Lights and Shadows of TORCH Infection Proteomics
Abstract
:1. Background
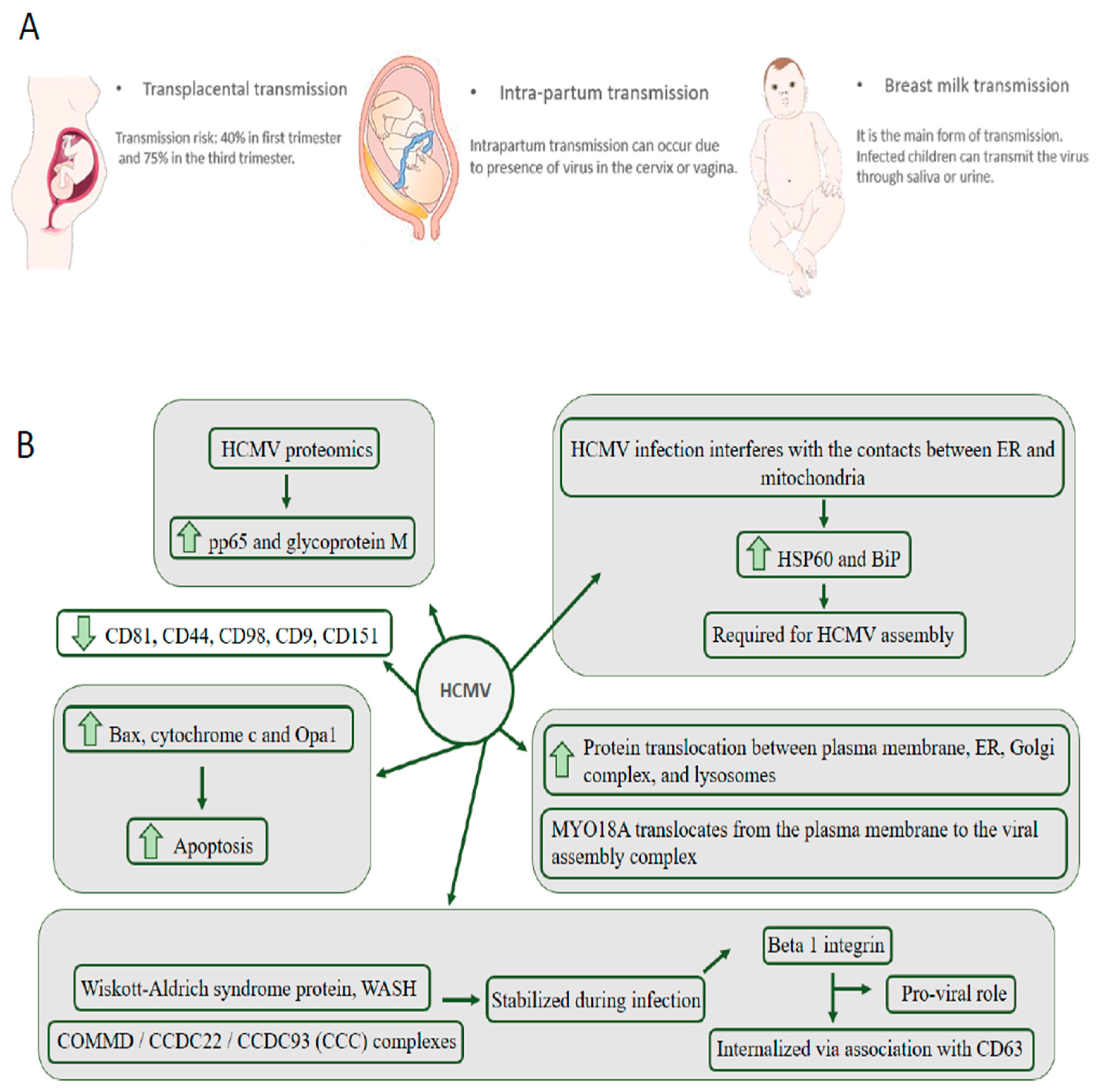
2. HCMV Is the Leading Cause of Congenital Neurological Disease by Transmission through the Placenta from the Mother to the Child
3. ZIKV: In 2015, the World Health Organization Reported Cases of Neurological Disorders in Infants Who Had Their Mothers Exposed to the Virus during Pregnancy
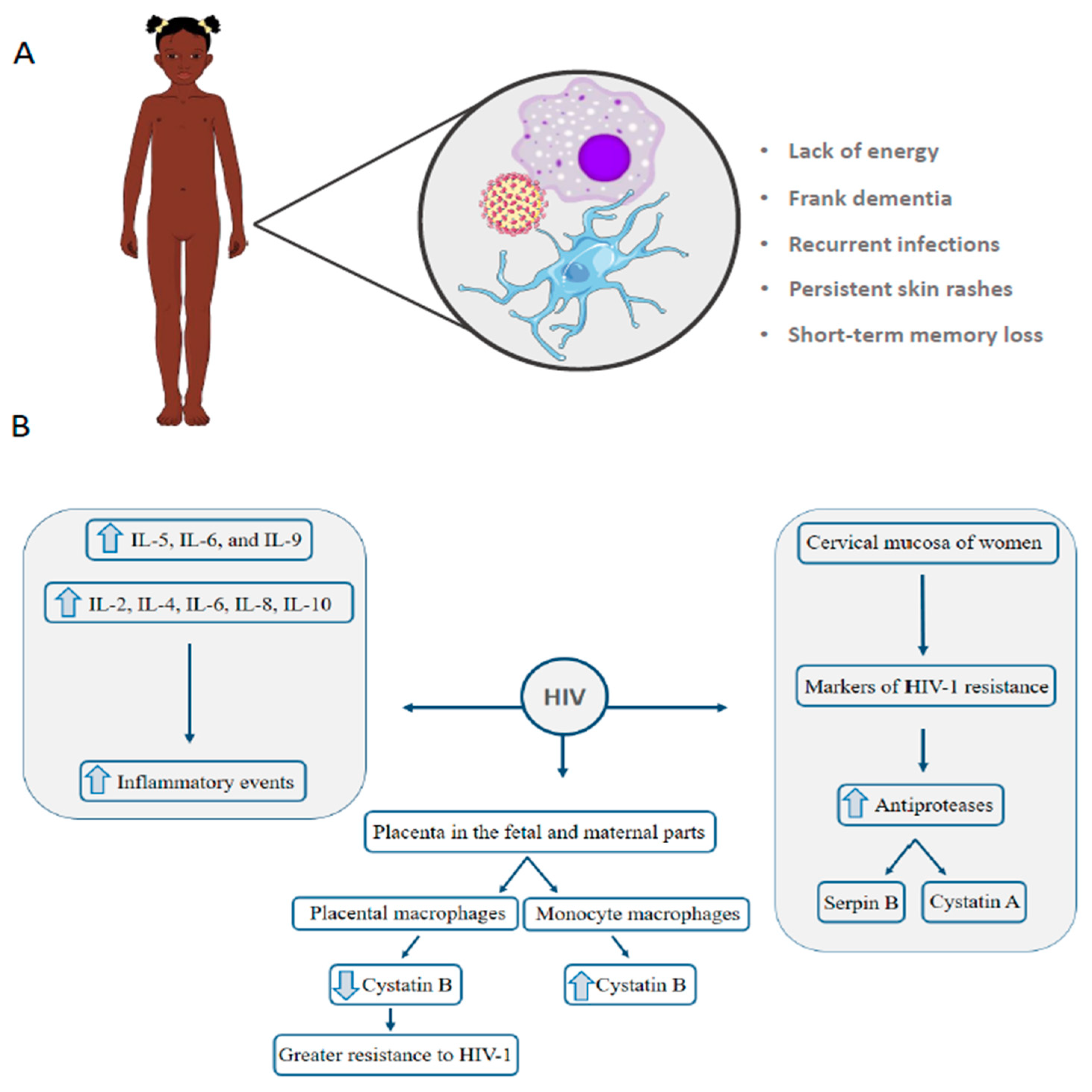
4. HIV: Vertical Transmission Is the Leading Cause of Infection in Children under 13 Years
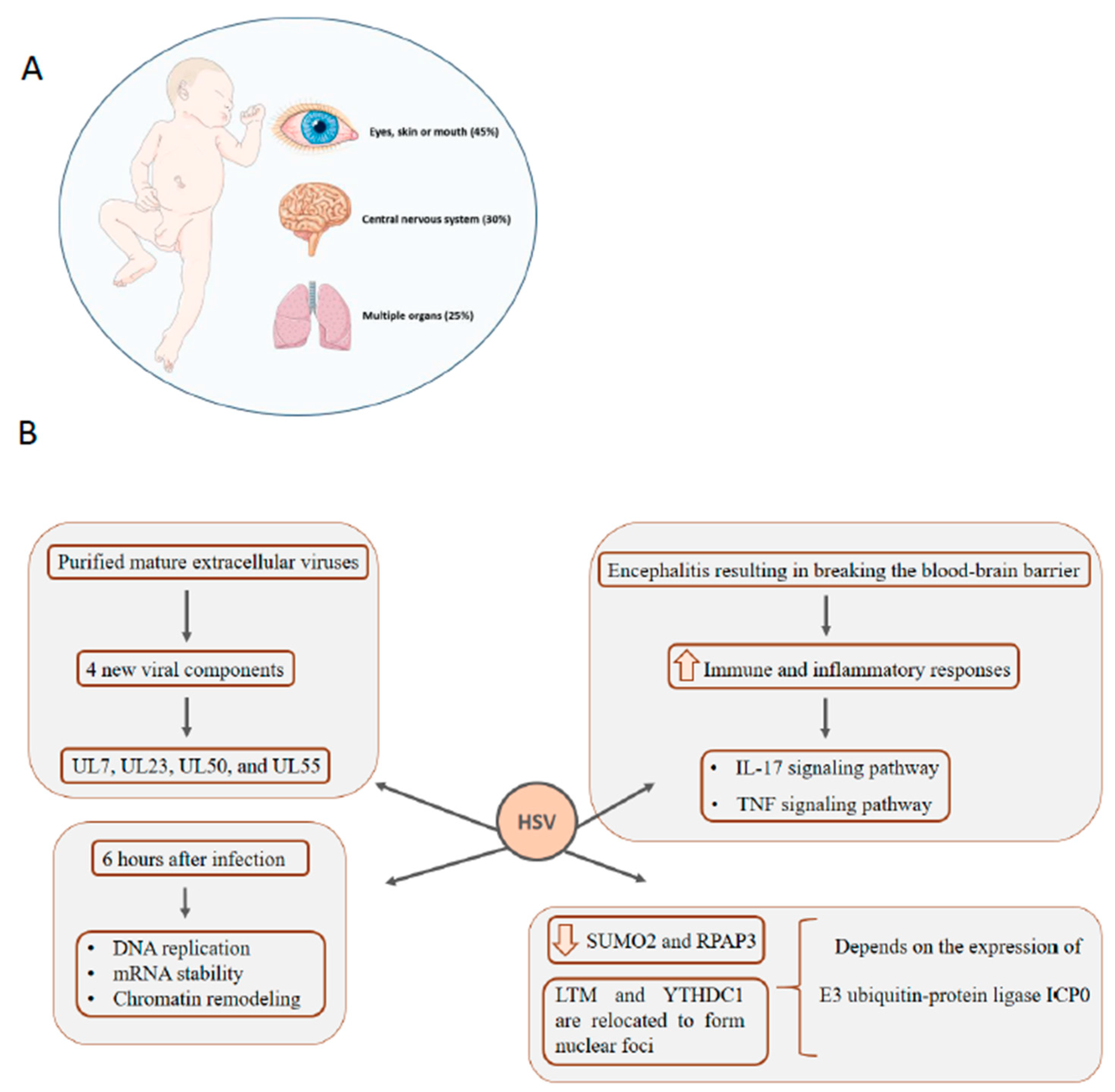
5. HSV: Infection in Newborns Can Affect Multiple Organs, Central Nervous System, Eyes, Skin, and Mouth
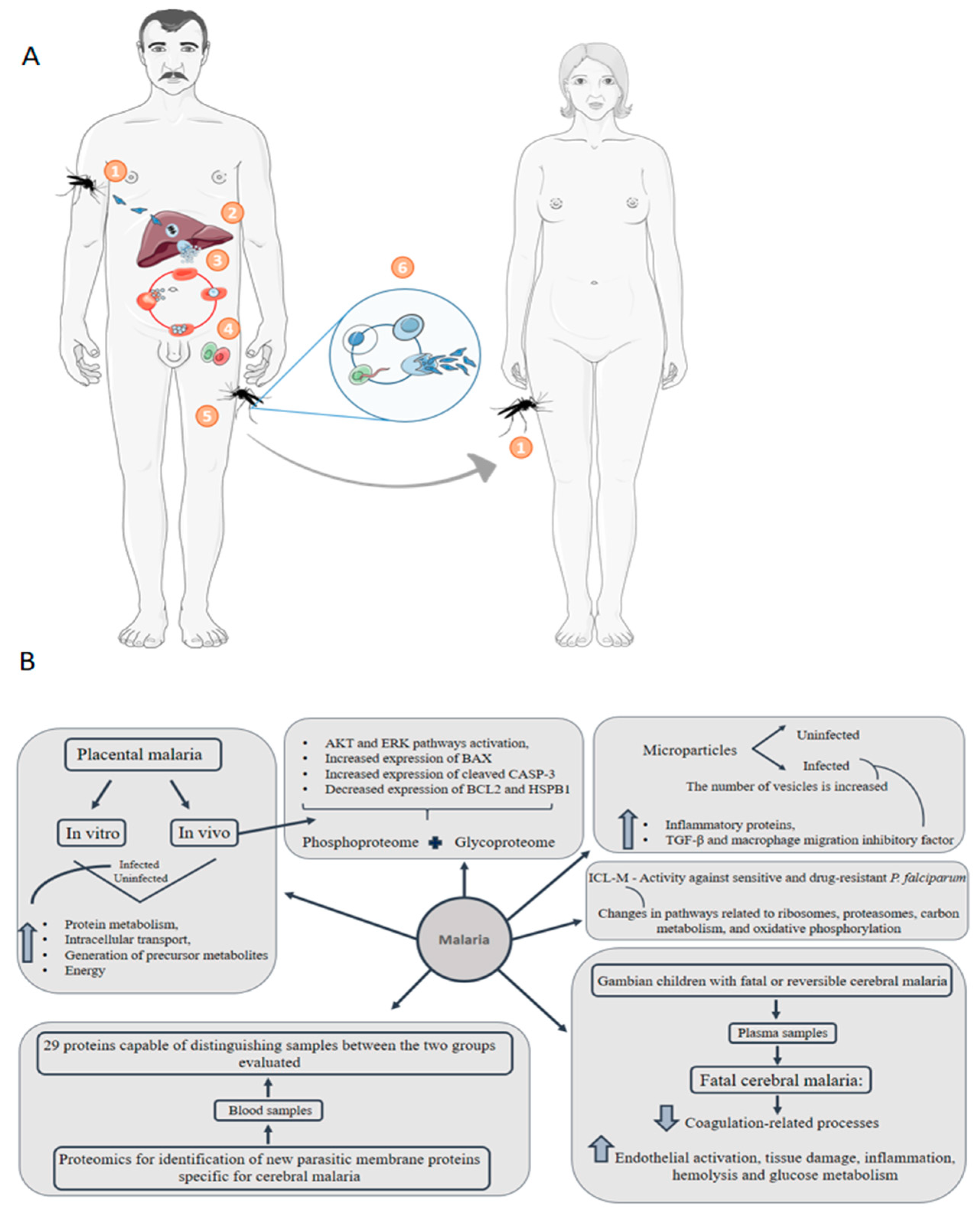
6. Malaria: Congenital Malaria Is Defined When the Parasite Is Identified in the Peripheral Blood of a Neonate in the First Week of Life
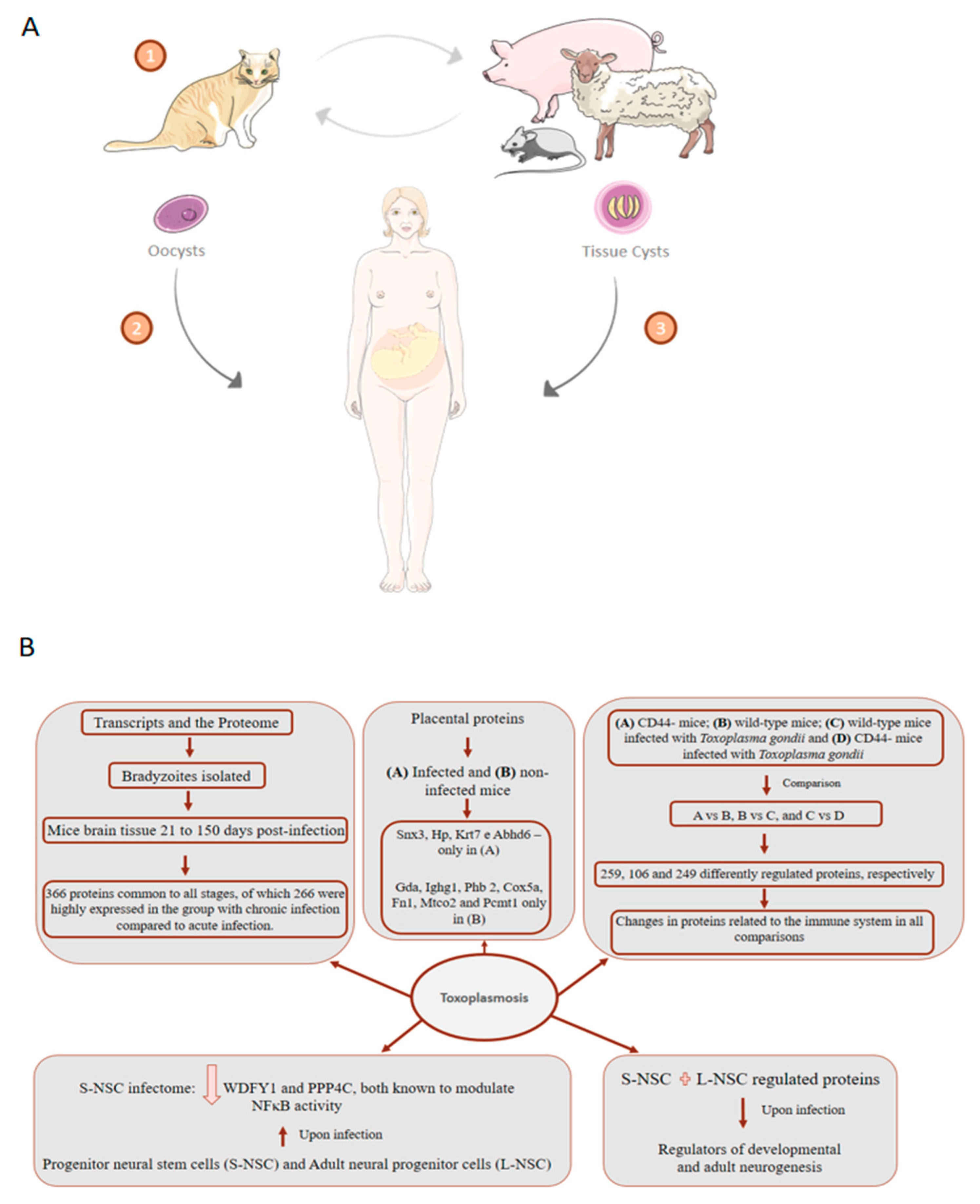
7. Toxoplasmosis: About 75% of Cases of Congenital Toxoplasmosis Have No Clinical Evidence, Making Early Treatment Difficult
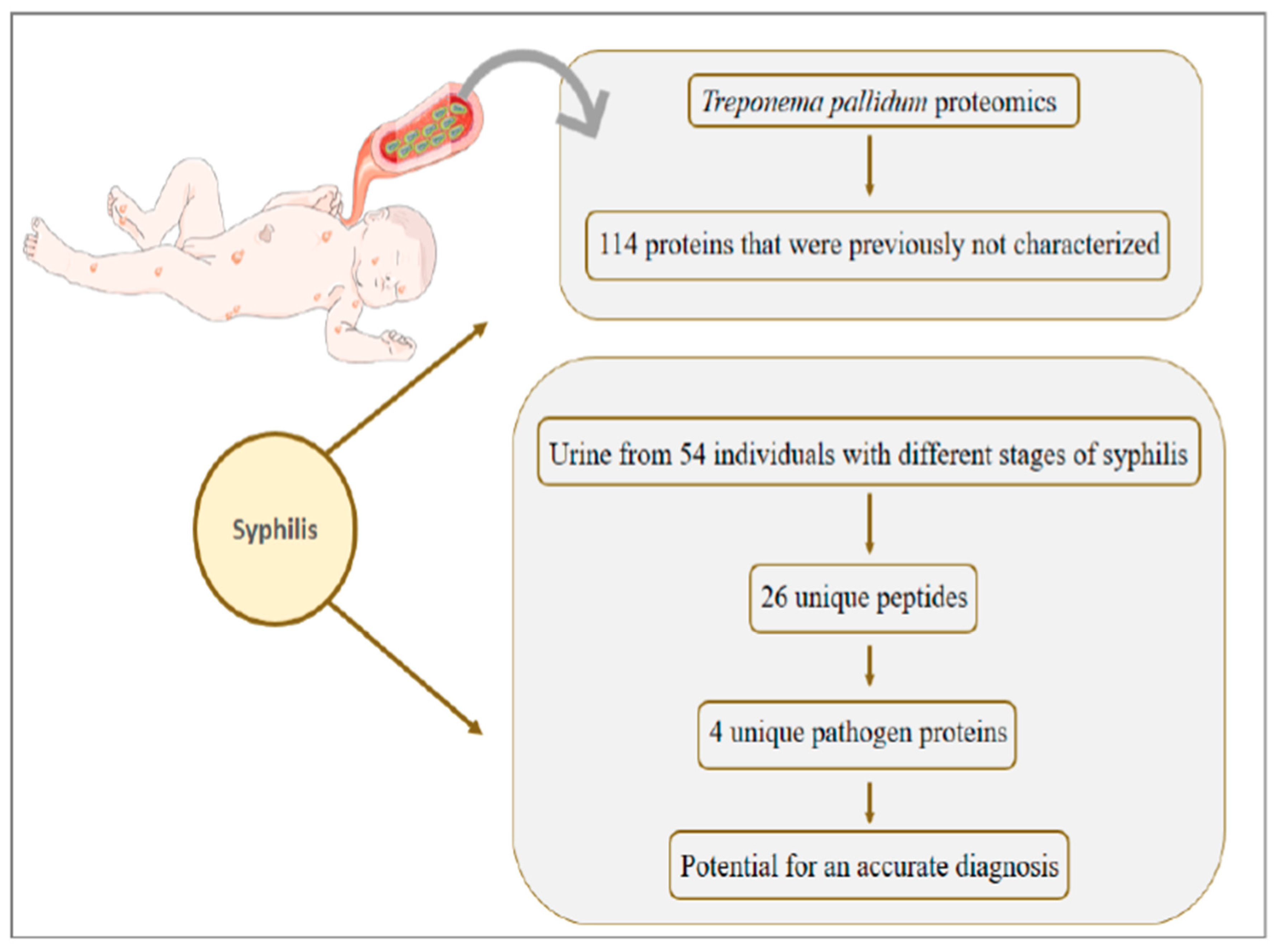
8. Syphilis: Congenital Syphilis Presents about One Million Cases per Year and Is Responsible for more than 300 Thousand Perinatal Deaths
9. Congenital Transmission of Varicella, Rubella, and Parvovirus B19 Has a Gap in Proteomic Studies
10. Critical Points of Proteomics Approaches Applied to Congenital Diseases
11. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, L. Congenital viral infection: Traversing the uterine-placental interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Investig. 2017, 127, 1591–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef]
- Stegmann, B.J.; Carey, J.C. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr. Womens Health Rep. 2002, 2, 253–258. [Google Scholar]
- Costa, M.L.; de Moraes Nobrega, G.; Antolini-Tavares, A. Key Infections in the Placenta. Obstet. Gynecol. Clin. N. Am. 2020, 47, 133–146. [Google Scholar] [CrossRef]
- Martin, G.P.; Marriott, C.; Kellaway, I.W. The effect of natural surfactants on the pheological properties of mucus. J. Pharm. Pharmacol. 1976, 28, 76. [Google Scholar]
- León-Juárez, M.; Martínez-Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Flores-Pliego, A.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [Green Version]
- Koi, H.; Zhang, J.; Makrigiannakis, A.; Getsios, S.; MacCalman, C.D.; Kopf, G.S.; Strauss, J.F.; Parry, S. Differential expression of the coxsackievirus and adenovirus receptor regulates adenovirus infection of the placenta. Biol. Reprod. 2001, 64, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Feire, A.L.; Koss, H.; Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 2004, 101, 15470–15475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aagaard, K.M.; Lahon, A.; Suter, M.A.; Arya, R.P.; Seferovic, M.D.; Vogt, M.B.; Hu, M.; Stossi, F.; Mancini, M.A.; Harris, R.A.; et al. Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication. Sci. Rep. 2017, 7, 41389. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.; Holder, J.; Strauss, J.F. Mechanisms of trophoblast-virus interaction. J. Reprod. Immunol. 1997, 37, 25–34. [Google Scholar] [CrossRef]
- Ranger-Rogez, S.; Alain, S.; Denis, F. Virus des hépatites: Transmission mère-enfant. Pathol. Biol. 2002, 50, 568–575. [Google Scholar] [CrossRef]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menendez, C. Malaria during pregnancy: A priority area of malaria research and control. Parasitol. Today 1995, 11, 178–183. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Faust, Z.S.; Varga, P.; Szereday, L.; Kelemen, K. The Immunological Pregnancy Protective Effect of Progesterone Is Manifested via Controlling Cytokine Production. Am. J. Reprod. Immunol. 1996, 35, 348–351. [Google Scholar] [CrossRef]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Marzi, M.; Vigano, A.; Trabattoni, D.; Villa, M.L.; Salvaggio, A.; Clerici, E.; Clerici, M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 1996, 106, 127–133. [Google Scholar] [CrossRef]
- Orton, D.; Doucette, A. Proteomic Workflows for Biomarker Identification Using Mass Spectrometry—Technical and Statistical Considerations during Initial Discovery. Proteomes 2013, 1, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.L.; Frappier, L. Viral Proteomics. Microbiol. Mol. Biol. Rev. 2007, 71, 398–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasinger, V.C.; Cordwell, S.J.; Cerpa-Poljak, A.; Yan, J.X.; Gooley, A.A.; Wilkins, M.R.; Duncan, M.W.; Harris, R.; Williams, K.L.; Humphery-Smith, I. Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis 1995, 16, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [Green Version]
- Catherman, A.D.; Skinner, O.S.; Kelleher, N.L. Top Down proteomics: Facts and perspectives. Biochem. Biophys. Res. Commun. 2014, 445, 683–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toby, T.K.; Fornelli, L.; Kelleher, N.L. Progress in Top-Down Proteomics and the Analysis of Proteoforms. Annu. Rev. Anal. Chem. (Palo Alto Calif.) 2016, 9, 499–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidoli, S.; Garcia, B.A. Middle-down proteomics: A still unexploited resource for chromatin biology. Expert Rev. Proteomics 2017, 14, 617–626. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Leney, A.C.; Heck, A.J.R. Native Mass Spectrometry: What is in the Name? J. Am. Soc. Mass Spectrom. 2017, 28, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Braun, P.; Gingras, A.-C. History of protein-protein interactions: From egg-white to complex networks. Proteomics 2012, 12, 1478–1498. [Google Scholar] [CrossRef]
