Identification and Characterization of Three Heat Shock Protein 90 (Hsp90) Homologs in the Brown Planthopper
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Identification of Hsp90 Homologs in BPH
2.3. Real-Time Quantitative PCR (RT-qPCR) Analysis
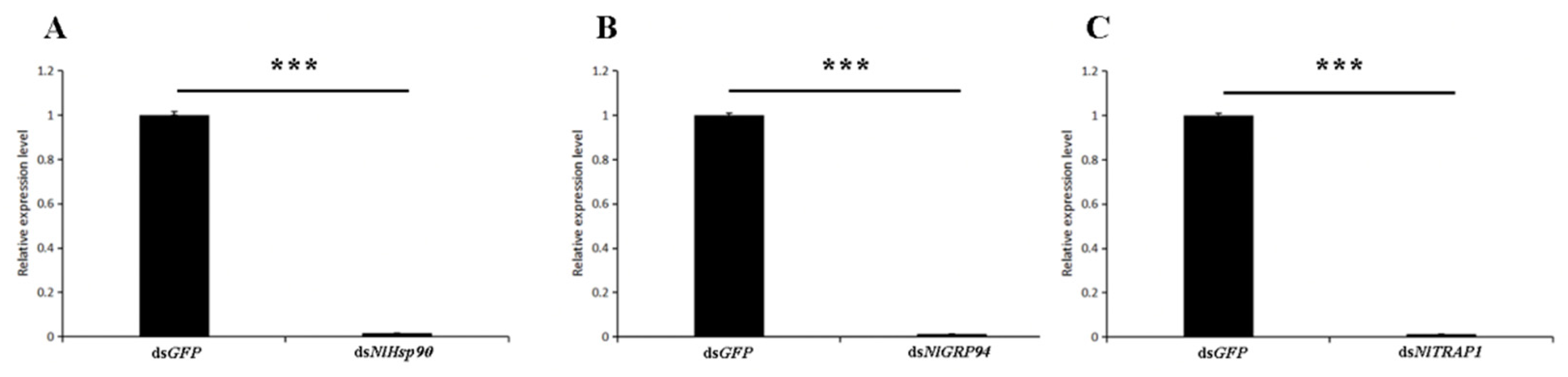
2.4. RNA Interference
2.5. Transmission Electron Microscopy (TEM) Observation
2.6. Statistical Analysis
3. Results
3.1. Identification and Analysis of the Hsp90 Homolog Gene Structure in BPH
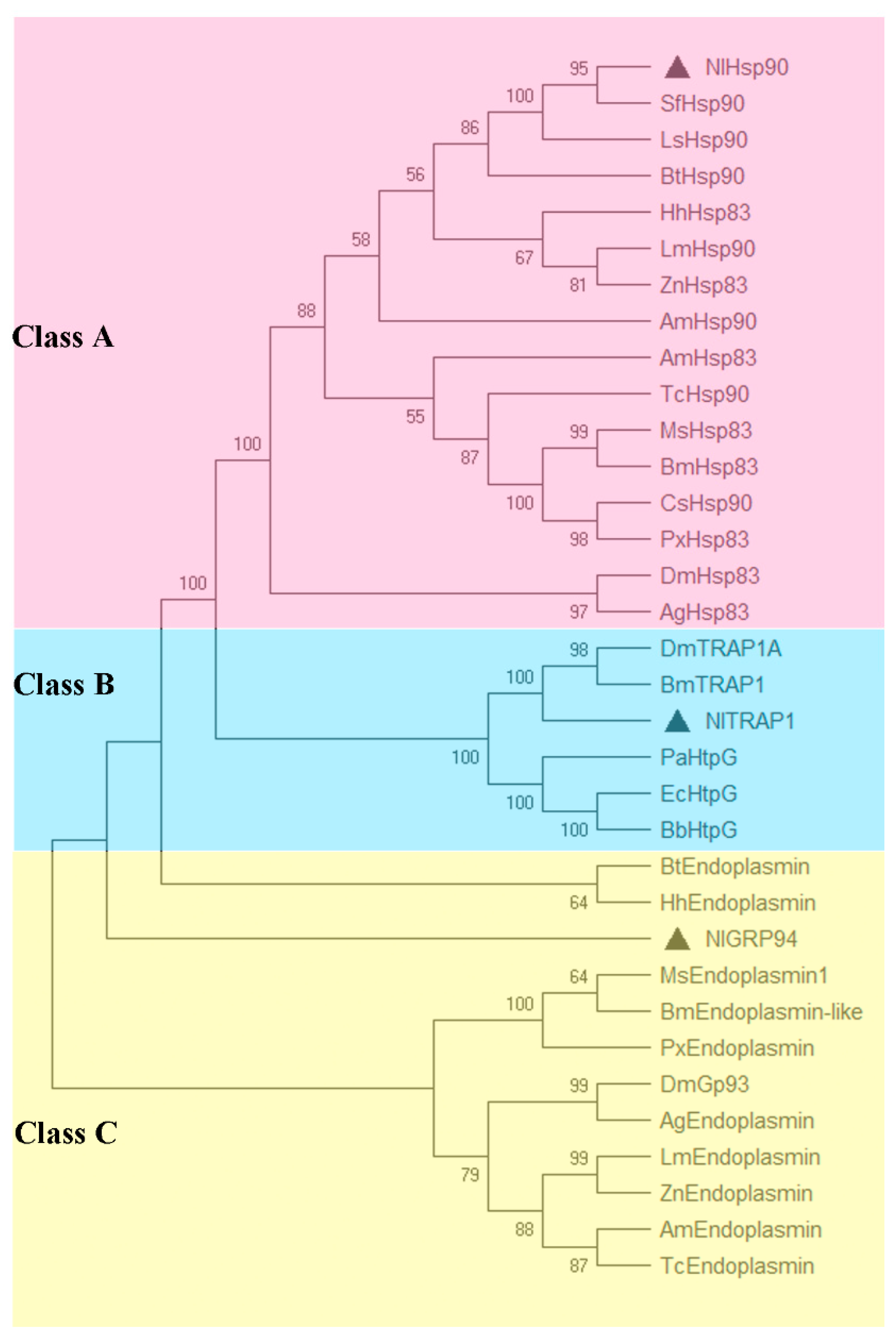
3.2. Phylogenetic Analysis
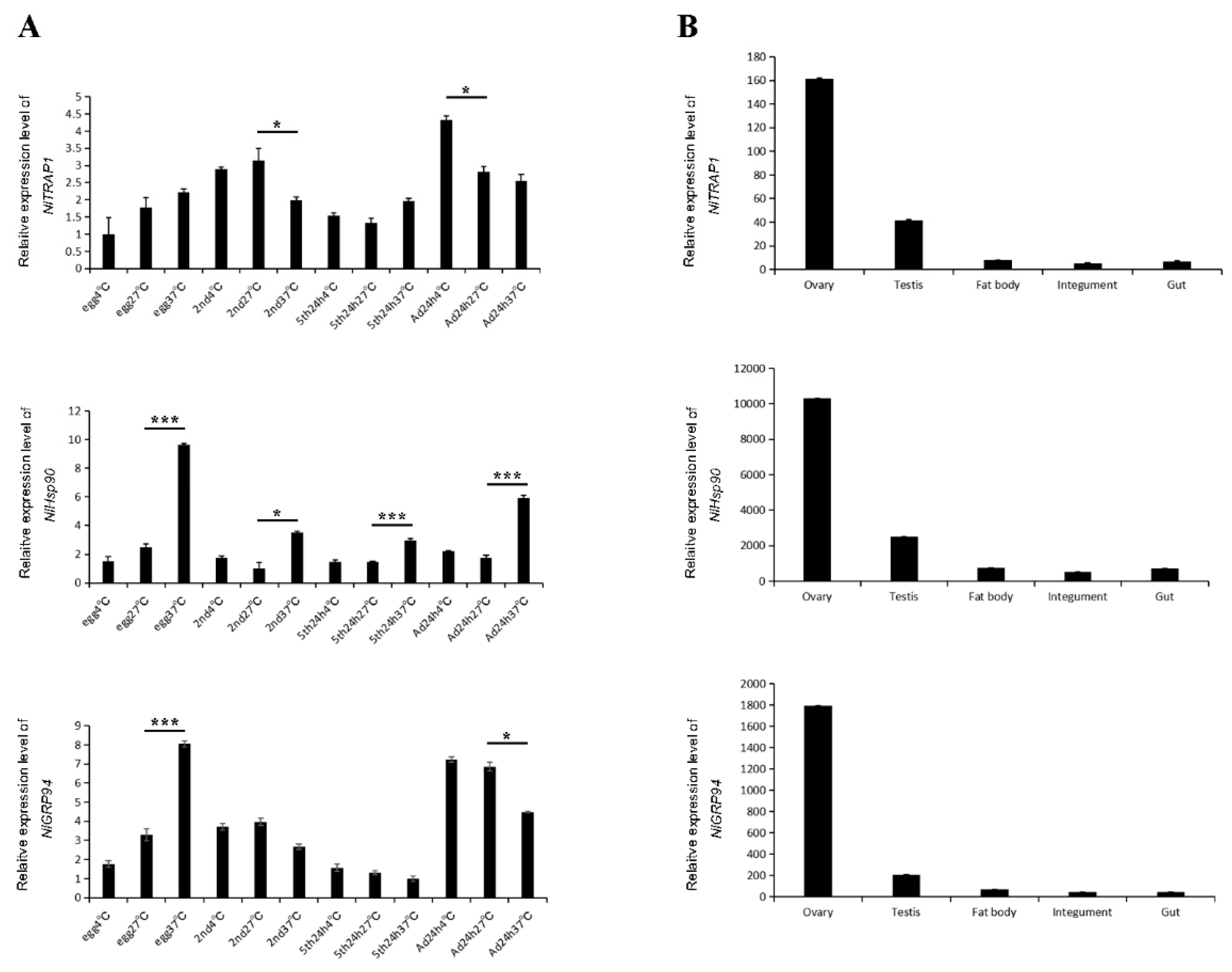
3.3. Temporospatial Expression Patterns
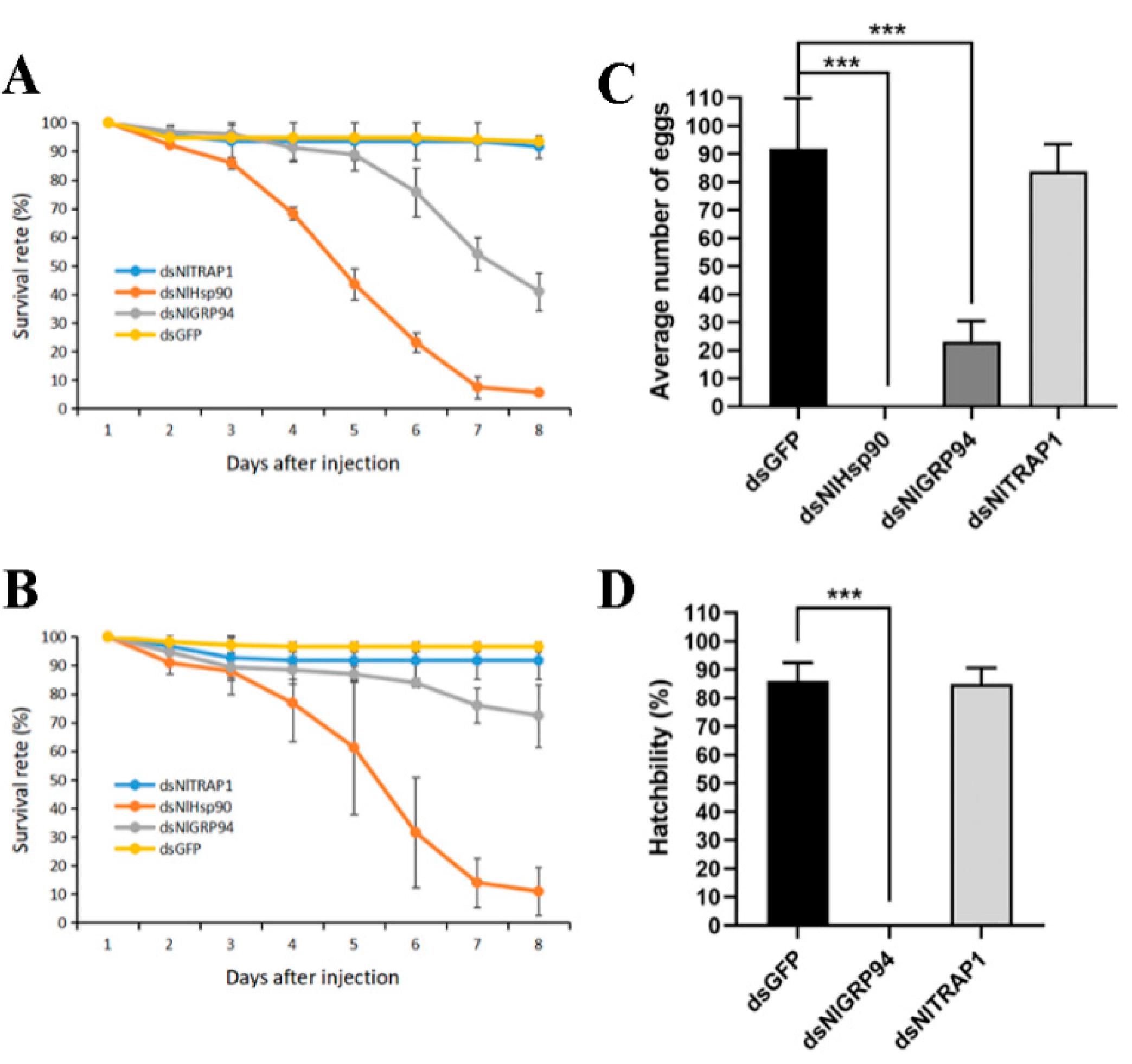
3.4. RNAi and Survival Assay under Normal Conditions
3.5. Effect of BPH Hsp90 Homologs on Oogenesis, Fecundity and Embryogenesis
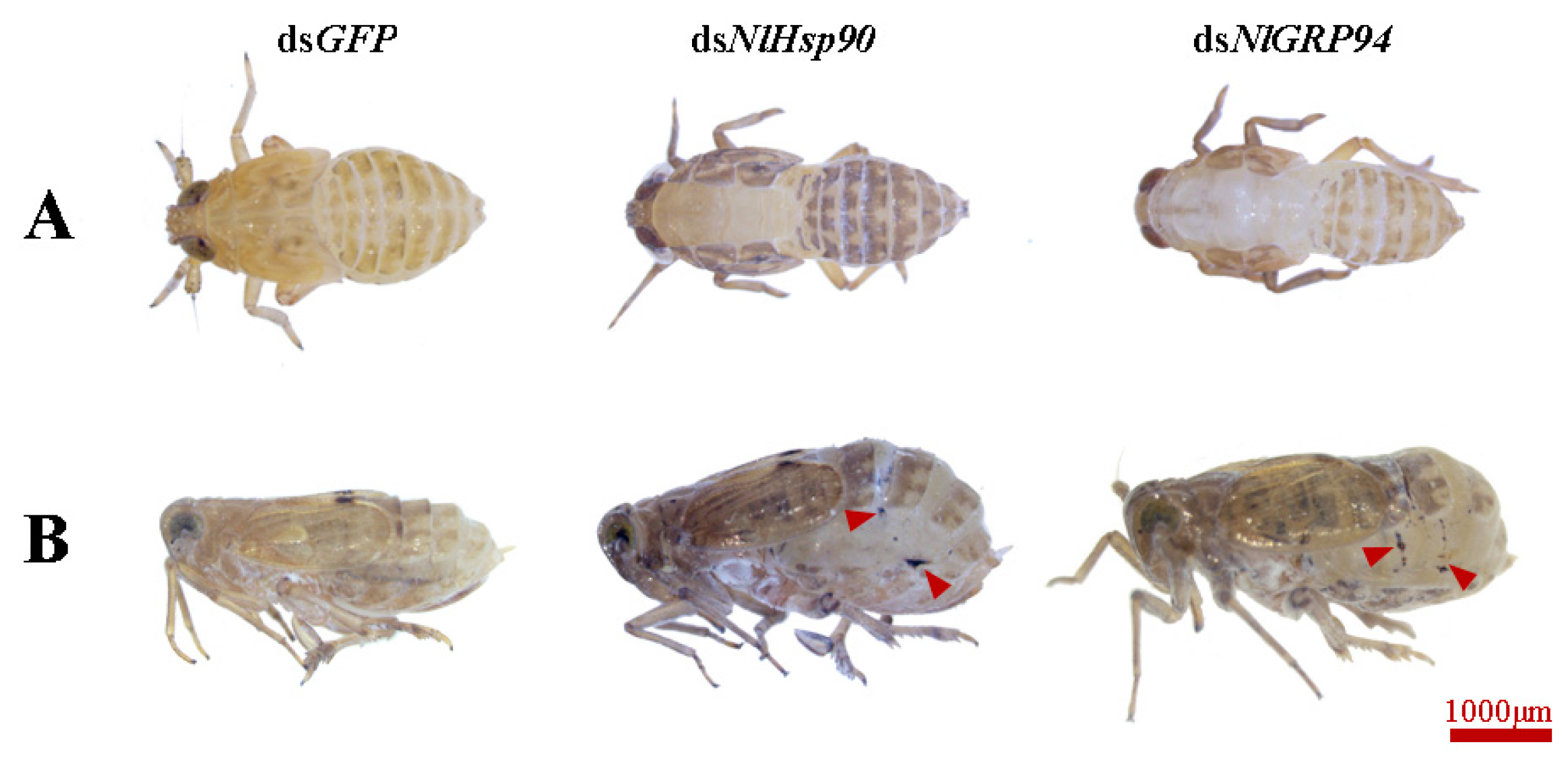
3.6. Electron Microscope Observations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borkovich, K.A.; Farrelly, F.W.; Finkelstein, D.B.; Taulien, J.; Lindquist, S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell Biol. 1989, 9, 3919–3930. [Google Scholar] [CrossRef] [PubMed]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 family: Structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef] [PubMed]
