Synthetic Pesticides Used in Agricultural Production Promote Genetic Instability and Metabolic Variability in Candida spp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Candida Strains, Experimental Conditions and Pesticides Tested
2.2. Testing Sensitivity to Pesticide Treatment
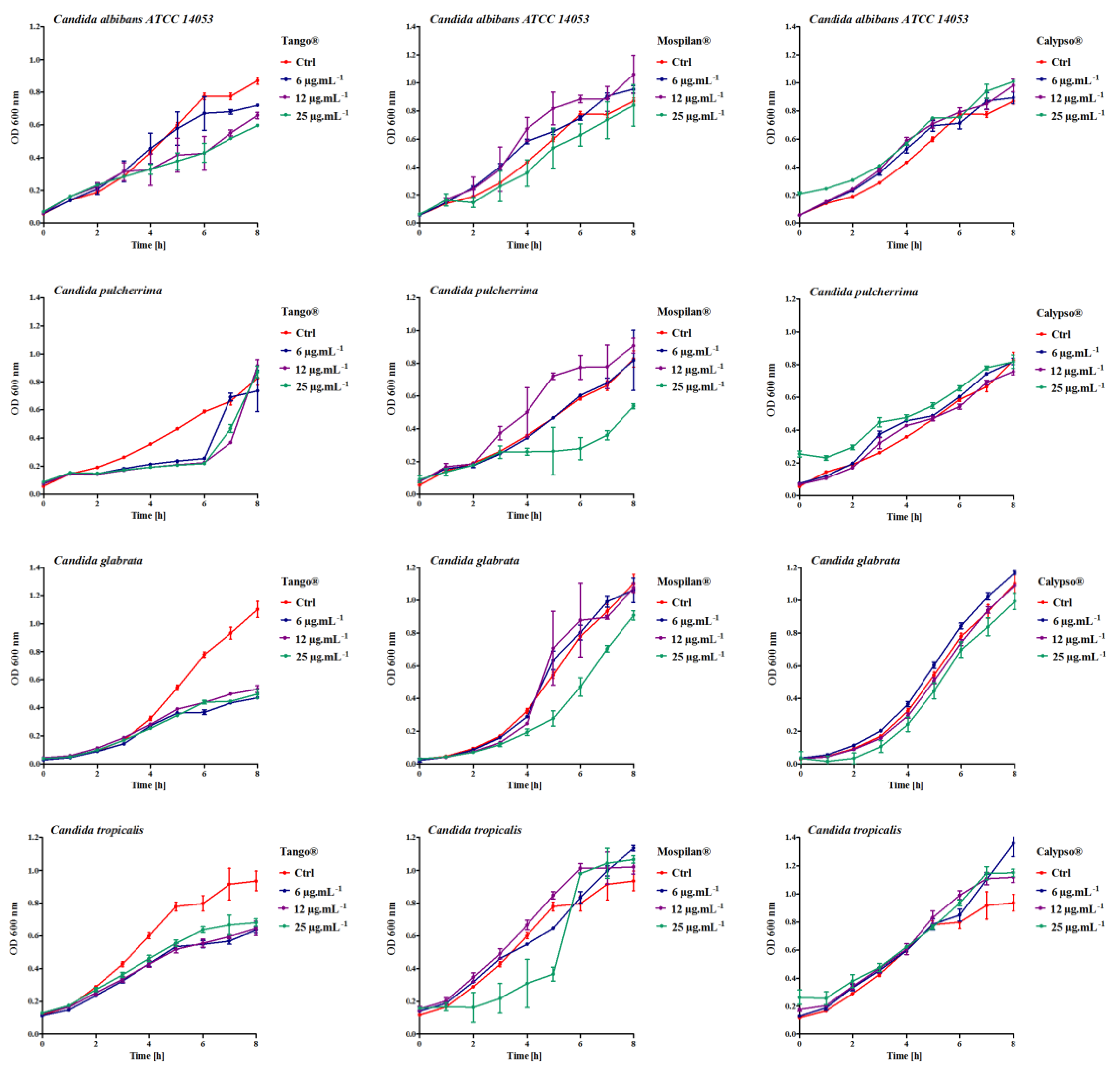
2.3. Growth Rate
2.4. Morphology Assessment
2.5. Effect on Candida Colony Aging
2.6. Biofilm Assay
2.7. Pesticide Content Analysis in Medium and Cell Pellet
2.8. Cell Viability Assays
2.9. Cell Cycle Phase Determination
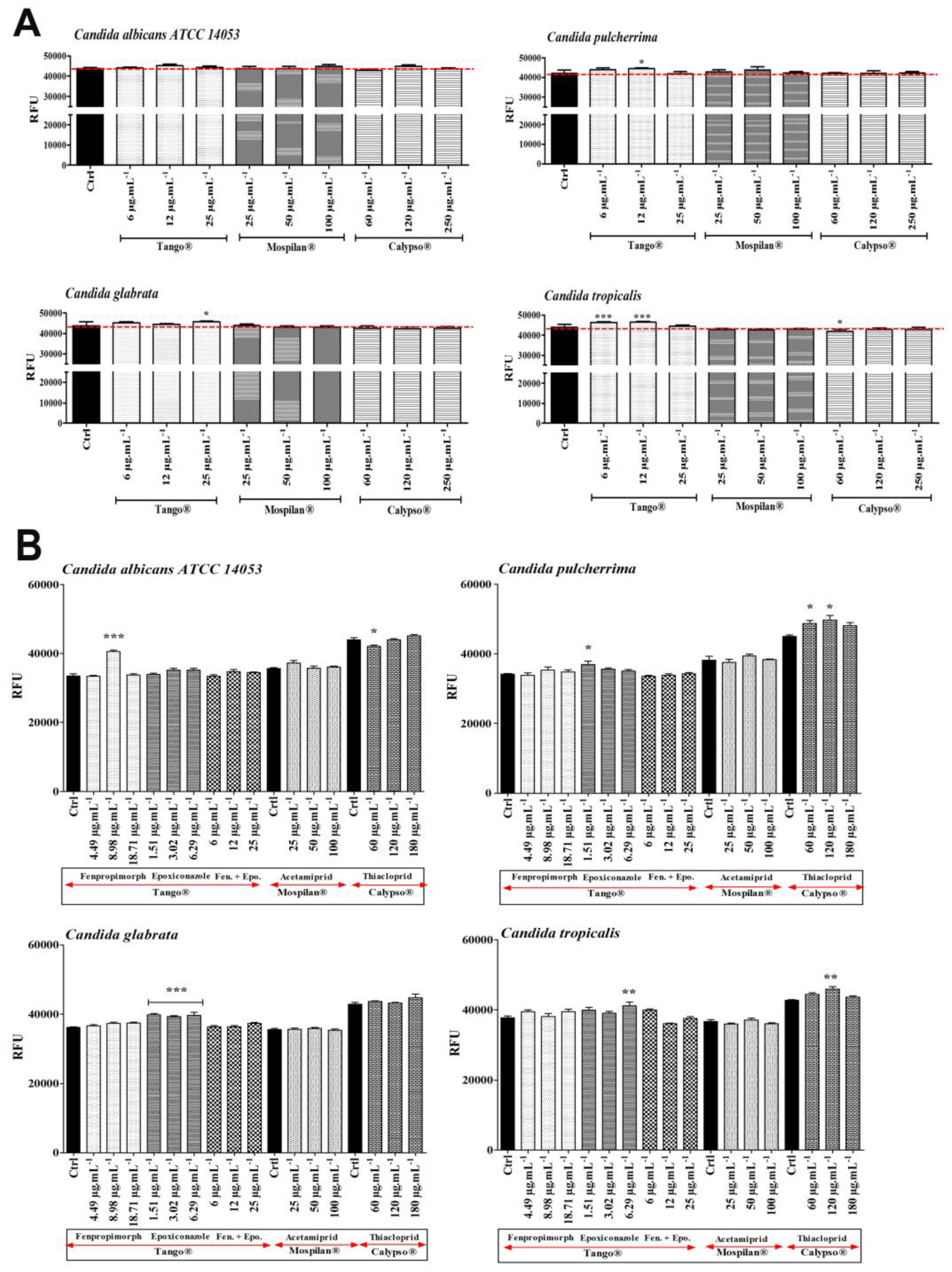
2.10. Oxidative Stress and Genotoxic Damage Assessment
2.11. Total Lipids Content Analysis
2.12. Phospholipid Determination by Phosphorus Assay
2.13. Glycogen Accumulation Analysis
2.14. Statistical Analysis
3. Results
3.1. Growth Change, Morphology and Aging
3.2. Bioavailability of Pesticides
3.3. Cytotoxicity and Changes in the Cell Cycle
3.4. Oxidative Stress and DNA Damage
3.5. Metabolic Activity Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lerro, C.C.; Koutros, S.; Andreotti, G.; Friesen, M.C.; Alavanja, M.C.; Blair, A.; Hoppin, J.A.; Sandler, D.P.; Lubin, J.H.; Ma, X.; et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2015, 72, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabello, G.; Valenzuela, M.; Vilaxa, A.; Duran, V.; Rudolph, I.; Hrepic, N.; Calaf, G. A rat mammary tumor model induced by the organophosphorous pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ. Health Perspect. 2001, 109, 471–479. [Google Scholar] [CrossRef]
- Melgarejo, M.; Mendiola, J.; Koch, H.M.; Monino-Garcia, M.; Noguera-Velasco, J.A.; Torres-Cantero, A.M. Associations between urinary organophosphate pesticide metabolite levels and reproductive parameters in men from an infertility clinic. Environ. Res. 2015, 137, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, S.; Nandi, S.; Gupta, P.S.; Ravindra, J.P. Effects of heavy metals and pesticides on buffalo (Bubalus bubalis) spermatozoa functions in vitro. Reprod. Domest. Anim. 2011, 46, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Lukowicz, C.; Ellero-Simatos, S.; Regnier, M.; Polizzi, A.; Lasserre, F.; Montagner, A.; Lippi, Y.; Jamin, E.L.; Martin, J.F.; Naylies, C.; et al. Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ. Health Perspect. 2018, 126, 067007. [Google Scholar] [CrossRef]
- Lopez, O.; Hernandez, A.F.; Rodrigo, L.; Gil, F.; Pena, G.; Serrano, J.L.; Parron, T.; Villanueva, E.; Pla, A. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicol. Lett. 2007, 171, 146–153. [Google Scholar] [CrossRef]
- Liu, Q.; Shao, W.; Zhang, C.; Xu, C.; Wang, Q.; Liu, H.; Sun, H.; Jiang, Z.; Gu, A. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ. Pollut. 2017, 226, 268–276. [Google Scholar] [CrossRef]
