Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function
Abstract
:1. An Introduction to Centromere Diversity
2. Centromere Organizational Diversity in Light of Evolution
Centromere Drive: From Conflicts to Benefits
3. Mapping Mutagenic Mechanisms by Following Their Evolutionary Footsteps on Centromere DNA
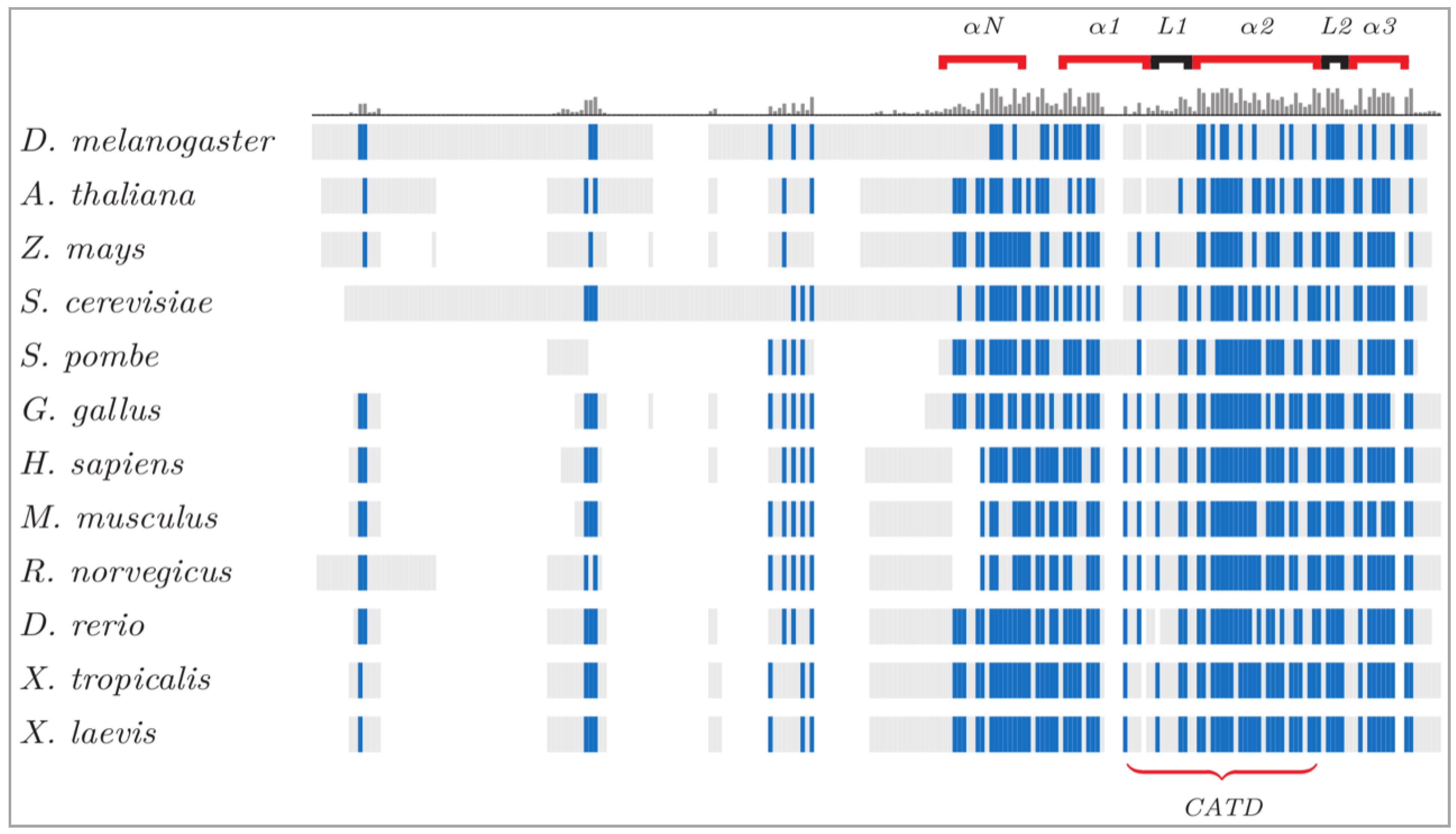
Formation of Human Centromeres through Evolutionary Mutagenesis
4. Changing Identity: Pathological Consequences of Rapid Centromere Evolution
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Flemming, W. Zellsubstanz, Kern und Zelltheilung. DMW—Dtsch. Med. Wochenschr. 1883, 9, 342. [Google Scholar] [CrossRef]
- Darlington, C.D.; Hallpike, C.S.; Hartridge, H.; Rawdon-Smith, A.F. The external mechanics of the chromosomes I—The scope of enquiry. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 1936, 121, 264–273. [Google Scholar] [CrossRef]
- Carbon, J.; Clarke, L. Structural and Functional Analysis of a Yeast Centromere (CEN3). J. Cell Sci. 1984, 1984, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Furuyama, S.; Biggins, S. Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc. Natl. Acad. Sci. USA 2007, 104, 14706–14711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willard, H.F. Centromeres: The missing link in the development of human artificial chromosomes. Curr. Opin. Genet. Dev. 1998, 8, 219–225. [Google Scholar] [CrossRef]
- Malik, H.S. Conflict begets complexity: The evolution of centromeres. Curr. Opin. Genet. Dev. 2002, 12, 711–718. [Google Scholar] [CrossRef]
- Henikoff, S.; Ahmad, K.; Malik, H.S. The Centromere Paradox: Stable Inheritance with Rapidly Evolving DNA. Science 2001, 293, 1098–1102. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.S. Gene Regulation, Epigenetics and Hormone Signaling; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Sanyal, K.; Baum, M.; Carbon, J. Centromeric DNA sequences in the pathogenic yeast Candida albicans are all different and unique. Proc. Natl. Acad. Sci. USA 2004, 101, 11374–11379. [Google Scholar] [CrossRef] [Green Version]
- Wood, V.; Gwilliam, R.; Rajandream, M.A.; Lyne, M.; Lyne, R.; Stewart, A.; Sgouros, J.; Peat, N.; Hayles, J.; Baker, S.; et al. The genome sequence of Schizosaccharomyces pombe. Nature 2002, 415, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Copenhaver, G.P. Genetic Definition and Sequence Analysis of Arabidopsis Centromeres. Science 1999, 286, 2468–2474. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Dong, F.; Langdon, T.; Ouyang, S.; Buell, C.R.; Gu, M.; Blattner, F.R.; Jiang, J. Functional Rice Centromeres Are Marked by a Satellite Repeat and a Centromere-Specific Retrotransposon. Plant Cell 2002, 14, 1691–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananiev, E.V.; Phillips, R.L.; Rines, H.W. Chromosome-specific molecular organization of maize (Zea mays L.) centromeric regions. Proc. Natl. Acad. Sci. USA 1998, 95, 13073–13078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, T.D.; Karpen, G.H. Localization of Centromere Function in a Drosophila Minichromosome. Cell 1995, 82, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Kipling, D.; Ackford, H.E.; Taylor, B.A.; Cooke, H.J. Mouse minor satellite DNA genetically maps to the centromere and is physically linked to the proximal telomere. Genomics 1991, 11, 235–241. [Google Scholar] [CrossRef]
- Lo, A.W.I.; Craig, J.M.; Saffery, R.; Kalitsis, P.; Irvine, D.V.; Earle, E.; Magliano, D.J.; Choo, K.H.A. A 330 kb CENP-A binding domain and altered replication timing at a human neocentromere. EMBO J. 2001, 20, 2087–2096. [Google Scholar] [CrossRef]
- Neumann, P.; Navrátilová, A.; Schroeder-Reiter, E.; Koblížková, A.; Steinbauerova, V.; Chocholová, E.; Novak, P.; Wanner, G.; Macas, J. Stretching the Rules: Monocentric Chromosomes with Multiple Centromere Domains. PLoS Genet. 2012, 8, e1002777. [Google Scholar] [CrossRef] [Green Version]
- Barlow, P.W.; Nevin, D. Quantitative karyology of some species of Luzula. Plant Syst. Evol. 1976, 125, 77–86. [Google Scholar] [CrossRef]
- The International Silkworm Genome; The International Silkworm Genome Consortium. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1036–1045. [Google Scholar] [CrossRef]
- Kawamoto, M.; Jouraku, A.; Toyoda, A.; Yokoi, K.; Minakuchi, Y.; Katsuma, S.; Fujiyama, A.; Kiuchi, T.; Yamamoto, K.; Shimada, T. High-quality genome assembly of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2019, 107, 53–62. [Google Scholar] [CrossRef]
- Waterston, R. Genome Sequence of the Nematode C. elegans: A Platform for Investigating Biology. Science 1998, 282, 2012–2018. [Google Scholar] [CrossRef]
- McKinley, K.L.; Cheeseman, I.M. The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol. 2015, 17, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. What makes a centromere? Exp. Cell Res. 2020, 389, 111895. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, G.A.; Gambogi, C.W.; Liskovykh, M.A.; Barrey, E.J.; Larionov, V.; Miga, K.H.; Heun, P.; Black, B.E. Human Artificial Chromosomes that Bypass Centromeric DNA. Cell 2019, 178, 624–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.F. A solid foundation: Functional specialization of centromeric chromatin. Curr. Opin. Genet. Dev. 2001, 11, 182–188. [Google Scholar] [CrossRef]
- Talbert, P.B.; Henikoff, S. Histone variants—Ancient wrap artists of the epigenome. Nat. Rev. Mol. Cell Biol 2010, 11, 264–275. [Google Scholar] [CrossRef]
- Saffery, R.; Earle, E.; Irvine, D.V.; Kalitsis, P.; Choo, K.H.A. Conservation of centromere protein in vertebrates. Chromosom. Res. 1999, 7, 261–265. [Google Scholar] [CrossRef]
- Meluh, P.B.; Yang, P.; Glowczewski, L.; Koshland, D.; Smith, M. Cse4p Is a Component of the Core Centromere of Saccharomyces cerevisiae. Cell 1998, 94, 607–613. [Google Scholar] [CrossRef] [Green Version]
- UniProt. Available online: https://www.uniprot.org/uniprot/P36012 (accessed on 17 June 2020).