- McClellan, A.J.; Xia, Y.; Deutschbauer, A.M.; Davis, R.W.; Gerstein, M.; Frydman, J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell 2007, 131, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.M.; Davey, M.; Hsu, Y.C.; Kaplanek, P.; Tong, A.; Parsons, A.B.; Krogan, N.; Cagney, G.; Mai, D.; Greenblatt, J.; et al. Navigating the chaperone network: An integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell 2005, 120, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 607–613. [Google Scholar] [CrossRef]
- Jackson, S.E. Hsp90: Structure and function. In Molecular Chaperones; Jackson, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 328, pp. 155–240. [Google Scholar]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Rehn, A.; Pelz, B.; Hellenkamp, B.; Richter, K.; Rief, M.; Buchner, J.; Hugel, T. The charged linker of the molecular chaperone Hsp90 modulates domain contacts and biological function. Proc. Natl. Acad. Sci. USA 2014, 111, 17881–17886. [Google Scholar] [CrossRef]
- Johnson, J.L.; Brown, C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones 2009, 14, 83–94. [Google Scholar] [CrossRef]
- Haslbeck, V.; Kaiser, C.J.O.; Richter, K. Hsp90 in non-mammalian metazoan model systems. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 712–721. [Google Scholar] [CrossRef]