- Stern-Ginossar, N.; Weisburd, B.; Michalski, A.; Le, V.T.K.; Hein, M.Y.; Huang, S.-X.; Ma, M.; Shen, B.; Qian, S.-B.; Hengel, H.; et al. Decoding human cytomegalovirus. Science 2012, 338, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Marsico, C.; Kimberlin, D.W. Congenital Cytomegalovirus infection: Advances and challenges in diagnosis, prevention and treatment. Ital. J. Pediatr. 2017, 43, 38. [Google Scholar] [CrossRef]
- Xu, W.-F.; Yuan, T.-M. A review on the prevention and treatment of congenital cytomegalovirus infection in mothers and infants. Zhongguo Dang Dai Er Ke Za Zhi 2018, 20, 870–875. [Google Scholar]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Dahle, A.J.; Fowler, K.B.; Wright, J.D.; Boppana, S.B.; Britt, W.J.; Pass, R.F. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J. Am. Acad. Audiol. 2000, 11, 283–290. [Google Scholar] [PubMed]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, M.; Sinclair, J. Aspects of human cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 2008, 325, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Robert, M. Oxygen affinity of haemoglobin (author’s transl). Bull. Physiopathol. Respir. (Nancy) 1975, 11, 79–170. [Google Scholar] [PubMed]
- Brizić, I.; Hiršl, L.; Britt, W.J.; Krmpotić, A.; Jonjić, S. Immune responses to congenital cytomegalovirus infection. Microbes Infect. 2018, 20, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J. Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection. Viruses 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Alford, C.A.; Hayes, K.; Britt, W. Primary Cytomegalovirus Infection in Pregnancy: Comparison of Antibody Responses to Virus-Encoded Proteins between Women with and without Intrauterine Infection. J. Infect. Dis. 1988, 158, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Lilleri, D.; Kabanova, A.; Revello, M.G.; Percivalle, E.; Sarasini, A.; Genini, E.; Sallusto, F.; Lanzavecchia, A.; Corti, D.; Gerna, G. Fetal Human Cytomegalovirus Transmission Correlates with Delayed Maternal Antibodies to gH/gL/pUL128-130-131 Complex during Primary Infection. PLoS ONE 2013, 8, e59863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pass, R.F.; Anderson, B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J. Pediatr. Infect. Dis. Soc. 2014, 3 (Suppl. 1), S2–S6. [Google Scholar] [CrossRef] [Green Version]
- Sinzger, C.; Digel, M.; Jahn, G. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 2008, 325, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Varnum, S.M.; Streblow, D.N.; Monroe, M.E.; Smith, P.; Auberry, K.J.; Pasa-Tolic, L.; Wang, D.; Camp, D.G.; Rodland, K.; Wiley, S.; et al. Identification of proteins in human cytomegalovirus (HCMV) particles: The HCMV proteome. J. Virol. 2004, 78, 10960–10966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean Beltran, P.M.; Cristea, I.M. The life cycle and pathogenesis of human cytomegalovirus infection: Lessons from proteomics. Expert Rev. Proteomics 2014, 11, 697–711. [Google Scholar] [CrossRef]
- Anderholm, K.M.; Bierle, C.J.; Schleiss, M.R. Cytomegalovirus vaccines: Current status and future prospects. Drugs 2016, 76, 1625–1645. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Boppana, S.B. Vaccination against the human cytomegalovirus. Vaccine 2019, 37, 7437–7442. [Google Scholar] [CrossRef]
- Viswanathan, K.; Verweij, M.C.; John, N.; Malouli, D.; Früh, K. Quantitative membrane proteomics reveals a role for tetraspanin enriched microdomains during entry of human cytomegalovirus. PLoS ONE 2017, 12, e0187899. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Martin, N.; Marcandalli, J.; Huang, C.S.; Arthur, C.P.; Perotti, M.; Foglierini, M.; Ho, H.; Dosey, A.M.; Shriver, S.; Payandeh, J.; et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 2018, 174, 1158–1171.e19. [Google Scholar] [CrossRef] [Green Version]
- Bozidis, P.; Williamson, C.D.; Colberg-Poley, A.M. Mitochondrial and secretory human cytomegalovirus UL37 proteins traffic into mitochondrion-associated membranes of human cells. J. Virol. 2008, 82, 2715–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Williamson, C.D.; Wong, D.S.; Bullough, M.D.; Brown, K.J.; Hathout, Y.; Colberg-Poley, A.M. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol. Cell. Proteomics 2011, 10, M111.009936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean Beltran, P.M.; Mathias, R.A.; Cristea, I.M. A portrait of the human organelle proteome in space and time during cytomegalovirus infection. Cell Syst. 2016, 3, 361–373.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Sheng, X.; Murray-Nerger, L.A.; Cristea, I.M. Temporal dynamics of protein complex formation and dissociation during human cytomegalovirus infection. Nat. Commun. 2020, 11, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, K.C.; Cristea, I.M. Location is everything: Protein translocations as a viral infection strategy. Curr. Opin. Chem. Biol. 2019, 48, 34–43. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Wang, B.; Yan, Z.; Qian, D.; Ding, S.; Song, X.; Bai, Z.; Li, L. Serum proteomics with SELDI-TOF-MS in congenital human cytomegalovirus hepatitis. J. Med. Virol. 2007, 79, 1500–1505. [Google Scholar] [CrossRef]