- Takahashi, K.; Chen, E.S.; Yanagida, M. Requirement of Mis6 Centromere Connector for Localizing a CENP-A-Like Protein in Fission Yeast. Science 2000, 288, 2215–2219. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprot/Q9Y812 (accessed on 17 June 2020).
- Henikoff, S.; Ahmad, K.; Platero, J.S.; Van Steensel, B. Heterochromatic deposition of centromeric histone H3-like proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 716–721. [Google Scholar] [CrossRef] [Green Version]
- UniProt. Available online: https://www.uniprot.org/uniprot/Q9V6Q2 (accessed on 17 June 2020).
- Lermontova, I.; Schubert, V.; Fuchs, J.; Klatte, S.; Macas, J.; Schubert, I. Loading of Arabidopsis Centromeric Histone CENH3 Occurs Mainly during G2 and Requires the Presence of the Histone Fold Domain. Plant Cell 2006, 18, 2443–2451. [Google Scholar] [CrossRef] [Green Version]
- UniProt. Available online: https://www.uniprot.org/uniprot/Q8RVQ9-1 (accessed on 17 June 2020).
- Buchwitz, B.J.; Ahmad, K.; Moore, L.L.; Roth, M.B.; Henikoff, S. A histone-H3-like protein in C. elegans. Nature 1999, 401, 547–548. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/uniprot/P34470 (accessed on 17 June 2020).
- Kalitsis, P.; Macdonald, A.C.; Newson, A.J.; Hudson, D.F.; Choo, K. Gene Structure and Sequence Analysis of Mouse Centromere Proteins A and C. Genomics 1998, 47, 108–114. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/uniprot/O35216 (accessed on 17 June 2020).
- Sullivan, K.F.; Hechenberger, M.; Masri, K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J. Cell Biol. 1994, 127, 581–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earnshaw, W.C.; Rothfield, N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 1985, 91, 313–321. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprot/P49450 (accessed on 17 June 2020).
- Mellone, B.G.; Grive, K.J.; Shteyn, V.; Bowers, S.R.; Oderberg, I.; Karpen, G. Assembly of Drosophila Centromeric Chromatin Proteins during Mitosis. PLoS Genet. 2011, 7, e1002068. [Google Scholar] [CrossRef] [Green Version]
- Talbert, P.B.; Henikoff, S. Phylogeny as the basis for naming histones. Trends Genet. 2013, 29, 499–500. [Google Scholar] [CrossRef]
- Neumann, P.; Pavlíková, Z.; Koblížková, A.; Fuková, I.; Jedličková, V.; Novak, P.; Macas, J. Centromeres off the Hook: Massive Changes in Centromere Size and Structure Following Duplication of CenH3 Gene in Fabeae Species. Mol. Biol. Evol. 2015, 32, 1862–1879. [Google Scholar] [CrossRef] [Green Version]
- Blower, M.D.; Sullivan, B.A.; Karpen, G. Conserved Organization of Centromeric Chromatin in Flies and Humans. Dev. Cell 2002, 2, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, B.A.; Karpen, G.H. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat. Struct. Mol. Biol. 2004, 11, 1076–1083. [Google Scholar] [CrossRef]
- Robledillo, L.Á.; Neumann, P.; Koblížková, A.; Novák, P.; Vrbová, I.; Macas, J. Extraordinary Sequence Diversity and Promiscuity of Centromeric Satellites in the Legume Tribe Fabeae. Mol. Biol. Evol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Earnshaw, W.C.; Migeon, B.R. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma 1985, 92, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Black, B.E.; Foltz, D.R.; Chakravarthy, S.; Luger, K.; Woods, V.L.; Cleveland, D.W. Structural determinants for generating centromeric chromatin. Nature 2004, 430, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Goutte-Gattat, D.; Shuaib, M.; Ouararhni, K.; Gautier, T.; Skoufias, D.A.; Hamiche, A.; Dimitrov, S. Phosphorylation of the CENP-A amino-terminus in mitotic centromeric chromatin is required for kinetochore function. Proc. Natl. Acad. Sci. USA 2013, 110, 8579–8584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incenp. Available online: https://incenp.org/research/cenpa.html (accessed on 26 June 2020).
- Akiyoshi, B.; Gull, K. Discovery of unconventional kinetochores in kinetoplastids. Cell 2014, 156, 1247–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drinnenberg, I.A.; deYoung, D.; Henikoff, S.; Malik, H.S. Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. eLife 2014, 3. [Google Scholar] [CrossRef]
- Oegema, K.A.; Hyman, A. Cell division. WormBook 2006, 1–40. [Google Scholar] [CrossRef]
- Cortes-Silva, N.; Ulmer, J.; Kiuchi, T.; Hsieh, E.; Cornilleau, G.; Ladid, I.; Dingli, F.; Loew, D.; Katsuma, S.; Drinnenberg, I.A. CenH3-Independent Kinetochore Assembly in Lepidoptera Requires CCAN, Including CENP-T. Curr. Biol. 2020, 30, 561–572.e10. [Google Scholar] [CrossRef]
- Ross, B.D.; Rosin, L.; Thomae, A.; Hiatt, M.A.; Vermaak, D.; De La Cruz, A.F.A.; Imhof, A.; Mellone, B.G.; Malik, H.S. Stepwise Evolution of Essential Centromere Function in a Drosophila Neogene. Science 2013, 340, 1211–1214. [Google Scholar] [CrossRef] [Green Version]
- Nerusheva, O.; Akiyoshi, B. Divergent polo box domains underpin the unique kinetoplastid kinetochore. Open Biol. 2016, 6, 150206. [Google Scholar] [CrossRef] [Green Version]
- D’Archivio, S.; Wickstead, B. Trypanosome outer kinetochore proteins suggest conservation of chromosome segregation machinery across eukaryotes. J. Cell Biol. 2016, 216, 379–391. [Google Scholar] [CrossRef]
- Talbert, P.B.; Bryson, T.D.; Henikoff, S. Adaptive evolution of centromere proteins in plants and animals. J. Biol. 2004, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldrup-MacDonald, M.E.; Sullivan, B.A. The Past, Present, and Future of Human Centromere Genomics. Genes 2014, 5, 33–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voullaire, L.E.; Slater, H.R.; Petrovic, V.; Choo, K.H. A functional marker centromere with no detectable α-satellite, satellite III, or CENP-B protein: Activation of a latent centromere? Am. J. Hum. Genet. 1993, 52, 1153–1163. [Google Scholar] [PubMed]
- Marshall, O.J.; Chueh, A.; Wong, L.H.; Choo, K.H.A. Neocentromeres: New Insights into Centromere Structure, Disease Development, and Karyotype Evolution. Am. J. Hum. Genet. 2008, 82, 261–282. [Google Scholar] [CrossRef] [Green Version]
- Westhorpe, F.G.; Straight, A.F. The Centromere: Epigenetic Control of Chromosome Segregation during Mitosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a015818. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, K.F.; Glass, C.A. CENP-B is a highly conserved mammalian centromere protein with homology to the helix-loop-helix family of proteins. Chromosoma 1991, 100, 360–370. [Google Scholar] [CrossRef]
- Tomkiel, J.; Cooke, C.A.; Saitoh, H.; Bernat, R.L.; Earnshaw, W.C. CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J. Cell Biol. 1994, 125, 531–545. [Google Scholar] [CrossRef]
- Giulotto, E.; Raimondi, E.; Sullivan, K.F. The Unique DNA Sequences Underlying Equine Centromeres. Adv. Biochem. Eng. Biotechnol. 2017, 56, 337–354. [Google Scholar] [CrossRef]
- Piras, F.M.; Nergadze, S.G.; Magnani, E.; Bertoni, L.; Attolini, C.; Khoriauli, L.; Raimondi, E.M.C.; Giulotto, E. Uncoupling of Satellite DNA and Centromeric Function in the Genus Equus. PLoS Genet. 2010, 6, e1000845. [Google Scholar] [CrossRef] [Green Version]
- Nergadze, S.G.; Piras, F.M.; Gamba, R.; Corbo, M.; Cerutti, F.; McCarter, J.G.; Cappelletti, E.; Gozzo, F.; Harman, R.M.; Antczak, D.F.; et al. Birth, evolution, and transmission of satellite-free mammalian centromeric domains. Genome Res. 2018, 28, 789–799. [Google Scholar] [CrossRef] [Green Version]