- Xu, J.; Shu, J.; Qiu, X.; Wang, Z.; Zhao, F.; Zhang, Z.; Zhang, Q. Effects of heat shock on ovary development and HSP83 expression in Tribolium castaneum (Coleoptera: Tenebrionidae). Arch. Insect Biochem. Physiol. 2009, 70, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shu, J.; Zhang, Q. Expression of the Tribolium castaneum (Coleoptera: Tenebrionidae) hsp83 gene and its relation to oogenesis during ovarian maturation. J. Genet. Genom. 2010, 37, 513–522. [Google Scholar] [CrossRef]
- Koch, G.; Smith, M.; Macer, D.; Webster, P.; Mortara, R. Endoplasmic-reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J. Cell Sci. 1986, 86, 217–232. [Google Scholar] [PubMed]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.C.; Pham, T.; Zheng, T.; Jockheck-Clark, A.; Rankin, H.B.; Newgard, C.B.; Spana, E.P.; Nicchitta, C.V. Gp93, the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev. Biol. 2010, 339, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol. Biol. Cell 2007, 18, 3764–3775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, S.; Li, C.; Sang, M.; Wu, W.; Yun, X.; Hu, X.; Li, B. Identification and characterization of novel ER-based hsp90 gene in the red flour beetle, Tribolium castaneum. Cell Stress Chaperones 2014, 19, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Chen, Y.M.; Dai, K.; Chen, P.L.; Riley, D.J.; Lee, W.H. A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol. Cell Biol. 1996, 16, 4691–4699. [Google Scholar] [CrossRef]