- Kindhauser, M.K.; Allen, T.; Frank, V.; Santhana, R.S.; Dye, C. Zika: The origin and spread of a mosquito-borne virus. Bull. World Health Organ. 2016, 94, 675C–686C. [Google Scholar] [CrossRef]
- Bailey, M.J.; Broecker, F.; Duehr, J.; Arumemi, F.; Krammer, F.; Palese, P.; Tan, G.S. Antibodies elicited by an NS1-based vaccine protect mice against zika virus. mBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Delaney, A.; Mai, C.; Smoots, A.; Cragan, J.; Ellington, S.; Langlois, P.; Breidenbach, R.; Fornoff, J.; Dunn, J.; Yazdy, M.; et al. Population-based surveillance of birth defects potentially related to zika virus infection—15 States and U.S. Territories, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 91–96. [Google Scholar] [CrossRef]
- Driggers, R.W.; Ho, C.-Y.; Korhonen, E.M.; Kuivanen, S.; Jääskeläinen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.M.E.; Pietrobon, A.J.; de Oliveira, L.M.; da Oliveira, L.M.S.; Sato, M.N. Maternal-Fetal Interplay in Zika Virus Infection and Adverse Perinatal Outcomes. Front. Immunol. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Tonnerre, P.; Melgaço, J.G.; Torres-Cornejo, A.; Pinto, M.A.; Yue, C.; Blümel, J.; de Sousa, P.S.F.; de da Mello, V.M.; Moran, J.; de Filippis, A.M.B.; et al. Evolution of the innate and adaptive immune response in women with acute Zika virus infection. Nat. Microbiol. 2020, 5, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, L.; Suthar, M.S.; Ahmed, R.; Wrammert, J. Humoral immune responses against zika virus infection and the importance of preexisting flavivirus immunity. J. Infect. Dis. 2017, 216, S906–S911. [Google Scholar] [CrossRef] [PubMed]
- Lesteberg, K.E.; Fader, D.S.; Beckham, J.D. Pregnancy alters innate immune responses to Zika virus infection in the genital tract. Immunology 2019. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Mohr, E.L.; Block, L.N.; Newman, C.M.; Stewart, L.M.; Koenig, M.; Semler, M.; Breitbach, M.E.; Teixeira, L.B.C.; Zeng, X.; Weiler, A.M.; et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS ONE 2018, 13, e0190617. [Google Scholar] [CrossRef] [Green Version]
- Souza, I.N.O.; Barros-Aragão, F.G.Q.; Frost, P.S.; Figueiredo, C.P.; Clarke, J.R. Late neurological consequences of zika virus infection: Risk factors and pharmaceutical approaches. Pharmaceuticals 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.E.; Galang, R.R.; Roth, N.M.; Ellington, S.R.; Moore, C.A.; Valencia-Prado, M.; Ellis, E.M.; Tufa, A.J.; Taulung, L.A.; Alfred, J.M.; et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—U.S. Territories and Freely Associated States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Prata-Barbosa, A.; Martins, M.M.; Guastavino, A.B.; da Cunha, A.J.L.A. Effects of Zika infection on growth. J. Pediatr. (Rio J.) 2019, 95 (Suppl. 1), 30–41. [Google Scholar] [CrossRef]
- Van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; van der Júnior, H.L.; Filho, E.L.R.; Ribeiro, E.M.; de Leal, M.C.; de Coimbra, P.P.A.; de Aragão, M.F.V.V.; et al. Description of 13 Infants Born During October 2015-January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth - Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- Smith, D.R.; Hollidge, B.; Daye, S.; Zeng, X.; Blancett, C.; Kuszpit, K.; Bocan, T.; Koehler, J.W.; Coyne, S.; Minogue, T.; et al. Neuropathogenesis of Zika Virus in a Highly Susceptible Immunocompetent Mouse Model after Antibody Blockade of Type I Interferon. PLoS Negl. Trop. Dis. 2017, 11, e0005296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Q.-L.; Deng, C.-L.; Chen, X.; Wang, J.; Wang, S.-B.; Wang, W.; Deng, F.; Zhang, B.; Xiao, G.; Zhang, L.-K. Quantitative Proteomic Analysis of Mosquito C6/36 Cells Reveals Host Proteins Involved in Zika Virus Infection. J. Virol. 2017, 91, e00554-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcez, P.P.; Minardi Nascimento, J.; Mota de Vasconcelos, J.; Madeiro da Costa, R.; Delvecchio, R.; Trindade, P.; Correia Loiola, E.; Higa, L.M.; Cassoli, J.; Vitória, G.; et al. Combined Proteome and Transcriptome Analyses Reveal That Zika Virus Circulating in Brazil Alters Cell Cycle and Neurogenic Programmes in Human Neurospheres. Available online: https://peerj.com/preprints/2033/ (accessed on 9 May 2016).
- Scaturro, P.; Stukalov, A.; Haas, D.A.; Cortese, M.; Draganova, K.; Płaszczyca, A.; Bartenschlager, R.; Götz, M.; Pichlmair, A. An orthogonal proteomic survey uncovers novel Zika virus host factors. Nature 2018, 561, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Fernandes, L.; Cugola, F.R.; Russo, F.B.; Kawahara, R.; de Melo Freire, C.C.; Leite, P.E.C.; Bassi Stern, A.C.; Angeli, C.B.; de Oliveira, D.B.L.; Melo, S.R.; et al. Zika Virus Impairs Neurogenesis and Synaptogenesis Pathways in Human Neural Stem Cells and Neurons. Front. Cell. Neurosci. 2019, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Robbiani, D.F.; Olsen, P.C.; Costa, F.; Wang, Q.; Oliveira, T.Y.; Nery, N.; Aromolaran, A.; do Rosário, M.S.; Sacramento, G.A.; Cruz, J.S.; et al. Risk of Zika microcephaly correlates with features of maternal antibodies. J. Exp. Med. 2019, 216, 2302–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allgoewer, K.; Zhao, A.; Maity, S.; Lashua, L.; Ramgopal, M.; Balkaran, B.N.; Liu, L.; Arévalo, M.T.; Ross, T.M.; Choi, H.; et al. High-resolution proteomics identifies potential new markers of Zika and dengue infections. Syst. Biol. 2019. [Google Scholar] [CrossRef]