- Stanaway, I.B.; Wallace, J.C.; Shojaie, A.; Griffith, W.C.; Hong, S.; Wilder, C.S.; Green, F.H.; Tsai, J.; Knight, M.; Workman, T.; et al. Human Oral Buccal Microbiomes Are Associated with Farmworker Status and Azinphos-Methyl Agricultural Pesticide Exposure. Appl. Environ. Microbiol. 2017, 83, e02149-16. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Sommer, F.; Stahlman, M.; Ilkayeva, O.; Arnemo, J.M.; Kindberg, J.; Josefsson, J.; Newgard, C.B.; Frobert, O.; Backhed, F. The Gut Microbiota Modulates Energy Metabolism in the Hibernating Brown Bear Ursus arctos. Cell Rep. 2016, 14, 1655–1661. [Google Scholar] [CrossRef] [Green Version]
- Tognini, P. Gut Microbiota: A Potential Regulator of Neurodevelopment. Front. Cell Neurosci. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 2017, 168, 928–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedmousavi, S.; Bosco, S.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56 (Suppl. 1), 165–187. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C.; Barros, L.; Silva, S.; Henriques, M. Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014, 177, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.E.; Lockhart, S.R. Recent Taxonomic Developments with Candida and Other Opportunistic Yeasts. Curr. Fungal Infect. Rep. 2012, 6, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.A.; Boekhout, T.; Scorzetti, G.; Fell, J.W.; Kurtzman, C.P. Candida Berkhout (1923). In Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volumes 1–3, pp. 987–1278. [Google Scholar]
- Fleet, G.H. Yeasts in foods and beverages: Impact on product quality and safety. Curr. Opin. Biotechnol. 2007, 18, 170–175. [Google Scholar] [CrossRef]
- Tournas, V.H.; Heeres, J.; Burgess, L. Moulds and yeasts in fruit salads and fruit juices. Food Microbiol. 2006, 23, 684–688. [Google Scholar] [CrossRef] [Green Version]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Bellini, M.I.; Pinelli, L.; Dos Santos, M.E.; Scavino, A.F. Bacterial consortia from raw water and sludges from water potabilization plants are able to degrade atrazine. Int. Biodeterior. Biodegrad. 2014, 90, 131–139. [Google Scholar] [CrossRef]
- Trinder, M.; Bisanz, J.E.; Burton, J.P.; Reid, G. Probiotic lactobacilli: A potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Benef. Microbes 2015, 6, 841–847. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casida, J.E. Pest toxicology: The primary mechanisms of pesticide action. Chem. Res. Toxicol. 2009, 22, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Ihara, M.; Buckingham, S.D.; Matsuda, K.; Sattelle, D.B. Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J. Neurochem. 2006, 99, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Zarn, J.A.; Bruschweiler, B.J.; Schlatter, J.R. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14 alpha-demethylase and aromatase. Environ. Health Perspect. 2003, 111, 255–261. [Google Scholar] [CrossRef]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of Action and Drug Resistance. Adv. Exp. Med. Biol. 2016, 892, 327–349. [Google Scholar]