- Felts, S.J.; Owen, B.A.L.; Nguyen, P.; Trepel, J.; Donner, D.B.; Toft, D.O. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J. Biol. Chem. 2000, 275, 3305–3312. [Google Scholar] [CrossRef]
- Masgras, I.; Sanchez-Martin, C.; Colombo, G.; Rasola, A. The chaperone TRAP1 as a modulator of the mitochondrial adaptations in cancer cells. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, Q.; Fan, Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J. Biol. Chem. 2007, 282, 20553–20560. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Loh, S.H.Y.; Martins, L.M. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of parkinson’s disease. Cell Death Dis. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Chen, X.; Liu, W.; Zhou, Q. Identification of a heat shock protein 90 gene involved in resistance to temperature stress in two wing-morphs of Nilaparvata lugens (stal). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 197, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.-L.; Ye, Y.-X.; Lou, Y.-H.; Lu, J.-B.; Cheng, C.; Shen, Y.; Moussian, B.; Zhang, C.-X. A comprehensive omics analysis and functional survey of cuticular proteins in the brown planthopper. Proc. Natl. Acad. Sci. USA 2018, 115, 5175–5180. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Xue, J.; Lu, B.; Zhang, X.-C.; Zhuo, J.-C.; He, S.-F.; Ma, X.-F.; Jiang, Y.-Q.; Fan, H.-W.; Xu, J.-Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Bao, Y.-Y.; Lao, S.-H.; Huang, X.-H.; Ye, Y.-Z.; Wu, J.-X.; Xu, H.-J.; Zhou, X.-P.; Zhang, C.-X. Rice ragged stunt virus-induced apoptosis affects virus transmission from its insect vector, the brown planthopper to the rice plant. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Stechmann, A.; Cavalier-Smith, T. Evolutionary origins of hsp90 chaperones and a deep paralogy in their bacterial ancestors. J. Eukaryot. Microbiol. 2004, 51, 364–373. [Google Scholar] [CrossRef]
- Ding, D.; Parkhurst, S.M.; Halsell, S.R.; Lipshitz, H.D. Dynamic Hsp83 RNA localization during Drosophila oogenesis and embryogenesis. Mol. Cell Biol. 1993, 13, 3773–3781. [Google Scholar] [CrossRef]
- Pisa, V.; Cozzolino, M.; Gargiulo, S.; Ottone, C.; Piccioni, F.; Monti, M.; Gigliotti, S.; Talamo, F.; Graziani, F.; Pucci, P.; et al. The molecular chaperone Hsp90 is a component of the cap-binding complex and interacts with the translational repressor Cup during Drosophila oogenesis. Gene 2009, 432, 67–74. [Google Scholar] [CrossRef]