- Song, G.; Rho, H.-S.; Pan, J.; Ramos, P.; Yoon, K.-J.; Medina, F.A.; Lee, E.M.; Eichinger, D.; Ming, G.; Muñoz-Jordan, J.L.; et al. Multiplexed Biomarker Panels Discriminate Zika and Dengue Virus Infection in Humans. Mol. Cell. Proteomics 2018, 17, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Luckay, A.; Sodora, D.L.; Telfer, P.; Reed, P.; Gettie, A.; Kanu, J.M.; Sadek, R.F.; Yee, J.; Ho, D.D.; et al. Human immunodeficiency virus type 2 (HIV-2) seroprevalence and characterization of a distinct HIV-2 genetic subtype from the natural range of simian immunodeficiency virus-infected sooty mangabeys. J. Virol. 1997, 71, 3953–3960. [Google Scholar] [CrossRef] [Green Version]
- Librelotto, C.S.; Gräf, T.; Simon, D.; de Almeida, S.E.M.; Lunge, V.R. HIV-1 epidemiology and circulating subtypes in the countryside of South Brazil. Rev. Soc. Bras. Med. Trop. 2015, 48, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef]
- Meléndez, L.M.; Colon, K.; Rivera, L.; Rodriguez-Franco, E.; Toro-Nieves, D. Proteomic Analysis of HIV-Infected Macrophages. J. Neuroimmune Pharmacol. 2011, 6, 89–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongertz, V. Vertical human immunodeficiency virus type 1—HIV-1–transmission—A review. Mem. Inst. Oswaldo Cruz. 2001, 96, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.Y.; Buehler, J.W.; Berkelman, R.L. Impact of the human immunodeficiency virus epidemic on mortality in women of reproductive age, United States. JAMA 1990, 264, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Santiago, M.R.; Souza, D.A.; Silva, G.E.B.; Chahud, F.; Quintana, S.M.; Mendes-Junior, C.T.; Donadi, E.A.; Fernandes, A.P.M. The role of the placenta in the vertical transmission of HIV-1. Med. (Ribeirao Preto. Online) 2016, 49, 80. [Google Scholar] [CrossRef]
- Cocker, A.T.H.; Shah, N.M.; Raj, I.; Dermont, S.; Khan, W.; Mandalia, S.; Imami, N.; Johnson, M.R. Pregnancy Gestation Impacts on HIV-1-Specific Granzyme B Response and Central Memory CD4 T Cells. Front. Immunol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallick, S.C.; Figari, I.S.; Morris, R.E.; Levinson, A.D.; Palladino, M.A. Immunoregulatory role of transforming growth factor beta (TGF-beta) in development of killer cells: Comparison of active and latent TGF-beta 1. J. Exp. Med. 1990, 172, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Burgener, A.; Boutilier, J.; Wachihi, C.; Kimani, J.; Carpenter, M.; Westmacott, G.; Cheng, K.; Ball, T.B.; Plummer, F. Identification of Differentially Expressed Proteins in the Cervical Mucosa of HIV-1-Resistant Sex Workers. J. Proteome Res. 2008, 7, 4446–4454. [Google Scholar] [CrossRef]
- Luciano-Montalvo, C.; Ciborowski, P.; Duan, F.; Gendelman, H.E.; Meléndez, L.M. Proteomic Analyses Associate Cystatin B with Restricted HIV-1 Replication in Placental Macrophages. Placenta 2008, 29, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- García, K.; García, V.; Pérez Laspiur, J.; Duan, F.; Meléndez, L.M. Characterization of the Placental Macrophage Secretome: Implications for Antiviral Activity. Placenta 2009, 30, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Soler-García, A.A.; Johnson, D.; Hathout, Y.; Ray, P.E. Iron-related proteins: Candidate urine biomarkers in childhood HIV-associated renal diseases. Clin. J. Am. Soc. Nephrol. 2009, 4, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Whitley, R.J.; Roizman, B. Herpes simplex virus infections. Lancet 2001, 357, 1513–1518. [Google Scholar] [CrossRef]
- Koelle, D.M.; Corey, L. Herpes Simplex: Insights on Pathogenesis and Possible Vaccines. Annu. Rev. Med. 2008, 59, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex. Trans. Infect. 2005, 81, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Mahnert, N.; Roberts, S.W.; Laibl, V.R.; Sheffield, J.S.; Wendel, G.D. The incidence of neonatal herpes infection. Am. J. Obstet. Gynecol. 2007, 196, e55–e56. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.D.; Backes, I.M.; Taylor, S.A.; Jiang, Y.; Marchant, A.; Pesola, J.M.; Coen, D.M.; Knipe, D.M.; Ackerman, M.E.; Leib, D.A. Maternal immunization confers protection against neonatal herpes simplex mortality and behavioral morbidity. Sci. Transl. Med. 2019, 11, eaau6039. [Google Scholar] [CrossRef]
- Purewal, R.; Costello, L.; Garlapati, S.; Mitra, S.; Mitchell, M.; Moffett, K.S. Congenital Herpes Simplex Virus in the Newborn: A Diagnostic Dilemma. J. Ped. Infect. Dis. 2016, 5, e21–e23. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, N.D.; Badri, T. Congenital Herpes Simplex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Loret, S.; Guay, G.; Lippé, R. Comprehensive characterization of extracellular herpes simplex virus type 1 virions. J. Virol. 2008, 82, 8605–8618. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, K.; Suzumura, E.; Hirata, K.; Majima, Y.; Sakakura, Y. Role of transepithelial ion transport as a determinant of mucus viscoelasticity in chronic inflammation of the maxillary sinus. Acta Otolaryngol. 1991, 111, 1133–1138. [Google Scholar] [CrossRef]