- Roberti, A.; Bensi, M.; Mazzagatti, A.; Piras, F.M.; Nergadze, S.G.; Giulotto, E.; Raimondi, E.M.C. Satellite DNA at the Centromere is Dispensable for Segregation Fidelity. Genes 2019, 10, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, C.M.; Giulotto, E.; Sigurdsson, S.; Zoli, M.; Gnerre, S.; Imsland, F.; Lear, T.L.; Adelson, D.L.; Bailey, E.; Bellone, R.R.; et al. Genome Sequence, Comparative Analysis, and Population Genetics of the Domestic Horse. Science 2009, 326, 865–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fachinetti, D.; Han, J.S.; McMahon, M.A.; Ly, P.; Abdullah, A.; Wong, A.J.; Cleveland, D.W. DNA Sequence-Specific Binding of CENP-B Enhances the Fidelity of Human Centromere Function. Dev. Cell 2015, 33, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, S.; Dumont, M.; Barra, V.; Ly, P.; Nechemia-Arbely, Y.; McMahon, M.A.; Hervé, S.; Cleveland, D.W.; Fachinetti, D. CENP-A Is Dispensable for Mitotic Centromere Function after Initial Centromere/Kinetochore Assembly. Cell Rep. 2016, 17, 2394–2404. [Google Scholar] [CrossRef] [Green Version]
- Earnshaw, W.C.; Sullivan, K.F.; Machlin, P.; Cooke, C.; Kaiser, D.; Pollard, T.; Rothfield, N.; Cleveland, D.W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J. Cell Biol. 1987, 104, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Black, B.E.; Cleveland, D.W. Epigenetic Centromere Propagation and the Nature of CENP-A Nucleosomes. Cell 2011, 144, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Mendiburo, M.J.; Padeken, J.; Fülöp, S.; Schepers, A.; Heun, P. Drosophila CENH3 Is Sufficient for Centromere Formation. Science 2011, 334, 686–690. [Google Scholar] [CrossRef]
- Hori, T.; Shang, W.H.; Takeuchi, K.; Fukagawa, T. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J. Cell Biol. 2012, 200, 45–60. [Google Scholar] [CrossRef]
- Barnhart, M.C.; Kuich, P.H.J.L.; Stellfox, M.E.; Ward, J.A.; Bassett, E.A.; Black, B.E.; Foltz, D.R. HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J. Cell Biol. 2011, 194, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zeng, Z.; Zhang, W.; Jiang, J. Three Potato Centromeres Are Associated with Distinct Haplotypes with or Without Megabase-Sized Satellite Repeat Arrays. Genetics 2013, 196, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wu, Y.; Zhang, W.; Dawe, R.K.; Jiang, J. Maize centromeres expand and adopt a uniform size in the genetic background of oat. Genome Res. 2013, 24, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, F.; Garcia, C.B.; Arruga, M.V.; Ferguson-Smith, M.A. Chromosome homology between chicken (Gallus gallus domesticus) and the red-legged partridge (Alectoris rufa); evidence of the occurrence of a neocentromere during evolution. Cytogenet. Genome Res. 2003, 102, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Fu, B.; O’Brien, P.C.M.; Nie, W.; Ryder, O.A.; Ferguson-Smith, M.A. Refined genome-wide comparative map of the domestic horse, donkey and human based on cross-species chromosome painting: Insight into the occasional fertility of mules. Chromosom. Res. 2004, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Purgato, S.; Belloni, E.; Piras, F.M.; Zoli, M.; Badiale, C.; Cerutti, F.; Mazzagatti, A.; Perini, G.; Della Valle, G.; Nergadze, S.G.; et al. Centromere sliding on a mammalian chromosome. Chromosoma 2014, 124, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Ventura, M.; Archidiacono, N.; Rocchi, M. Centromere Emergence in Evolution. Genome Res. 2001, 11, 595–599. [Google Scholar] [CrossRef] [Green Version]
- du Sart, D.; Cancilla, M.R.; Earle, E.; Mao, J.I.; Saffery, R.; Tainton, K.M.; Kalitsis, P.; Martyn, J.; Barry, A.E.; Choo, K.H.A. A functional neo-centromere formed through activation of a latent human centromere and consisting of non-α-satellite DNA. Nat. Genet. 1997, 16, 144–153. [Google Scholar] [CrossRef]
- Montefalcone, G.; Tempesta, S.; Rocchi, M.; Archidiacono, N. Centromere Repositioning. Genome Res. 1999, 9, 1184–1188. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, M.; Archidiacono, N.; Schempp, W.; Capozzi, O.; Stanyon, R. Centromere repositioning in mammals. Heredity 2011, 108, 59–67. [Google Scholar] [CrossRef]
- Amor, D.J.; Bentley, K.; Ryan, J.; Perry, J.K.; Wong, L.H.; Slater, H.; Choo, K.H.A. Human centromere repositioning “in progress”. Proc. Natl. Acad. Sci. USA 2004, 101, 6542–6547. [Google Scholar] [CrossRef] [Green Version]
- Amor, D.J.; Choo, K.H.A. Neocentromeres: Role in Human Disease, Evolution, and Centromere Study. Am. J. Hum. Genet. 2002, 71, 695–714. [Google Scholar] [CrossRef] [Green Version]
- Burrack, L.S.; Berman, J. Neocentromeres and epigenetically inherited features of centromeres. Chromosom. Res. 2012, 20, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Stimpson, K.M.; Matheny, J.E.; Sullivan, B.A. Dicentric chromosomes: Unique models to study centromere function and inactivation. Chromosom. Res. 2012, 20, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nechemia-Arbely, Y.; Miga, K.H.; Shoshani, O.; Aslanian, A.; McMahon, M.A.; Lee, A.Y.; Fachinetti, D.; Yates, J.R.; Ren, B.; Cleveland, D.W. DNA replication acts as an error correction mechanism to maintain centromere identity by restricting CENP-A to centromeres. Nature 2019, 21, 743–754. [Google Scholar] [CrossRef]
- Zeitlin, S.G.; Baker, N.M.; Chapados, B.R.; Soutoglou, E.; Wang, J.Y.J.; Berns, M.W.; Cleveland, D.W. Double-strand DNA breaks recruit the centromeric histone CENP-A. Proc. Natl. Acad. Sci. USA 2009, 106, 15762–15767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.C.; Murphy, T.D.; Goldberg, M.L.; Karpen, G. Neocentromere activity of structurally acentric mini-chromosomes in Drosophila. Nat. Genet. 1998, 18, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Maggert, K.A.; Karpen, G.H. The activation of a neocentromere in Drosophila requires proximity to an endogenous centromere. Genetics 2001, 158, 1615–1628. [Google Scholar]
- Olszak, A.M.; Van Essen, M.; Pereira, A.; Diehl, S.; Manke, T.; Maiato, H.; Saccani, S.; Heun, P. Heterochromatin boundaries are hotspots for de novo kinetochore formation. Nature 2011, 13, 799–808. [Google Scholar] [CrossRef]
- Piacentini, L.; Marchetti, M.; Bucciarelli, E.; Casale, A.M.; Cappucci, U.; Bonifazi, P.; Renda, F.; Fanti, L. A role of the Trx-G complex in Cid/CENP-A deposition at Drosophila melanogaster centromeres. Chromosoma 2019, 128, 503–520. [Google Scholar] [CrossRef]
- Topp, C.N.; Okagaki, R.; Melo, J.; Kynast, R.; Phillips, R.; Dawe, R. Identification of a maize neocentromere in an oat-maize addition line. Cytogenet. Genome Res. 2009, 124, 228–238. [Google Scholar] [CrossRef] [Green Version]
- Leo, L.; Marchetti, M.; Giunta, S.; Fanti, L. Epigenetics as an Evolutionary Tool for Centromere Flexibility. Genes 2020, 11, 809. [Google Scholar] [CrossRef]
- Malik, H.S.; Henikoff, S. Major Evolutionary Transitions in Centromere Complexity. Cell 2009, 138, 1067–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henikoff, S.; Henikoff, J.G. “Point” centromeres of Saccharomyces harbor single centromere-specific nucleosomes. Genetics 2012, 190, 1575–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegemann, J.H.; Fleig, U.N. The centromere of budding yeast. BioEssays 1993, 15, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Krassovsky, K.; Henikoff, J.G.; Henikoff, S. Tripartite organization of centromeric chromatin in budding yeast. Proc. Natl. Acad. Sci. USA 2011, 109, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Tatchell, K.; Van Holde, K.E. Nucleosome reconstitution: Effect of DNA length on nucleosome structure. Biochemistry 1979, 18, 2871–2880. [Google Scholar] [CrossRef]