- Will, T.; Schmidtberg, H.; Skaljac, M.; Vilcinskas, A. Heat shock protein 83 plays pleiotropic roles in embryogenesis, longevity, and fecundity of the pea aphid Acyrthosiphon pisum. Dev. Genes Evol. 2017, 227, 1–9. [Google Scholar] [CrossRef]
- Yue, L.; Karr, T.L.; Nathan, D.F.; Swift, H.; Srinivasan, S.; Lindquist, S. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 1999, 151, 1064–1079. [Google Scholar]
- Schnorrer, F.; Schoenbauer, C.; Langer, C.C.H.; Dietzl, G.; Novatchkova, M.; Schernhuber, K.; Fellner, M.; Azaryan, A.; Radolf, M.; Stark, A.; et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 2010, 464, 287–291. [Google Scholar] [CrossRef]
- Knorr, E.; Vilcinskas, A. Post-embryonic functions of Hsp90 in Tribolium castaneum include the regulation of compound eye development. Dev. Genes Evol. 2011, 221, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Arbeitman, M.N.; Hogness, D.S. Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell 2000, 101, 67–77. [Google Scholar] [CrossRef]
- Subjeck, J.R.; Shyy, T.T. Stress protein systems of mammalian-cells. Am. J. Physiol. 1986, 250, C1–C17. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-X.; Zhao, F.-Y.; Zhang, Y.; Zhu, Y.-J.; Ma, M.-S.; Mao, H.-L.; Hu, C.-Y. Overexpression of Hsp90 from grass carp (Ctenopharyngodon idella) increases thermal protection against heat stress. Fish Shellfish. Immunol. 2012, 33, 42–47. [Google Scholar] [CrossRef]