- Liu, H.; Huang, C.-X.; He, Q.; Li, D.; Luo, M.-H.; Zhao, F.; Lu, W. Proteomics analysis of HSV-1-induced alterations in mouse brain microvascular endothelial cells. J. Neurovirol. 2019, 25, 525–539. [Google Scholar] [CrossRef]
- Antrobus, R.; Grant, K.; Gangadharan, B.; Chittenden, D.; Everett, R.D.; Zitzmann, N.; Boutell, C. Proteomic analysis of cells in the early stages of herpes simplex virus type-1 infection reveals widespread changes in the host cell proteome. Proteomics 2009, 9, 3913–3927. [Google Scholar] [CrossRef]
- Berard, A.R.; Coombs, K.M.; Severini, A. Quantification of the host response proteome after herpes simplex virus type 1 infection. J. Proteome Res. 2015, 14, 2121–2142. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.D. HSV-1 biology and life cycle. Methods Mol. Biol. 2014, 1144, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kulej, K.; Avgousti, D.C.; Sidoli, S.; Herrmann, C.; Della Fera, A.N.; Kim, E.T.; Garcia, B.A.; Weitzman, M.D. Time-resolved Global and Chromatin Proteomics during Herpes Simplex Virus Type 1 (HSV-1) Infection. Mol. Cell Proteomics 2017, 16, S92–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drayman, N.; Karin, O.; Mayo, A.; Danon, T.; Shapira, L.; Rafael, D.; Zimmer, A.; Bren, A.; Kobiler, O.; Alon, U. Dynamic Proteomics of Herpes Simplex Virus Infection. mBio 2017, 8, e01612-17. [Google Scholar] [CrossRef] [Green Version]
- Sloan, E.; Tatham, M.H.; Groslambert, M.; Glass, M.; Orr, A.; Hay, R.T.; Everett, R.D. Analysis of the SUMO2 Proteome during HSV-1 Infection. PLoS Pathog. 2015, 11, e1005059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- Bertin, G.I.; Sabbagh, A.; Argy, N.; Salnot, V.; Ezinmegnon, S.; Agbota, G.; Ladipo, Y.; Alao, J.M.; Sagbo, G.; Guillonneau, F.; et al. Proteomic analysis of Plasmodium falciparum parasites from patients with cerebral and uncomplicated malaria. Sci. Rep. 2016, 6, 26773. [Google Scholar] [CrossRef] [Green Version]
- Bloland, P.B.; Williams, H.A.; National Research Council (US) Committee on Population; Program on Forced Migration and Health at the Mailman School of Public Health, C.U. Malaria Control during Mass Population Movements and Natural Disasters; National Academies Press (US): Washington, DC, USA, 2002; ISBN 978-0-309-08615-8. [Google Scholar]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef]
- Aly, A.S.I.; Vaughan, A.M.; Kappe, S.H.I. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol. 2009, 63, 195–221. [Google Scholar] [CrossRef] [Green Version]
- Lacey, R.W. Basic medical microbiology (4th edition). J. Hosp. Infect. 1992, 20, 135–136. [Google Scholar] [CrossRef]
- Bhatia, R.; Rajwaniya, D.; Agrawal, P. Congenital Malaria due to Plasmodium Vivax Infection in a Neonate. Case Rep. Pediatr. 2016, 2016, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.E.; Duffy, P.E. Congenital malaria: Rare but potentially fatal. Pediatr. Health 2008, 2, 235–248. [Google Scholar] [CrossRef]
- Dombrowski, J.G.; de Souza, R.M.; Lima, F.A.; Bandeira, C.L.; Murillo, O.; de Costa, D.S.; Peixoto, E.P.M.; dos Cunha, M.P.; de Zanotto, P.M.A.; Bevilacqua, E.; et al. Association of Malaria Infection During Pregnancy with Head Circumference of Newborns in the Brazilian Amazon. JAMA Netw. Open 2019, 2, e193300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, L.; Shukla, G. Placental Malaria: A New Insight into the Pathophysiology. Front. Med. (Lausanne) 2017, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Desai, M.; ter Kuile, F.O.; Nosten, F.; McGready, R.; Asamoa, K.; Brabin, B.; Newman, R.D. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 2007, 7, 93–104. [Google Scholar] [CrossRef]
- Rogerson, S.J.; Hviid, L.; Duffy, P.E.; Leke, R.F.G.; Taylor, D.W. Malaria in pregnancy: Pathogenesis and immunity. Lancet Infect. Dis. 2007, 7, 105–117. [Google Scholar] [CrossRef]
- Darmstadt, G.L.; Zaidi, A.K.M.; Stoll, B.J. Neonatal Infections. In Infectious Diseases of the Fetus and Newborn; Elsevier: Philadelphia, PA, USA, 2011; pp. 24–51. ISBN 978-1-4160-6400-8. [Google Scholar]
- Dobbs, K.R.; Dent, A.E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology 2016, 143, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Odorizzi, P.M.; Feeney, M.E. Impact of In Utero Exposure to Malaria on Fetal T Cell Immunity. Trends Mol. Med. 2016, 22, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.S.; Barboza, R.; Murillo, O.; Barateiro, A.; Peixoto, E.P.M.; Lima, F.A.; Gomes, V.M.; Dombrowski, J.G.; Leal, V.N.C.; Araujo, F.; et al. Inflammasome activation and IL-1 signaling during placental malaria induce poor pregnancy outcomes. Sci. Adv. 2020, 6, eaax6346. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, R.; Rosa-Fernandes, L.; Dos Santos, A.F.; Bandeira, C.L.; Dombrowski, J.G.; Souza, R.M.; Da Fonseca, M.P.; Festuccia, W.T.; Labriola, L.; Larsen, M.R.; et al. Integrated proteomics reveals apoptosis-related mechanisms associated with placental malaria. Mol. Cell. Proteomics 2019, 18, 182–199. [Google Scholar] [CrossRef] [Green Version]