- Brogaard, K.; Xi, L.; Wang, J.P.; Widom, J. A map of nucleosome positions in yeast at base-pair resolution. Nature 2012, 486, 496–501. [Google Scholar] [CrossRef]
- Henikoff, S.; Ramachandran, S.; Krassovsky, K.; Bryson, T.D.; Codomo, C.A.; Brogaard, K.; Widom, J.; Wang, J.P.; Henikoff, J.G. The budding yeast Centromere DNA Element II wraps a stable Cse4 hemisome in either orientation in vivo. Abstract 2014, 3, e01861. [Google Scholar] [CrossRef]
- Aravamudhan, P.; Felzer-Kim, I.; Joglekar, A. The budding yeast point centromere associates with two Cse4 molecules during mitosis. Curr. Biol. 2013, 23, 770–774. [Google Scholar] [CrossRef] [Green Version]
- Mizuguchi, G.; Xiao, H.; Wiśniewski, J.; Smith, M.M.; Wu, C. Nonhistone Scm3 and Histones CenH3-H4 Assemble the Core of Centromere-Specific Nucleosomes. Cell 2007, 129, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Lochmann, B.; Ivanov, D. Histone H3 Localizes to the Centromeric DNA in Budding Yeast. PLoS Genet. 2012, 8, e1002739. [Google Scholar] [CrossRef] [Green Version]
- Henikoff, S.; Furuyama, T. The unconventional structure of centromeric nucleosomes. Chromosoma 2012, 121, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuyama, T.; Codomo, C.A.; Henikoff, S. Reconstitution of hemisomes on budding yeast centromeric DNA. Nucleic Acids Res. 2013, 41, 5769–5783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaseko, Y.; Adachi, Y.; Funahashi, S.I.; Niwa, O.; Yanagida, M. Chromosome walking shows a highly homologous repetitive sequence present in all the centromere regions of fission yeast. EMBO J. 1986, 5, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wahlstrom, J.; Karpen, G.H. Molecular Structure of a Functional Drosophila Centromere. Cell 1997, 91, 1007–1019. [Google Scholar] [CrossRef] [Green Version]
- Round, E.K.; Flowers, S.K.; Richards, E.J. Arabidopsis thaliana Centromere Regions: Genetic Map Positions and Repetitive DNA Structure. Genome Res. 1997, 7, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Miller, J.T.; Jackson, S.A.; Wang, G.L.; Ronald, P.; Jiang, J. Rice (Oryza sativa) centromeric regions consist of complex DNA. Proc. Natl. Acad. Sci. USA 1998, 95, 8135–8140. [Google Scholar] [CrossRef] [Green Version]
- Nagaki, K.; Talbert, P.B.; Zhong, C.X.; Dawe, R.K.; Henikoff, S.; Jiang, J. Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 2003, 163, 1221–1225. [Google Scholar]
- Vig, B.K.; Latour, D.; Frankovich, J. Dissociation of minor satellite from the centromere in mouse. J. Cell Sci. 1994, 107, 3091–3095. [Google Scholar]
- Joseph, A.; Mitchell, A.; Miller, O. The organization of the mouse satellite DNA at centromeres. Exp. Cell Res. 1989, 183, 494–500. [Google Scholar] [CrossRef]
- Sullivan, L.L.; Chew, K.; Sullivan, B.A. α satellite DNA variation and function of the human centromere. Nucleus 2017, 8, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Melters, D.; Paliulis, L.V.; Korf, I.F.; Chan, S.W.L. Holocentric chromosomes: Convergent evolution, meiotic adaptations, and genomic analysis. Chromosom. Res. 2012, 20, 579–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinchcomb, D.T.; Shaw, J.E.; Carr, S.H.; Hirsh, D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol. Cell. Biol. 1985, 5, 3484–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henikoff, S.; Dalal, Y. Centromeric chromatin: What makes it unique? Curr. Opin. Genet. Dev. 2005, 15, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lawrimore, J.; Bloom, K. The regulation of chromosome segregation via centromere loops. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 352–370. [Google Scholar] [CrossRef]
- Roach, K.C.; Ross, B.; Malik, H.S. Rapid evolution of centromeres and centromeric/kinetochore proteins. In Rapidly Evolving Genes and Genetic Systems; Oxford University Press (OUP): Oxford, UK, 2012; pp. 83–93. [Google Scholar]
- Malik, H.S.; Bayes, J. Genetic conflicts during meiosis and the evolutionary origins of centromere complexity. Biochem. Soc. Trans. 2006, 34, 569–573. [Google Scholar] [CrossRef]
- Kursel, L.E.; Malik, H.S. The cellular mechanisms and consequences of centromere drive. Curr. Opin. Cell Biol. 2018, 52, 58–65. [Google Scholar] [CrossRef]
- Henikoff, S.; Malik, H.S. Centromeres: Selfish drivers. Nature 2002, 417, 227. [Google Scholar] [CrossRef]
- Akera, T.; Chmatal, L.; Trimm, E.; Yang, K.; Aonbangkhen, C.; Chenoweth, D.M.; Janke, C.; Schultz, R.M.; Lampson, M.A. Spindle asymmetry drives non-Mendelian chromosome segregation. Science 2017, 358, 668–672. [Google Scholar] [CrossRef] [Green Version]
- Lampson, M.A.; Black, B.E. Cellular and Molecular Mechanisms of Centromere Drive. Cold Spring Harb. Symp. Quant. Biol. 2017, 82, 249–257. [Google Scholar] [CrossRef]
- Malik, H.S. The Centromere-Drive Hypothesis: A Simple Basis for Centromere Complexity. Silicon Biominer. 2009, 48, 33–52. [Google Scholar] [CrossRef]
- Vermaak, D.; Hayden, H.S.; Henikoff, S. Centromere Targeting Element within the Histone Fold Domain of Cid. Mol. Cell. Biol. 2002, 22, 7553–7561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata-Otsubo, A.; Dawicki-McKenna, J.M.; Akera, T.; Falk, S.J.; Chmátal, L.; Yang, K.; Sullivan, B.A.; Schultz, R.M.; Lampson, M.A.; Black, B.E. Expanded Satellite Repeats Amplify a Discrete CENP-A Nucleosome Assembly Site on Chromosomes that Drive in Female Meiosis. Curr. Biol. 2017, 27, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Smoak, E.M.; Linares-Saldana, R.; Lampson, M.A.; Black, B.E. Centromere inheritance through the germline. Chromosoma 2017, 126, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; Tan, E.H.; West, A.; Franklin, F.C.H.; Comai, L.; Chan, S.W.L. Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of Hybrids. PLoS Genet. 2015, 11, e1004970. [Google Scholar] [CrossRef] [Green Version]
- Carbone, L.; Nergadze, S.G.; Magnani, E.; Misceo, D.; Cardone, M.F.; Roberto, R.; Bertoni, L.; Attolini, C.; Piras, M.F.; de Jong, P.; et al. Evolutionary movement of centromeres in horse, donkey, and zebra. Genomics 2006, 87, 777–782. [Google Scholar] [CrossRef] [Green Version]
- Finseth, F.R.; Dong, Y.; Saunders, A.; Fishman, L. Duplication and Adaptive Evolution of a Key Centromeric Protein in Mimulus, a Genus with Female Meiotic Drive. Mol. Biol. Evol. 2015, 32, 2694–2706. [Google Scholar] [CrossRef] [Green Version]
- Kursel, L.E.; Welsh, F.C.; Malik, H.S. Ancient Coretention of Paralogs of Cid Centromeric Histones and Cal1 Chaperones in Mosquito Species. Mol. Biol. Evol. 2020, 37, 1949–1963. [Google Scholar] [CrossRef]
- Kursel, L.E.; Malik, H.S. Recurrent Gene Duplication Leads to Diverse Repertoires of Centromeric Histones in Drosophila Species. Mol. Biol. Evol. 2017, 34, 1445–1462. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.D.; O’Neill, R.J. Chromosomes, Conflict, and Epigenetics: Chromosomal Speciation Revisited. Annu. Rev. Genom. Hum. Genet. 2010, 11, 291–316. [Google Scholar] [CrossRef]
- Black, E.M.; Giunta, S. Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases. Genes 2018, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Giunta, S.; Funabiki, H. Integrity of the human centromere DNA repeats is protected by CENP-A., CENP-C., and CENP-T. Proc. Natl. Acad. Sci. USA 2017, 114, 1928–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sax, K. Chromosome structure and the mechanism of crossing over. J. Arnold. Arb. 1930, 13, 180–212. [Google Scholar]