- Wachowska, U.; Irzykowski, W.; Jedryczka, M. Agrochemicals: Effect on genetic resistance in yeasts colonizing winter wheat kernels. Ecotoxicol. Environ. Saf. 2018, 162, 77–84. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Podbielska, M.; Kus-Liskiewicz, M.; Jagusztyn, B.; Piechowicz, B.; Sadlo, S.; Slowik-Borowiec, M.; Twaruzek, M.; Szpyrka, E. Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies. Molecules 2020, 25, 1421. [Google Scholar] [CrossRef] [Green Version]
- Schneemann, I.; Kajahn, I.; Ohlendorf, B.; Zinecker, H.; Erhard, A.; Nagel, K.; Wiese, J.; Imhoff, J.F. Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea. J. Nat. Prod. 2010, 73, 1309–1312. [Google Scholar] [CrossRef]
- Schwarzbacherova, V.; Wnuk, M.; Lewinska, A.; Potocki, L.; Zebrowski, J.; Koziorowski, M.; Holeckova, B.; Sivikova, K.; Dianovsky, J. Evaluation of cytotoxic and genotoxic activity of fungicide formulation Tango((R)) Super in bovine lymphocytes. Environ. Pollut. 2017, 220, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Wang, Z.; Klaunig, J.E. Alkaline Comet Assay for Assessing DNA Damage in Individual Cells. Curr. Protoc. Toxicol. 2015, 65, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Miedziak, B.; Wnuk, M. Assessment of yeast chromosome XII instability: Single chromosome comet assay. Fungal. Genet. Biol. 2014, 63, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Durand, R.E.; Banath, J.P.; Johnston, P.J. Analysis of DNA damage in individual cells. Methods Cell. Biol. 2001, 64, 235–249. [Google Scholar] [PubMed]
- van Wychen, S.; Laurens, L.M.L. Determination of total lipids as fatty acid methyl esters (FAME) by in situ transesterification. Contract 2013, 303, 300–375. [Google Scholar]
- Chester, V.E. Heritable glycogen-storage deficiency in yeast and its induction by ultra-violet light. J. Gen. Microbiol. 1968, 51, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Kosel, J.; Raspor, P.; Čadež, N. Maximum residue limit of fungicides inhibits the viability and growth of desirable non-Saccharomyces wine yeasts. Aust. J. Grape Wine Res. 2019, 25, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Vachova, L.; Cap, M.; Palkova, Z. Yeast colonies: A model for studies of aging, environmental adaptation, and longevity. Oxid. Med. Cell. Longev. 2012, 2012, 601836. [Google Scholar] [CrossRef] [Green Version]
- Palkova, Z.; Vachova, L. Life within a community: Benefit to yeast long-term survival. FEMS Microbiol. Rev. 2006, 30, 806–824. [Google Scholar] [CrossRef] [Green Version]
- Palkova, Z.; Vachova, L. Ammonia signaling in yeast colony formation. Int. Rev. Cytol. 2003, 225, 229–272. [Google Scholar] [PubMed]