- Antwi-Baffour, S.; Adjei, J.K.; Agyemang-Yeboah, F.; Annani-Akollor, M.; Kyeremeh, R.; Asare, G.A.; Gyan, B. Proteomic analysis of microparticles isolated from malaria positive blood samples. Proteome Sci. 2016, 15, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moussa, E.M.; Huang, H.; Thézénas, M.L.; Fischer, R.; Ramaprasad, A.; Sisay-Joof, F.; Jallow, M.; Pain, A.; Kwiatkowski, D.; Kessler, B.M.; et al. Proteomic profiling of the plasma of Gambian children with cerebral malaria. Malar. J. 2018, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Wendler, J.P.; Mutabingwa, T.K.; Duffy, P.E. Mass spectrometric analysis ofPlasmodium falciparum erythrocyte membrane protein-1 variants expressed by placental malaria parasites. Proteomics 2004, 4, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Gonzales Hurtado, P.A.; Morrison, R.; Ribeiro, J.M.C.; Magale, H.; Attaher, O.; Diarra, B.S.; Mahamar, A.; Barry, A.; Dicko, A.; Duffy, P.E.; et al. Proteomics Pipeline for Identifying Variant Proteins in Plasmodium falciparum Parasites Isolated from Children Presenting with Malaria. J. Proteome Res. 2019, 18, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.G.; Korsik, M.; Todd, M.H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rujimongkon, K.; Mungthin, M.; Tummatorn, J.; Ampawong, S.; Adisakwattana, P.; Boonyuen, U.; Reamtong, O. Proteomic analysis of Plasmodium falciparum response to isocryptolepine derivative. PLoS ONE 2019, 14, e0220871. [Google Scholar] [CrossRef] [Green Version]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saadatnia, G.; Golkar, M. A review on human toxoplasmosis. Scand. J. Infect. Dis. 2012, 44, 805–814. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Pacheco-Vega, S.J.; Hernández-Tinoco, J.; Centeno-Tinoco, M.M.; Beristain-García, I.; Sánchez-Anguiano, L.F.; Liesenfeld, O.; Rábago-Sánchez, E.; Berumen-Segovia, L.O. Miscarriage history and Toxoplasma gondii infection: A cross-sectional study in women in Durango City, Mexico. Eur. J. Microbiol. Immunol. (Bp.) 2014, 4, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Freeman, K.; Oakley, L.; Pollak, A.; Buffolano, W.; Petersen, E.; Semprini, A.E.; Salt, A.; Gilbert, R. European Multicentre Study on Congenital Toxoplasmosis Association between congenital toxoplasmosis and preterm birth, low birthweight and small for gestational age birth. BJOG 2005, 112, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chávez, F.; Cañedo-Solares, I.; Ortiz-Alegría, L.B.; Flores-García, Y.; Luna-Pastén, H.; Figueroa-Damián, R.; Mora-González, J.C.; Correa, D. Maternal Immune Response During Pregnancy and Vertical Transmission in Human Toxoplasmosis. Front. Immunol. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.B. Congenital Toxoplasmosis. J. Pediatr. Infect. Dis. Soc. 2014, 3 (Suppl. 1), S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Darde, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.N. Toxoplasmosis: A review. J. Med. 1986, 17, 373–396. [Google Scholar]
- Ngô, H.M.; Zhou, Y.; Lorenzi, H.; Wang, K.; Kim, T.-K.; Zhou, Y.; El Bissati, K.; Mui, E.; Fraczek, L.; Rajagopala, S.V.; et al. Toxoplasma Modulates Signature Pathways of Human Epilepsy, Neurodegeneration & Cancer. Sci. Rep. 2017, 7, 11496. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-H.; Zhang, Y.; Jiang, L.-Q.; Wang, S.; Lei, C.-Q.; Sun, M.-S.; Shu, H.-B.; Liu, Y. WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep. 2015, 16, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Schlüter, D.; Barragan, A. Advances and Challenges in Understanding Cerebral Toxoplasmosis. Front. Immunol. 2019, 10, 242. [Google Scholar] [CrossRef]
- Garfoot, A.L.; Wilson, G.M.; Coon, J.J.; Knoll, L.J. Proteomic and transcriptomic analyses of early and late-chronic Toxoplasma gondii infection shows novel and stage specific transcripts. BMC Genom. 2019, 20, 859. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Du, F.; Zhou, X.; Wang, L.; Li, S.; Fang, R.; Zhao, J. Brain proteomic differences between wild-type and CD44- mice induced by chronic Toxoplasma gondii infection. Parasitol. Res. 2018, 117, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Zhang, D.; Jiang, M.; Mi, J.; Liu, X.; Zhang, H.; Hu, Z.; Xu, X.; Hu, X. Label-free proteomic analysis of placental proteins during Toxoplasma gondii infection. J. Proteom. 2017, 150, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordon, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dooren, G.G.; Stimmler, L.M.; McFadden, G.I. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol. Rev. 2006, 30, 596–630. [Google Scholar] [CrossRef] [Green Version]
- Seidi, A.; Muellner-Wong, L.S.; Rajendran, E.; Tjhin, E.T.; Dagley, L.F.; Aw, V.Y.; Faou, P.; Webb, A.I.; Tonkin, C.J.; van Dooren, G.G. Elucidating the mitochondrial proteome of Toxoplasma gondii reveals the presence of a divergent cytochrome c oxidase. eLife 2018, 7, e38131. [Google Scholar] [CrossRef]