- Mather, K. CROSSING-OVER. Biol. Rev. 1938, 13, 252–292. [Google Scholar] [CrossRef]
- Mahtani, M.M.; Willard, H.F. A primary genetic map of the pericentromeric region of the human X chromosome. Genomics 1988, 2, 294–301. [Google Scholar] [CrossRef]
- Choo, K.H.A. Why Is the Centromere So Cold? Genome Res. 1998, 8, 81–82. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.A. Difference in the Behaviour of Eu- and Hetero-chromatin: Crossing-over. Nature 1965, 205, 725–726. [Google Scholar] [CrossRef]
- Jaco, I.; Canela, A.; Vera, E.; Blasco, M.A. Centromere mitotic recombination in mammalian cells. J. Cell Biol. 2008, 181, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Roizès, G. Human centromeric alphoid domains are periodically homogenized so that they vary substantially between homologues. Mechanism and implications for centromere functioning. Nucleic Acids Res. 2006, 34, 1912–1924. [Google Scholar] [CrossRef] [Green Version]
- Pironon, N.; Puechberty, J.; Roizès, G. Molecular and evolutionary characteristics of the fraction of human α satellite DNA associated with CENP-A at the centromeres of chromosomes 1, 5, 19, and 21. BMC Genom. 2010, 11, 195. [Google Scholar] [CrossRef] [Green Version]
- Langley, S.A.; Miga, K.H.; Karpen, G.H.; Langley, C.H. Haplotypes spanning centromeric regions reveal persistence of large blocks of archaic DNA. eLife 2019. [Google Scholar] [CrossRef]
- Giunta, S. Centromere Chromosome Orientation Fluorescent in situ Hybridization (Cen-CO-FISH) Detects Sister Chromatid Exchange at the Centromere in Human Cells. Bio Protoc. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Aze, A.; Sannino, V.; Soffientini, P.; Bachi, A.; Costanzo, V. Centromeric DNA replication reconstitution reveals DNA loops and ATR checkpoint suppression. Nature 2016, 18, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasinathan, S.; Henikoff, S. Non-B-Form DNA Is Enriched at Centromeres. Mol. Biol. Evol. 2018, 35, 949–962. [Google Scholar] [CrossRef] [Green Version]
- Smith, G. Evolution of repeated DNA sequences by unequal crossover. Science 1976, 191, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Henikoff, S. Centromeres Convert but Don’t Cross. PLoS Biol. 2010, 8, e1000326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wolf, S.E.; Burke, J.M.; Presting, G.G.; Ross-Ibarra, J.; Dawe, R.K. Widespread Gene Conversion in Centromere Cores. PLoS Biol. 2010, 8, e1000327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.D.M.; Dover, G.A. Conservation of segmental variants of satellite DNA of Mus musculus in a related species: Mus spretus. Nature 1980, 285, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Stahl, F.W. Gene Conversion. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Academic Press: San Diego, CA, USA, 2013; ISBN 9780080961569. [Google Scholar]
- Taghian, D.G.; Nickoloff, J.A. Chromosomal double-strand breaks induce gene conversion at high frequency in mammalian cells. Mol. Cell. Biol. 1997, 17, 6386–6393. [Google Scholar] [CrossRef] [Green Version]
- Elliott, B.; Richardson, C.; Winderbaum, J.; Nickoloff, J.A.; Jasin, M. Gene Conversion Tracts from Double-Strand Break Repair in Mammalian Cells. Mol. Cell. Biol. 1998, 18, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Richardson, C.; Moynahan, M.E.; Jasin, M. Double-strand break repair by interchromosomal recombination: Suppression of chromosomal translocations. Genes Dev. 1998, 12, 3831–3842. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.D.; Jasin, M. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 2000, 19, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.A.; Innan, H. Neutral and Non-Neutral Evolution of Duplicated Genes with Gene Conversion. Genes 2011, 2, 191–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.M.; Cooper, D.N.; Chuzhanova, N.; Férec, C.; Patrinos, G.P. Gene conversion: Mechanisms, evolution and human disease. Nat. Rev. Genet. 2007, 8, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Birchler, J.A.; Presting, G.G. Retrotransposon insertion targeting: A mechanism for homogenization of centromere sequences on nonhomologous chromosomes. Genes Dev. 2012, 26, 638–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.J.; O’Neill, R.J. Transposable elements: Genome innovation, chromosome diversity, and centromere conflict. Chromosom. Res. 2018, 26, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Suntronpong, A.; Singchat, W.; Kruasuwan, W.; Prakhongcheep, O.; Sillapaprayoon, S.; Muangmai, N.; Somyong, S.; Indananda, C.; Kraichak, E.; Peyachoknagul, S.; et al. Characterization of centromeric satellite DNAs (MALREP) in the Asian swamp eel (Monopterus albus) suggests the possible origin of repeats from transposable elements. Genomics 2020. [Google Scholar] [CrossRef]
- Macas, J.; Koblízková, A.; Navrátilová, A.; Neumann, P. Hypervariable 3′ UTR region of plant LTR-retrotransposons as a source of novel satellite repeats. Gene 2009, 448, 198–206. [Google Scholar] [CrossRef]
- Carone, D.M.; Zhang, C.; Hall, L.E.; Obergfell, C.; Carone, B.R.; O’Neill, M.J.; O’Neill, R.J. Hypermorphic expression of centromeric retroelement-encoded small RNAs impairs CENP-A loading. Chromosom. Res. 2013, 21, 49–62. [Google Scholar] [CrossRef]
- Corless, S.; Höcker, S.; Erhardt, S. Centromeric RNA and Its Function at and Beyond Centromeric Chromatin. J. Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Niedenthal, R.; Stoll, R.; Hegemann, J.H. In vivo characterization of the Saccharomyces cerevisiae centromere DNA element I, a binding site for the helix-loop-helix protein CPF1. Mol. Cell. Biol. 1991, 11, 3545–3553. [Google Scholar] [CrossRef] [Green Version]
- Kheiavi, E.K.; Ahmadikhah, A. Genome Mining of Rice (Oryza sativa subsp. indica) for Detection and Characterization of Long Palindromic Sequences. J. Data Min. Genom. Proteom. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Lago, M.; Bergman, C.M.; De Pablos, B.; Tracey, A.; Whitehead, S.L.; Villasante, A. A Large Palindrome with Interchromosomal Gene Duplications in the Pericentromeric Region of the D. melanogaster Y Chromosome. Mol. Biol. Evol. 2011, 28, 1967–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuzhanova, N.; Chen, J.M.; Bacolla, A.; Patrinos, G.P.; Férec, C.; Wells, R.D.; Cooper, D.N. Gene conversion causing human inherited disease: Evidence for involvement of non-B-DNA-forming sequences and recombination-promoting motifs in DNA breakage and repair. Hum. Mutat. 2009, 30, 1189–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, J.B. Persistence of Tandem Arrays: Implications for Satellite and Simple-Sequence Dnas. Genetics 1987, 115, 553–567. [Google Scholar] [PubMed]
- Zhao, J.; Bacolla, A.; Wang, G.; Vasquez, K.M. Non-B DNA structure-induced genetic instability and evolution. Cell. Mol. Life Sci. 2009, 67, 43–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Chou, S.H.; Reid, B.R. A single G-to-C change causes human centromere TGGAA repeats to fold back into hairpins. Proc. Natl. Acad. Sci. USA 1996, 93, 12159–12164. [Google Scholar] [CrossRef] [Green Version]
- Ohno, M.; Fukagawa, T.; Lee, J.S.; Ikemura, T. Triplex-forming DNAs in the human interphase nucleus visualized in situ by polypurine/polypyrimidine DNA probes and antitriplex antibodies. Chromosoma 2002, 111, 201–213. [Google Scholar] [CrossRef]
- Garavís, M.; Escaja, N.; Gabelica, V.; Villasante, A.; Gonzalez, C. Centromeric α-Satellite DNA Adopts Dimeric i-Motif Structures Capped by AT Hoogsteen Base Pairs. Chem. Eur. J. 2015, 21, 9816–9824. [Google Scholar] [CrossRef] [Green Version]