- Sellam, A.; Whiteway, M. Recent advances on Candida albicans biology and virulence. F1000Research 2016, 5, 2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, R.; Delaney, C.; Rajendran, R.; Sherry, L.; Metcalfe, R.; Thomas, R.; McLean, W.; Williams, C.; Ramage, G. Gaining Insights from Candida Biofilm Heterogeneity: One Size Does Not Fit All. J. Fungi 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel, B.D.; White, T.C. Accumulation of Azole Drugs in the Fungal Plant Pathogen Magnaporthe oryzae Is the Result of Facilitated Diffusion Influx. Front. Microbiol. 2017, 8, 1320. [Google Scholar] [CrossRef] [Green Version]
- Mansfield, B.E.; Oltean, H.N.; Oliver, B.G.; Hoot, S.J.; Leyde, S.E.; Hedstrom, L.; White, T.C. Azole drugs are imported by facilitated diffusion in Candida albicans and other pathogenic fungi. PLoS Pathog. 2010, 6, e1001126. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.J.; Ji, W.W.; Chen, T.; Zhang, W.J.; Liu, Z.H.; Ge, F.; Yuan, S. Metabolism of the Neonicotinoid insecticides acetamiprid and thiacloprid by the yeast Rhodotorula mucilaginosa strain IM-2. J. Agric. Food Chem. 2010, 58, 2419–2425. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Sláviková, E. Influence of Pesticides on Yeasts Colonizing Leaves. Z. Nat. C J. Biosci. 2011, 66, 588–594. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Isuwa, M.M.; Savinkova, O.V.; Derevshchikova, M.I.; Popov, V.N. The Effect of Pesticides on the Microbiome of Animals. Agriculture 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- Komin, N.; Skupin, A. How to address cellular heterogeneity by distribution biology. Curr. Opin. Syst. Biol. 2017, 3, 154–160. [Google Scholar] [CrossRef]
- Thompson, D.S.; Carlisle, P.L.; Kadosh, D. Coevolution of morphology and virulence in Candida species. Eukaryot. Cell 2011, 10, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Berman, J.; Sudbery, P.E. Candida albicans: A molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 2002, 3, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Ali, S.; Ahmed, L. Biological Study of Candida Species and Virulence Factor. Int. J. Adv. Res. Technol. 2018, 1, 8–16. [Google Scholar]
- Marichal, P.; Gorrens, J.; Van Cutsem, J.; Vanden Bossche, H. Culture media for the study of the effects of azole derivatives on germ tube formation and hyphal growth of C. albicans. Mykosen 1986, 29, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.C.; White, T.C. Effects of azole antifungal drugs on the transition from yeast cells to hyphae in susceptible and resistant isolates of the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 1999, 43, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Čadež, N.; Zupan, J.; Raspor, P. The effect of fungicides on yeast communities associated with grape berries. FEMS Yeast Res. 2010, 10, 619–630. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Martínez, M.; López-Ribot, J.L.; Kirkpatrick, W.R.; Bachmann, S.P.; Perea, S.; Ruesga, M.T.; Patterson, T.F. Heterogeneous mechanisms of azole resistance in Candida albicans clinical isolates from an HIV-infected patient on continuous fluconazole therapy for oropharyngeal candidosis. J. Antimicrob. Chemother. 