- Xiao, J.; Yolken, R.H. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol. (Oxf.) 2015, 213, 828–845. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.-H.; Wang, Z.-X.; Zhou, C.-X.; He, S.; Elsheikha, H.M.; Zhu, X.-Q. Comparative proteomic analysis of virulent and avirulent strains of Toxoplasma gondii reveals strain-specific patterns. Oncotarget 2017, 8, 80481–80491. [Google Scholar] [CrossRef] [Green Version]
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018, 5, 24–38. [Google Scholar] [CrossRef]
- Hook, E.W. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Primers 2017, 3, 17073. [Google Scholar] [CrossRef]
- De Cerqueira, L.R.P.; Monteiro, D.L.M.; Taquette, S.R.; Rodrigues, N.C.P.; Trajano, A.J.B.; de Souza, F.M.; Araújo, B.D.M. The magnitude of syphilis: From prevalence to vertical transmission. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Lancet, null Congenital syphilis in the USA. Lancet 2018, 392, 1168. [CrossRef]
- Bowen, V.; Su, J.; Torrone, E.; Kidd, S.; Weinstock, H. Increase in incidence of congenital syphilis—United States, 2012–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Sánchez, P.J. Congenital syphilis. Semin. Perinatol. 2018, 42, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Samson, G.R.; Beatty, D.W.; Malan, A.F. Immune studies in infants with congenital syphilis. Clin. Exp. Immunol. 1990, 81, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.R.; Ford-Jones, E.L. Congenital syphilis: A guide to diagnosis and management. Paediatr. Child Health 2000, 5, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Osbak, K.K.; Houston, S.; Lithgow, K.V.; Meehan, C.J.; Strouhal, M.; Šmajs, D.; Cameron, C.E.; Van Ostade, X.; Kenyon, C.R.; Van Raemdonck, G.A. Characterizing the Syphilis-Causing Treponema pallidum ssp. pallidum Proteome Using Complementary Mass Spectrometry. PLoS Negl. Trop. Dis. 2016, 10, e0004988. [Google Scholar] [CrossRef] [Green Version]
- Ratnam, S. The laboratory diagnosis of syphilis. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Osbak, K.K.; Van Raemdonck, G.A.; Dom, M.; Cameron, C.E.; Meehan, C.J.; Deforce, D.; Ostade, X.V.; Kenyon, C.R.; Dhaenens, M. Candidate Treponema pallidum biomarkers uncovered in urine from individuals with syphilis using mass spectrometry. Future Microbiol. 2018, 13, 1497–1510. [Google Scholar] [CrossRef]
- Abdel-Razeq, S.S.; Cross, S.N.; Lipkind, H.S.; Copel, J.A. Cytomegalovirus, Rubella, Toxoplasmosis, Herpes Simplex Virus, and Varicella. In Obstetric Imaging: Fetal Diagnosis and Care; Elsevier: Minneapolis, MN, USA, 2018; pp. 666–681.e3. ISBN 978-0-323-44548-1. [Google Scholar]
- David, S.; Khandhar, P.B. Double-Blind Study. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Baron, S. (Ed.) Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Enders, G.; Bolley, I.; Miller, E.; Cradock-Watson, J.; Ridehalgh, M. Consequences of varicella and herpes zoster in pregnancy: Prospective study of 1739 cases. Lancet 1994, 343, 1548–1551. [Google Scholar] [CrossRef]
- Koren, G. Risk of varicella infection during late pregnancy. Can. Fam. Phys. 2003, 49, 1445–1446. [Google Scholar]
- Harger, J.H.; Ernest, J.M.; Thurnau, G.R.; Moawad, A.; Thom, E.; Landon, M.B.; Paul, R.; Miodovnik, M.; Dombrowski, M.; Sibai, B.; et al. Frequency of congenital varicella syndrome in a prospective cohort of 347 pregnant women. Obstet. Gynecol. 2002, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Savarese, I.; De Carolis, M.P.; Costa, S.; De Rosa, G.; De Carolis, S.; Lacerenza, S.; Romagnoli, C. Atypical manifestations of congenital parvovirus B19 infection. Eur. J. Pediatr. 2008, 167, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, E.D.; Brown, K.E. Human parvovirus B19. Clin. Microbiol. Rev. 2002, 15, 485–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, J.; Bager, P.; Wohlfahrt, J.; Bottiger, B.; Melbye, M. Parvovirus B19 infection in pregnancy and subsequent morbidity and mortality in offspring. Int. J. Epidemiol. 2013, 42, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Vílchez, J.A.; Albaladejo-Otón, M.D. New Trends in Biomarkers and Diseases: An Overview. Bentham Sci. Publ. 2017, 1, 63–64. [Google Scholar] [CrossRef]
- Li, D.; Chan, D.W. Proteomic cancer biomarkers from discovery to approval: It’s worth the effort. Expert Rev. Proteom. 2014, 11, 135–136. [Google Scholar] [CrossRef] [Green Version]
- Finehout, E.J.; Franck, Z.; Choe, L.H.; Relkin, N.; Lee, K.H. Cerebrospinal fluid proteomic biomarkers for Alzheimer’s disease. Ann. Neurol. 2007, 61, 120–129. [Google Scholar] [CrossRef]
- Santamaria, C.; Chatelain, E.; Jackson, Y.; Miao, Q.; Ward, B.J.; Chappuis, F.; Ndao, M. Serum biomarkers predictive of cure in Chagas disease patients after nifurtimox treatment. BMC Infect. Dis. 2014, 14, 302. [Google Scholar] [CrossRef] [Green Version]
- Weekes, M.P.; Tomasec, P.; Huttlin, E.L.; Fielding, C.A.; Nusinow, D.; Stanton, R.J.; Wang, E.C.Y.; Aicheler, R.; Murrell, I.; Wilkinson, G.W.G.; et al. Quantitative temporal viromics: An approach to investigate host-pathogen interaction. Cell 2014, 157, 1460–1472. [Google Scholar] [CrossRef] [Green Version]

| Disease | Intracranial Calcifications | Hearing Loss | Eye Impairment | Microcephaly | Bone Lesions | CNS Damage |
|---|---|---|---|---|---|---|
| Toxoplasmosis | + | - | + | + | - | + |