- Garavís, M.; Méndez-Lago, M.; Gabelica, V.; Whitehead, S.L.; Gonzalez, C.; Villasante, A. The structure of an endogenous Drosophila centromere reveals the prevalence of tandemly repeated sequences able to form i-motifs. Sci. Rep. 2015, 5, 13307. [Google Scholar] [CrossRef] [Green Version]
- Jonstrup, A.T.; Thomsen, T.; Wang, Y.; Knudsen, B.R.; Koch, J.; Andersen, A.H. Hairpin structures formed by α satellite DNA of human centromeres are cleaved by human topoisomerase II. Nucleic Acids Res. 2008, 36, 6165–6174. [Google Scholar] [CrossRef] [Green Version]
- Madireddy, A.; Gerhardt, J. Replication through Repetitive DNA Elements and Their Role in Human Diseases. In Retinal Degenerative Diseases; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 549–581. [Google Scholar]
- Available online: https://www.biorxiv.org/content/10.1101/731471v1 (accessed on 10 August 2019). [CrossRef] [Green Version]
- Maccaroni, K.; Balzano, E.; Mirimao, F.; Giunta, S.; Pelliccia, F. Impaired Replication Timing Promotes Tissue-Specific Expression of Common Fragile Sites. Genes 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2015, 26, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Callen, E.; Zong, D.; Wu, W.; Wong, N.; Stanlie, A.; Ishikawa, M.; Pavani, R.; Dumitrache, L.C.; Byrum, A.K.; Mendez-Dorantes, C.; et al. 53BP1 Enforces Distinct Pre- and Post-resection Blocks on Homologous Recombination. Mol. Cell 2020, 77, 26–38. [Google Scholar] [CrossRef]
- Malkova, A.; Ira, G. Break-induced replication: Functions and molecular mechanism. Curr. Opin. Genet. Dev. 2013, 23, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Khajavi, M.; Connolly, A.M.; Towne, C.F.; Batish, S.D.; Lupski, J.R. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat. Genet. 2009, 41, 849–853. [Google Scholar] [CrossRef] [Green Version]
- Hastings, P.J.; Ira, G.; Lupski, J.R. A Microhomology-Mediated Break-Induced Replication Model for the Origin of Human Copy Number Variation. PLoS Genet. 2009, 5, e1000327. [Google Scholar] [CrossRef] [Green Version]
- Llorente, B.; Smith, C.E.; Symington, L.S. Break-induced replication: What is it and what is it for? Cell Cycle 2008, 7, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, A.H.; Humayun, M.Z.; Karfopoulos, S.G.; Rush, M.G. Molecular characterization of small polydisperse circular DNA from an African green monkey cell line. Biochemistry 1982, 21, 2076–2085. [Google Scholar] [CrossRef]
- Baumann, P.; West, S.C. Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci. 1998, 23, 247–251. [Google Scholar] [CrossRef]
- Available online: https://www.biorxiv.org/content/10.1101/768887v1.full (accessed on 23 September 2019). [CrossRef]
- Bhargava, R.; Onyango, D.O.; Stark, J.M. Regulation of Single-Strand Annealing and its Role in Genome Maintenance. Trends Genet. 2016, 32, 566–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnan, S.; Kockler, Z.; Evans, R.; Downing, B.D.; Malkova, A. Single-strand annealing between inverted DNA repeats: Pathway choice, participating proteins, and genome destabilizing consequences. PLoS Genet. 2018, 14, e1007543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, A.W.; Liao, G.C.C.; Rocchi, M.; Choo, K.H.A. Extreme Reduction of Chromosome-Specific α-Satellite Array Is Unusually Common in Human Chromosome 21. Genome Res. 1999, 9, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Nakano, M.; Ohzeki, J.I.; Larionov, V.; Masumoto, H. A minimal CENP-A core is required for nucleation and maintenance of a functional human centromere. EMBO J. 2007, 26, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodor, D.L.; Mata, J.F.; Sergeev, M.; David, A.F.; Salimian, K.J.; Panchenko, T.; Cleveland, D.W.; Black, B.E.; Shah, J.V.; Jansen, L.E.T. The quantitative architecture of centromeric chromatin. eLife 2014, 3, e2137. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.biorxiv.org/content/10.1101/731430v1 (accessed on 10 August 2019). [CrossRef] [Green Version]
- Guin, K.; Chen, Y.; Mishra, R.; Muzaki, S.R.B.M.; Thimmappa, B.C.; O’Brien, C.E.; Butler, G.; Sanyal, A.; Sanyal, K. Spatial inter-centromeric interactions facilitated the emergence of evolutionary new centromeres. eLife 2020, 9. [Google Scholar] [CrossRef]
- Stephan, W. Tandem-repetitive noncoding DNA: Forms and forces. Mol. Biol. Evol. 1989, 6, 198–212. [Google Scholar] [CrossRef]
- Stephan, W.; Cho, S. Possible Role of Natural Selection in the Formation of Tandem-Repetitive Noncoding DNA. Genetics 1994, 136, 333–341. [Google Scholar]
- Britten, R.J. Divergence between samples of chimpanzee and human DNA sequences is 5%, counting indels. Proc. Natl. Acad. Sci. USA 2002, 99, 13633–13635. [Google Scholar] [CrossRef] [Green Version]
- Haaf, T.; Willard, H.F. Chromosome-specific α-satellite DNA from the centromere of chimpanzee chromosome 4. Chromosoma 1997, 106, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Archidiacono, N.; Antonacci, R.; Marzella, R.; Finelli, P.; Lonoce, A.; Rocchi, M. Comparative mapping of human alphoid sequences in great apes using fluorescence in situ hybridization. Genomics 1995, 25, 477–484. [Google Scholar] [CrossRef]
- Haaf, T.; Mater, A.G.; Wienberg, J.; Ward, D.C.; Matera, A.A. Presence and abundance of CENP-B box sequences in great ape subsets of primate-specific α-satellite DNA. J. Mol. Evol. 1995, 41, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, I.; Kazakov, A.; Tumeneva, I.; Shepelev, V.; Yurov, Y.B. α-satellite DNA of primates: Old and new families. Chromosoma 2001, 110, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Cacheux, L.; Ponger, L.; Gerbault-Seureau, M.; Loll, F.; Gey, D.; Richard, F.A.; Escudé, C. The Targeted Sequencing of α Satellite DNA in Cercopithecus pogonias Provides New Insight into the Diversity and Dynamics of Centromeric Repeats in Old World Monkeys. Genome Biol. Evol. 2018, 10, 1837–1851. [Google Scholar] [CrossRef]
- Cacheux, L.; Ponger, L.; Gerbault-Seureau, M.; Richard, F.A.; Escudé, C. Diversity and distribution of α satellite DNA in the genome of an Old World monkey: Cercopithecus solatus. BMC Genom. 2016, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Alves, G.; Seuánez, H.N.; Fanning, T. A Clade of New World Primates with Distinctive Alphoid Satellite DNAs. Mol. Phylogenetics Evol. 1998, 9, 220–224. [Google Scholar] [CrossRef]
- Alves, G.; Seuánez, H.N.; Fanning, T. α satellite DNA in neotropical primates (Platyrrhini). Chromosoma 1994, 103, 262–267. [Google Scholar] [CrossRef]
- Maio, J.J.; Brown, F.L.; Musich, P.R. Toward a molecular paleontology of primate genomes. Chromosoma 1981, 83, 103–125. [Google Scholar] [CrossRef]
- Musich, P.R.; Brown, F.L.; Maio, J.J. Highly repetitive component α and related alphoid DNAs in man and monkeys. Chromosoma 1980, 80, 331–348. [Google Scholar] [CrossRef]
- Baldini, A.; Miller, D.A.; Miller, O.J.; Ryder, O.A.; Mitchell, A.R. A chimpanzee-derived chromosome-specific α satellite DNA sequence conserved between chimpanzee and human. Chromosoma 1991, 100, 156–161. [Google Scholar] [CrossRef]
- Warburton, P.E.; Haaf, T.; Gosden, J.; Lawson, D.; Willard, H.F. Characterization of a Chromosome-Specific Chimpanzee α Satellite Subset: Evolutionary Relationship to Subsets on Human Chromosomes. Genomics 1996, 33, 220–228. [Google Scholar] [CrossRef]