2002, 49, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Mondon, P.; Petter, R.; Amalfitano, G.; Luzzati, R.; Concia, E.; Polacheck, I.; Kwon-Chung, K.J. Heteroresistance to Fluconazole and Voriconazole in Cryptococcus neoformans. Antimicrob. Agents Chemother. 1999, 43, 1856–1861. [Google Scholar] [CrossRef] [Green Version]
- Ginovart, M.; Carbó, R.; Blanco, M.; Portell, X. Digital Image Analysis of Yeast Single Cells Growing in Two Different Oxygen Concentrations to Analyze the Population Growth and to Assist Individual-Based Modeling. Front. Microbiol. 2018, 8, 2628. [Google Scholar] [CrossRef]
- Chung, Y.M.; Bae, Y.S.; Lee, S.Y. Molecular ordering of ROS production, mitochondrial changes, and caspase activation during sodium salicylate-induced apoptosis. Free Radic. Biol. Med. 2003, 34, 434–442. [Google Scholar] [CrossRef]
- Franco, R.; Li, S.; Rodriguez-Rocha, H.; Burns, M.; Panayiotidis, M.I. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2010, 188, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shekhova, E.; Kniemeyer, O.; Brakhage, A.A. Induction of Mitochondrial Reactive Oxygen Species Production by Itraconazole, Terbinafine, and Amphotericin B as a Mode of Action against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e00978-17. [Google Scholar] [CrossRef] [Green Version]
- Schwarzbacherova, V.; Wnuk, M.; Deregowska, A.; Holeckova, B.; Lewinska, A. In vitro exposure to thiacloprid-based insecticide formulation promotes oxidative stress, apoptosis and genetic instability in bovine lymphocytes. Toxicol. In Vitro 2019, 61, 104654. [Google Scholar] [CrossRef] [PubMed]
- Tafforeau, L.; Le Blastier, S.; Bamps, S.; Dewez, M.; Vandenhaute, J.; Hermand, D. Repression of ergosterol level during oxidative stress by fission yeast F-box protein Pof14 independently of SCF. EMBO J. 2006, 25, 4547–4556. [Google Scholar] [CrossRef] [PubMed]
- Arana, D.M.; Nombela, C.; Pla, J. Fluconazole at subinhibitory concentrations induces the oxidative- and nitrosative-responsive genes TRR1, GRE2 and YHB1, and enhances the resistance of Candida albicans to phagocytes. J. Antimicrob. Chemother. 2009, 65, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; François, J.-M.; Capp, J.-P. Gene Expression Noise Produces Cell-to-Cell Heterogeneity in Eukaryotic Homologous Recombination Rate. Front. Genet. 2019, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Palková, Z.; Wilkinson, D.; Váchová, L. Aging and differentiation in yeast populations: Elders with different properties and functions. FEMS Yeast Res. 2014, 14, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Zarei Mahmoudabadi, P.A.; Drucker, D.B. Effect of amphotericin B, nystatin and miconazole on the polar lipids of Candida albicans and Candida dubliniensis. Indian J. Pharmacol. 2006, 38, 423. [Google Scholar] [CrossRef]
