.) | Moslavac (C) | Tzimliansky belyi | 20/1 | 34.67 | |||
| Pošip b. (C) | ♀ Zlatarica blatska (C) | Bratkovina bijela (D) | 19/0 | 34.47 | 13/1 | 45/1 | [63] |
| Moslavac (C) | Alba imputotato | Belina starohrvatska (C) | 20/0 | 34.43 | |||
| Glavinuša (A) | Plavac mali (D) | ♀ Vugava (A) | 20/0 | 34.05 | 14/2 | [14] | |
| Crnka (D) | Plavac mali (D) | Bratkovina crvena (D) | 20/0 | 33.30 | 14/0 | 45/0 | |
| Volovina (n.d.) | Sciaccarello = Mammolo | Muškat momjanski=Muscat a pet. grains | 20/0 | 32.94 | [14] | ||
| Belina šemnička (C) | Kovacs Kreger | Belina starohrvatska (C) | 18/0 | 32.51 | |||
| Bljuzgavac (D) | Bratkovina crvena (D) | Gyöngy feher | 20/0 | 32.31 | [14] | ||
| Plavec žuti (C) | Bljuzgavac (D) | ♀ Belina starohrvatska (C) | 20/0 | 31.63 | 12/1 | ||
| Plavina istarska (C) | Pinella bianca | Bljuzgavac (D) | 20/0 | 29.55 | |||
| Ranfol (C) | Bljuzgavac (D) | ♀ Belina starohrvatska (C) | 20/0 | 28.84 | 13/2 | 45/0 | [14] |
| Svjetljak (C) | Bljuzgavac (D) | ♀ Belina starohrvatska (C) | 20/0 | 27.80 | 12/2 | 45/1 |
| Cultivar 1 | Cultivar 2 | SSR Genotyped/ Mismatch | LOD Score | Comments |
|---|---|---|---|---|
| Bljuzgavac | Žlahtina | 20/0 | 13.22 | Possible only if cultivar 1 is genitor of cultivar 2 |
| Bašćan | Bombino bianco | 20/0 | 12.31 | |
| Belina Hižakovec | Kozjak bijeli | 19/0 | 15.42 | |
| Belina Mala | Moslavac | 20/0 | 7.63 | |
| Belina Mala | Sacy [14] | 20/0 | 9.16 | |
| Belina Šemnička | Kozjak bijeli | 18/0 | 12.13 | Cultivar 2 grandparent of cultivar 1 |
| Bogdanuša | Palagružanka | 20/0 | 11.85 | |
| Bombino bianco | Glavanjuša | 20/0 | 10.68 | |
| Bombino bianco | Mladenka | 20/0 | 15.63 | |
| Bombino bianco | Žutozelen | 19/1 | 13.61 | |
| Bratkovina bijela | Pošip crni | 19/0 | 13.61 | |
| Dišeća ranina | Kozjak bijeli | 19/0 | 7.24 | Possible only if cultivar 2 is genitor of cultivar 1 |
| Dišeća ranina | Urmi dinka [14] | 19/0 | 10.8 | |
| Draganela | Plavčina | 20/0 | 15.22 | |
| Drnekuša mala | Plavac mali | 20/0 | 15.66 | |
| Glavanjuša | Plavac mali | 20/0 | 20.41 | |
| Jarbola | Belina starohrvatska | 20/0 | 9.37 | |
| Klešćec | Argant [14] | 20/0 | 19.87 | |
| Malvazija župska | Belina starohrvatska | 20/0 | 11.45 | |
| Plava lovora | Plavac mali | 19/0 | 16.37 | |
| Plavac mali | Siložder | 20/0 | 15.24 | |
| Plavac mali | Šemperinka bijela | 18/0 | 16.1 | |
| Plavac mali | Vugava b. omiška | 19/0 | 17.87 | |
| Plavac mali | Zelenjak | 20/0 | 18.64 | |
| Pošip crni | Vugava bijela omiška | 19/0 | 11.38 | |
| Pršljivka | Alba imputotato [14] | 20/0 | 11.28 | |
| Sansigot | Tribidrag | 20/0 | 13.87 | |
| Silbijanac | Alba imputotato [14] | 20/0 | 17.97 | |
| Škrlet | Argant [14] | 20/0 | 18.17 | |
| Teran | Barbera [14] | 17/0 | 6.05 | |
| Teran | Greco nero [14] | 20/0 | 19.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žulj Mihaljević, M.; Maletić, E.; Preiner, D.; Zdunić, G.; Bubola, M.; Zyprian, E.; Pejić, I. Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm. Genes 2020, 11, 737. https://doi.org/10.3390/genes11070737
Žulj Mihaljević M, Maletić E, Preiner D, Zdunić G, Bubola M, Zyprian E, Pejić I. Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm. Genes. 2020; 11(7):737. https://doi.org/10.3390/genes11070737
Chicago/Turabian StyleŽulj Mihaljević, Maja, Edi Maletić, Darko Preiner, Goran Zdunić, Marijan Bubola, Eva Zyprian, and Ivan Pejić. 2020. "Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm" Genes 11, no. 7: 737. https://doi.org/10.3390/genes11070737
APA StyleŽulj Mihaljević, M., Maletić, E., Preiner, D., Zdunić, G., Bubola, M., Zyprian, E., & Pejić, I. (2020). Genetic Diversity, Population Structure, and Parentage Analysis of Croatian Grapevine Germplasm. Genes, 11(7), 737. https://doi.org/10.3390/genes11070737