- Waye, J.S.; Willard, H.F. Chromosome specificity of satellite DNAs: Short- and long-range organization of a diverged dimeric subset of human α satellite from chromosome 3. Chromosoma 1989, 97, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Durfy, S.J.; Willard, H.F. Concerted evolution of primate α satellite DNA. Evidence for an ancestral sequence shared by gorilla and human X chromosome α satellite. J. Mol. Biol. 1990, 216, 555–566. [Google Scholar] [CrossRef]
- Haaf, T.; Willard, H.F. Orangutan α-satellite monomers are closely related to the human consensus sequence. Mamm. Genome 1998, 9, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Rudd, M.K.; Matera, A.G.; Willard, H.F.; Hunt, P.A.; Schwartz, S.; Tartakoff, A. Organization, Evolution and Function of α Satellite DNA at Human Centromeres. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2005. [Google Scholar]
- Willard, H.F.; Waye, J.S. Hierarchical order in chromosome-specific human α satellite DNA. Trends Genet. 1987, 3, 192–198. [Google Scholar] [CrossRef]
- Romanova, L.; Deriagin, G.; Mashkova, T.; Tumeneva, I.; Mushegian, A.R.; Kisselev, L.; Alexandrov, I. Evidence for Selection in Evolution of α Satellite DNA: The Central Role of CENP-B/pJα Binding Region. J. Mol. Biol. 1996, 261, 334–340. [Google Scholar] [CrossRef]
- Tyler-Smith, C.; Brown, W.R. Structure of the major block of alphoid satellite DNA on the human Y chromosome. J. Mol. Biol. 1987, 195, 457–470. [Google Scholar] [CrossRef]
- Laurent, A.; Puechberty, J.; Roizès, G. Hypothesis: For the worst and for the best, L1Hs retrotransposons actively participate in the evolution of the human centromeric alphoid sequences. Chromosom. Res. 1999, 7, 305–317. [Google Scholar] [CrossRef]
- Schindelhauer, D.; Schwarz, T. Evidence for a Fast, Intrachromosomal Conversion Mechanism from Mapping of Nucleotide Variants within a Homogeneous α-Satellite DNA Array. Genome Res. 2002, 12, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Shepelev, V.A.; Alexandrov, A.A.; Yurov, Y.B.; Alexandrov, I.A. The Evolutionary Origin of Man Can Be Traced in the Layers of Defunct Ancestral α Satellites Flanking the Active Centromeres of Human Chromosomes. PLoS Genet. 2009, 5, e1000641. [Google Scholar] [CrossRef] [PubMed]
- Csink, A.K.; Henikoff, S. Something from nothing: The evolution and utility of satellite repeats. Trends Genet. 1998, 14, 200–204. [Google Scholar] [CrossRef]
- Otake, K.; Ohzeki, J.I.; Shono, N.; Kugou, K.; Okazaki, K.; Nagase, T.; Yamakawa, H.; Kouprina, N.; Larionov, V.; Kimura, H.; et al. CENP-B creates alternative epigenetic chromatin states permissive for CENP-A or heterochromatin assembly. J. Cell Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Miga, K.H.; Newton, Y.; Jain, M.; Altemose, N.; Willard, H.F.; Kent, W.J. Centromere reference models for human chromosomes X and Y satellite arrays. Genome Res. 2014, 24, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, K.H.; Vissel, B.; Nagy, A.; Earle, E.; Kalitsis, P. A survey of the genomic distribution of α satellite DNA on all the human chromosomes, and derivation of a new consensus sequence. Nucleic Acids Res. 1991, 19, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, M.K.; Wray, G.A.; Willard, H.F. The evolutionary dynamics of α satellite. Genome Res. 2005, 16, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Carine, K.; Jacquemin-Sablon, A.; Waltzer, E.; Mascarello, J.; Scheffler, I.E. Molecular characterization of human minichromosomes with centromere from chromosome 1 in human-hamster hybrid cells. Somat. Cell Mol. Genet. 1989, 15, 445–460. [Google Scholar] [CrossRef]
- Willard, H.F.; Waye, J.S. Chromosome-specific subsets of human α satellite DNA: Analysis of sequence divergence within and between chromosomal subsets and evidence for an ancestral pentameric repeat. J. Mol. Evol. 1987, 25, 207–214. [Google Scholar] [CrossRef]
- Fukagawa, T.; Earnshaw, W.C. The Centromere: Chromatin Foundation for the Kinetochore Machinery. Dev. Cell 2014, 30, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Hartley, G.; O’Neill, R.J. Centromere Repeats: Hidden Gems of the Genome. Genes 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Glunčić, M.; Vlahović, I.; Paar, V. Discovery of 33mer in chromosome 21—The largest α satellite higher order repeat unit among all human somatic chromosomes. Sci. Rep. 2019, 9, 12629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziccardi, W.; Zhao, C.; Shepelev, V.; Uralsky, L.; Alexandrov, I.; Andreeva, T.; Rogaev, E.; Bun, C.; Miller, E.; Putonti, C.; et al. Clusters of α satellite on human chromosome 21 are dispersed far onto the short arm and lack ancient layers. Chromosom. Res. 2016, 24, 421–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldrup-MacDonald, M.E.; Kuo, M.E.; Sullivan, L.L.; Chew, K.; Sullivan, B.A. Genomic variation within α satellite DNA influences centromere location on human chromosomes with metastable epialleles. Genome Res. 2016, 26, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barra, V.; Fachinetti, D. The dark side of centromeres: Types, causes and consequences of structural abnormalities implicating centromeric DNA. Nat. Commun. 2018, 9, 4340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, M.; Gamba, R.; Gestraud, P.; Klaasen, S.; Worrall, J.T.; De Vries, S.G.; Boudreau, V.; Salinas-Luypaert, C.; Maddox, P.S.; Lens, S.M.; et al. Human chromosome-specific aneuploidy is influenced by DNA-dependent centromeric features. EMBO J. 2019, 39, e102924. [Google Scholar] [CrossRef]
- Amon, J.D.; Koshland, D. RNase H enables efficient repair of R-loop induced DNA damage. eLife 2016, 5, 115. [Google Scholar] [CrossRef]
- Okita, A.K.; Zafar, F.; Su, J.; Weerasekara, D.; Kajitani, T.; Takahashi, T.S.; Kimura, H.; Murakami, Y.; Masukata, H.; Nakagawa, T. Heterochromatin suppresses gross chromosomal rearrangements at centromeres by repressing Tfs1/TFIIS-dependent transcription. Commun. Biol. 2019, 2, 17. [Google Scholar] [CrossRef]
- Blat, Y.; Kleckner, N. Cohesins Bind to Preferential Sites along Yeast Chromosome III, with Differential Regulation along Arms versus the Centric Region. Cell 1999, 98, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Weber, S.A.; Gerton, J.L.; Polancic, J.E.; DeRisi, J.L.; Koshland, D.; Megee, P.C. The Kinetochore Is an Enhancer of Pericentric Cohesin Binding. PLoS Biol. 2004, 2, e260. [Google Scholar] [CrossRef]
- González-Barrios, R.; Soto-Reyes, E.; Herrera, L.A. Assembling pieces of the centromere epigenetics puzzle. Epigenetics 2012, 7, 3–13. [Google Scholar] [CrossRef]
- Available online: https://www.biorxiv.org/content/10.1101/2020.06.04.133272v1 (accessed on 4 June 2020). [CrossRef]
- Wijmenga, C.; Scott Hansen, R.; Gimelli, G.; Björck, E.J.; Graham Davies, E.; Valentine, D.; Belohradsky, B.H.; Van Dongen, J.J.; Smeets, D.F.C.M.; Van Den Heuvel, L.P.W.J.; et al. Genetic variation in ICF syndrome: Evidence for genetic heterogeneity. Hum. Mutat. 2000. [Google Scholar] [CrossRef]
- Thijssen, P.E.; Ito, Y.; Grillo, G.; Wang, J.; Velasco, G.; Nitta, H.; Unoki, M.; Yoshihara, M.; Suyama, M.; Sun, Y.; et al. Mutations in CDCA7 and HELLS cause immunodeficiency–centromeric instability–facial anomalies syndrome. Nat. Commun. 2015, 6, 7870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unoki, M.; Funabiki, H.; Velasco, G.; Francastel, C.; Sasaki, H. CDCA7 and HELLS mutations undermine nonhomologous end joining in centromeric instability syndrome. J. Clin. Investig. 2018, 129, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Jenness, C.; Giunta, S.; Müller, M.M.; Kimura, H.; Muir, T.W.; Funabiki, H. HELLS and CDCA7 comprise a bipartite nucleosome remodeling complex defective in ICF syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, E876–E885. [Google Scholar] [CrossRef] [Green Version]