| Syphilis | - | - | - | + | + | + |
| ZIKV | + | + | + | + | + | + |
| HIV | - | - | - | - | + | + |
| Varicella | - | - | + | - | - | + |
| CVM | + | + | + | + | - | + |
| HSV | - | + | + | + | + | + |
| Rubella | + | + | + | + | - | + |
| Disease | Matrix | MS Approach | Total Identifications | Reference |
|---|---|---|---|---|
| HCMV | Human serum | Label-free quantification with SELDI-TOF-MS | Not available | [56] |
| primary human fetal foreskin fibroblasts | TMT quantification and LC-MS/MS on Orbitrap Elite and Fusion | >8000 cellular proteins and 139 canonical and 14 ORFs viral proteins | [177] | |
| ARPE-19 and Expi293F cells | Easy nLC 1000 HPLC system coupled to an Orbitrap Elite mass spectrometer | 1297 | [50] | |
| Purified HCMV AD169 virions | Label-free quantification on a Finnigan LCQ ion trap MS | 59 | [45] | |
| MRC5 human lung fibroblasts | Label-free quantification and TMT labeling on a LTQ-Orbitrap XL | 4000 host and 100 viral proteins | [53] | |
| HFFs cells | SILAC labeling with 2D–LC-MS/MS (MudPIT) on a LCQ Deca XP Plus mass | 504 | [49] | |
| HFFs cells | SILAC labeling with LC-MS/MS on a LTQ Orbitrap | 1719 | [52] | |
| MRC5 cells | TMT labeling with nLC-MS/MS on a Q-Exactive HF | 5300 | [54] | |
| ZIKV | HeLa and HFFs cells | iTRAQ labeling with LC–MS/MS on a TripleTOF 5600 | 3544 | [72] |
| NPCs and iPSCs | TMT labeling with nLC-MS/MS on a Q-Exactive HF-Hybrid Quadrupole-Orbitrap | 6080 | [75] | |
| Neurospheres | Label-free quantification on a 2D-RP/RP Synapt G2-Si mass spectrometer | Not available | [73] | |
| NPCs and SK-N-BEB2 cell line | Label-free quantification with AP–LC–MS/MS on a LTQ-Orbitrap XL and Orbitrap Q Exactive HF | 386 ZIKV-interacting proteins and 1216 phosphorylation sites | [74] | |
| Human serum | Label-free quantification with EASY-nLC 1000 on a Q Exactive High | 300 | [77] | |
| HIV | Vaginal discharge | Label-free quantification with 2D-DIGE Nanoflow LC/MSMS on a QStar XL Qq-TOF | 72 protein spots with change in volume | [88] |
| Monocytes and placental macrophages | Label-free quantification with SELDI-TOF and (LC MS/MS) | Not available | [89] | |
| Placenta | Label-free quantification with LC–MS/MS on a LTQ XL | Not available | [90] | |
| HSV | Purified virions | Label-free quantification with ESI-MS/MS on a QTRAP 4000 linear ion trap mass spectrometer | 37 | [99] |
| HEp-2 cells line | Label-free quantification with 2-DE and LC-MS/MS on a Q-TOF 1 Mass Spectrometer | 103 protein spot changes | [102] | |
| HEK293 cells | SILAC labeling with LC-MS/MS on a Q-Star Elite mass | At 4 hpi, 2178; At 24 hpi, 1947; At 10 hpi, 2099 | [103] | |
| HFF cells | Label-free quantification with LC-MS/MS on a Orbitrap Fusion Tribrid mass spectrometer | 4000 | [105] | |
| bEnd.3 cells | TMT labeling with nanoLC-MS/MS on a Q-Exactive Orbitrap | 6761 | [101] | |
| Malaria | Human blood | Label-free quantification with LC-MS/MS on a Linear Trap Quadrupole-Orbitrap Velos | 1527 | [109] |
| Human plasma | Label-free quantification with 2D LC-MS on a LTQ ion trap | 1806 | [125] | |
| Human plasma | Label-free quantification with Nano-LC–MS/MS on a LTQ-Orbitrap Velos | 504 | [126] | |
| Human blood | Label-free quantification on a LTQ Orbitrap Velos | Not available | [128] | |
| Infected placentas | TMT labeling with nano-LC-MS/MS on a Orbitrap Fusion | 2946 | [124] | |
| Human erythrocytes cell culture | Label-free quantification on a micrOTOF-Q | 668 | [130] | |
| Toxoplasmosis | Cysts from brain and muscle tissues of pigs | iTRAQ labeling with LC–MS/MS on a Q Exactive Orbitrap | 2551 | [151] |
| Primary, neuronal and monocytic stem cells | iTRAQ labeling with LC/MS/MS on a LTQ Orbitrap Velos | 4367 | [140] | |
| Brain mice | iTRAQ labeling with 2D-LC-MS/MS on a Orbitrap LC-MS | 2612 | [145] | |
| Brain mice | Label-free quantification with LC-MS/MS on a Q-IT-OT Fusion Lumos | 1683 | [144] | |
| T. gondii-infected and -uninfected placentas of pregnant mice | Label-free quantification on a Q-Exactive Plus Orbitrap mass | 792 | [146] | |
| Mitochondria from parasites | Label-free quantification on a Q-Exactive Orbitrap | 400 | [149] | |
| Syphilis | Urine | Label-free quantification on a 2D-LC-MALDI TOF/TOF and LC/ESI-IM-Q-TOF/HDMS | Not available | [163] |
| DAL-1 strain bacteria isolated from rabbits | Label-free quantification on a MALDI-TOF/TOF and ESI-LTQ-Orbitrap | 557 | [161] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo-da-Silva, J.; Marinho, C.R.F.; Palmisano, G.; Rosa-Fernandes, L. Lights and Shadows of TORCH Infection Proteomics. Genes 2020, 11, 894. https://doi.org/10.3390/genes11080894
Macedo-da-Silva J, Marinho CRF, Palmisano G, Rosa-Fernandes L. Lights and Shadows of TORCH Infection Proteomics. Genes. 2020; 11(8):894. https://doi.org/10.3390/genes11080894
Chicago/Turabian StyleMacedo-da-Silva, Janaina, Claudio Romero Farias Marinho, Giuseppe Palmisano, and Livia Rosa-Fernandes. 2020. "Lights and Shadows of TORCH Infection Proteomics" Genes 11, no. 8: 894. https://doi.org/10.3390/genes11080894
APA StyleMacedo-da-Silva, J., Marinho, C. R. F., Palmisano, G., & Rosa-Fernandes, L. (2020). Lights and Shadows of TORCH Infection Proteomics. Genes, 11(8), 894. https://doi.org/10.3390/genes11080894