- Sharma, S.; Khuller, G.K. Changes in the cellular composition of Candida albicans resistant to miconazole. Indian J. Biochem. Biophys. 1996, 33, 420–424. [Google Scholar]
- Mishra, N.N.; Prasad, T.; Sharma, N.; Gupta, D.K. Membrane fluidity and lipid composition of fluconazole resistant and susceptible strains of Candida albicans isolated from diabetic patients. Braz. J. Microbiol. 2008, 39, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Kapoor, K. Multidrug resistance in yeast Candida. Int. Rev. Cytol. 2005, 242, 215–248. [Google Scholar] [PubMed]
- Zeitz, M.A.; Tanveer, Z.; Openshaw, A.T.; Schmidt, M. Genetic Regulators and Physiological Significance of Glycogen Storage in Candida albicans. J. Fungi 2019, 5, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potocki, L.; Depciuch, J.; Kuna, E.; Worek, M.; Lewinska, A.; Wnuk, M. FTIR and Raman Spectroscopy-Based Biochemical Profiling Reflects Genomic Diversity of Clinical Candida Isolates That May Be Useful for Diagnosis and Targeted Therapy of Candidiasis. Int. J. Mol. Sci. 2019, 20, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Strains | Characteristics |
|---|---|
| Candida albicans ATCC 14053 | Control strain |
| Candida pulcherrima VKM Y-955 | Environmental isolate, strains were kindly provided from the Institute of Cell Biology NASU Lviv, Ukraine |
| Candida glabrata | Urinary isolate, female; Identification (API® Candida Biochemical Test) and DNA sequencing–GenBank Accession Number–LC389261.1 |
| Candida tropicalis | Bronchoalveolar lavage isolate, male; Identification (API®Candida Biochemical Test) and DNA sequencing–GenBankAccession Number-KX664669.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potocki, L.; Baran, A.; Oklejewicz, B.; Szpyrka, E.; Podbielska, M.; Schwarzbacherová, V. Synthetic Pesticides Used in Agricultural Production Promote Genetic Instability and Metabolic Variability in Candida spp. Genes 2020, 11, 848. https://doi.org/10.3390/genes11080848
Potocki L, Baran A, Oklejewicz B, Szpyrka E, Podbielska M, Schwarzbacherová V. Synthetic Pesticides Used in Agricultural Production Promote Genetic Instability and Metabolic Variability in Candida spp. Genes. 2020; 11(8):848. https://doi.org/10.3390/genes11080848
Chicago/Turabian StylePotocki, Leszek, Aleksandra Baran, Bernadetta Oklejewicz, Ewa Szpyrka, Magdalena Podbielska, and Viera Schwarzbacherová. 2020. "Synthetic Pesticides Used in Agricultural Production Promote Genetic Instability and Metabolic Variability in Candida spp." Genes 11, no. 8: 848. https://doi.org/10.3390/genes11080848
APA StylePotocki, L., Baran, A., Oklejewicz, B., Szpyrka, E., Podbielska, M., & Schwarzbacherová, V. (2020). Synthetic Pesticides Used in Agricultural Production Promote Genetic Instability and Metabolic Variability in Candida spp. Genes, 11(8), 848. https://doi.org/10.3390/genes11080848