- De Greef, J.C.; Wang, J.; Balog, J.; Dunnen, J.T.D.; Frants, R.R.; Straasheijm, K.R.; Aytekin, C.; Van Der Burg, M.; Duprez, L.; Ferster, A.; et al. Mutations in ZBTB24 Are Associated with Immunodeficiency, Centromeric Instability, and Facial Anomalies Syndrome Type 2. Am. J. Hum. Genet. 2011, 88, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Weemaes, C.M.R.; Van Tol, M.J.; Wang, J.; Dam, M.M.V.O.T.; Van Eggermond, M.C.; Thijssen, P.E.; Aytekin, C.; Brunetti-Pierri, N.; Van Der Burg, M.; Davies, E.G.; et al. Heterogeneous clinical presentation in ICF syndrome: Correlation with underlying gene defects. Eur. J. Hum. Genet. 2013, 21, 1219–1225. [Google Scholar] [CrossRef]
- Ohzeki, J.I.; Bergmann, J.H.; Kouprina, N.; Noskov, V.N.; Nakano, M.; Kimura, H.; Earnshaw, W.C.; Larionov, V.; Masumoto, H. Breaking the HAC Barrier: Histone H3K9 acetyl/methyl balance regulates CENP-A assembly. EMBO J. 2012, 31, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Sathyan, K.M.; Fachinetti, D.; Foltz, D.R. α-amino trimethylation of CENP-A by NRMT is required for full recruitment of the centromere. Nat. Commun. 2017, 8, 14678. [Google Scholar] [CrossRef]
- Hedouin, S.; Grillo, G.; Ivkovic, I.; Velasco, G.; Francastel, C. CENP-A chromatin disassembly in stressed and senescent murine cells. Sci. Rep. 2017, 7, 42520. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Itkin-Ansari, P.; Levine, F. CENP-A, a protein required for chromosome segregation in mitosis, declines with age in islet but not exocrine cells. Aging 2010, 2, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Ly, D.H.; Lockhart, D.J.; Lerner, R.A.; Schultz, P.G. Mitotic Misregulation and Human Aging. Science 2000, 287, 2486–2492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narita, M.; Narita, M.; Krizhanovsky, V.; Nũnez, S.; Chicas, A.; Hearn, S.A.; Myers, M.P.; Lowe, S.W. A Novel Role for High-Mobility Group A Proteins in Cellular Senescence and Heterochromatin Formation. Cell 2006, 126, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Nye, J.; Sturgill, D.; Athwal, R.; Dalal, Y. HJURP antagonizes CENP-A mislocalization driven by the H3.3 chaperones HIRA and DAXX. PLoS ONE 2018, 13, e205948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaratiegui, M.; Vaughn, M.; Irvine, D.V.; Goto, D.; Watt, S.; Bähler, J.; Arcangioli, B.; Martienssen, R.A. CENP-B preserves genome integrity at replication forks paused by retrotransposon LTR. Nature 2010, 469, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, S.L.; Wulaningsih, W.; Lehmann, U. Transposable Elements in Human Cancer: Causes and Consequences of Deregulation. Int. J. Mol. Sci. 2017, 18, 974. [Google Scholar] [CrossRef] [Green Version]
- Ting, D.T.; Lipson, R.; Paul, S.; Brannigan, B.W.; Akhavanfard, S.; Coffman, E.J.; Contino, G.; Deshpande, V.; Iafrate, A.J.; Letovsky, S.; et al. Aberrant Overexpression of Satellite Repeats in Pancreatic and Other Epithelial Cancers. Science 2011, 331, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Bersani, F.; Lee, E.; Kharchenko, P.V.; Xu, A.W.; Liu, M.; Xega, K.; MacKenzie, O.C.; Brannigan, B.W.; Wittner, B.S.; Jung, H.; et al. Pericentromeric satellite repeat expansions through RNA-derived DNA intermediates in cancer. In Proc. Natl. Acad. Sci. USA 2015, 112, 15148–15153. [Google Scholar] [CrossRef] [Green Version]
- Kishikawa, T.; Otsuka, M.; Yoshikawa, T.; Ohno, M.; Yamamoto, K.; Yamamoto, N.; Kotani, A.; Koike, K. Quantitation of circulating satellite RNAs in pancreatic cancer patients. JCI Insight 2016, 1, e86646. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Hoong, N.; Aslanian, A.; Hara, T.; Benner, C.; Heinz, S.; Miga, K.H.; Ke, E.; Verma, S.; Soroczynski, J.; et al. Heterochromatin-Encoded Satellite RNAs Induce Breast Cancer. Mol. Cell 2018, 70, 842–853. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Jinks-Robertson, S. Transcription as a source of genome instability. Nat. Rev. Genet. 2012, 13, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Su, H.; Zhang, J.; Liu, Y.; Feng, C.; Han, F. Back-spliced RNA from retrotransposon binds to centromere and regulates centromeric chromatin loops in maize. PLoS Biol. 2020, 18, e3000582. [Google Scholar] [CrossRef] [PubMed]
- DuPraw, E.J. Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 1968; ISBN -10: 012224950X. [Google Scholar]
- Hagen, K.G.T.; Gilbert, D.M.; Willard, H.F.; Cohen, S.N. Replication timing of DNA sequences associated with human centromeres and telomeres. Mol. Cell. Biol. 1990, 10, 6348–6355. [Google Scholar] [CrossRef] [PubMed]
- Shelby, R.D.; Vafa, O.; Sullivan, K.F. Assembly of CENP-A into Centromeric Chromatin Requires a Cooperative Array of Nucleosomal DNA Contact Sites. J. Cell Biol. 1997, 136, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Erliandri, I.; Fu, H.; Nakano, M.; Kim, J.H.; Miga, K.H.; Liskovykh, M.; Earnshaw, W.C.; Masumoto, H.; Kouprina, N.; Aladjem, M.I.; et al. Replication of α-satellite DNA arrays in endogenous human centromeric regions and in human artificial chromosome. Nucleic Acids Res. 2014, 42, 11502–11516. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Centromere Type | Species | Size | References |
|---|---|---|---|
| Point centromere | fungi | [4] | |
| Saccharomyces cerevisiae | ~125 bp | ||
| Short regional centromere | fungi | [9,10] | |
| Candida albicans | ~3–5 kb | ||
| Schizosaccharomyces pombe | ~35–110 kb | ||
| Long regional centromere | viridiplantae | [11,12,13] | |
| Arabidopsis thaliana | ~400 kb–1.4 Mb | ||
| Oryza sativa | ~65 kb–2 Mb | ||
| Zea mays | ~180 kb | ||
| metazoa | [14,15,16] | ||
| Drosophila melanogaster | ~420 kb | ||
| Mus musculus | ~1 Mb | ||
| Homo sapiens | ~0.5 to 5 Mb | ||
| Meta-polycentric centromere | tracheobionta | [17] | |
| Pisum sativum | ~69–107 Mb | ||
| Holocentromere | viriplantae | [18] | |
| Luzula nivea | ~100 Mb | ||
| metazoa | [19,20,21] | ||
| Bombyx mori | ~8–21 Mb | ||
| Caenorhabditis elegans | ~14–21 Mb |
| H3-Like Centromeric Protein A Homologues | Model Organism | Size |
|---|---|---|
| Chromosome segregation 4 (Cse4) | Saccharomyces cerevisiae [28] | ~26 kDa [29] |
| Centromere-specific histone H3 (Cnp1) | Schizosaccharomyces pombe [30] | ~13 kDa [31] |
| Centromere identifier (Cid) | Drosophila melanogaster [32] | ~25 kDa [33] |
| Centromeric histone 3 (CenH3) | Arabidopsis thaliana [34] | ~19 kDa [35] |
| Histone H3-like centromeric protein (HCP-3) | Caenorhabditis elegans [36] | ~32 kDa [37] |
| Centromeric protein A (Cenpa) | Mus musculus [38] | ~15 kDa [39] |
| Centromeric protein A (CENP-A) | Homo sapiens [40,41] | ~15 kDa [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzano, E.; Giunta, S. Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function. Genes 2020, 11, 912. https://doi.org/10.3390/genes11080912
Balzano E, Giunta S. Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function. Genes. 2020; 11(8):912. https://doi.org/10.3390/genes11080912
Chicago/Turabian StyleBalzano, Elisa, and Simona Giunta. 2020. "Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function" Genes 11, no. 8: 912. https://doi.org/10.3390/genes11080912
APA StyleBalzano, E., & Giunta, S. (2020). Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function. Genes, 11(8), 912. https://doi.org/10.3390/genes11080912