- Melnick, J.; Dul, J.L.; Argon, Y. Sequential interaction of the chaperones bip and GRP94 with immunoglobulin-chains in the endoplasmic-reticulum. Nature 1994, 370, 373–375. [Google Scholar] [CrossRef]
- Luo, M.; Li, D.; Wang, Z.; Guo, W.; Kang, L.; Zhou, S. Juvenile hormone differentially regulates two Grp78 genes encoding protein chaperones required for insect fat body cell homeostasis and vitellogenesis. J. Biol. Chem. 2017, 292, 8823–8834. [Google Scholar] [CrossRef]
- Mao, C.; Wang, M.; Luo, B.; Wey, S.; Dong, D.; Wesselschmidt, R.; Rawlings, S.; Lee, A.S. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS ONE 2010, 5, e10852. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, Z.-D.; Dai, Y.-T.; Jiang, M.-X.; Zhang, C.-X. Identification and Characterization of Three Heat Shock Protein 90 (Hsp90) Homologs in the Brown Planthopper. Genes 2020, 11, 1074. https://doi.org/10.3390/genes11091074
Chen X, Li Z-D, Dai Y-T, Jiang M-X, Zhang C-X. Identification and Characterization of Three Heat Shock Protein 90 (Hsp90) Homologs in the Brown Planthopper. Genes. 2020; 11(9):1074. https://doi.org/10.3390/genes11091074
Chicago/Turabian StyleChen, Xuan, Ze-Dong Li, Yi-Ting Dai, Ming-Xing Jiang, and Chuan-Xi Zhang. 2020. "Identification and Characterization of Three Heat Shock Protein 90 (Hsp90) Homologs in the Brown Planthopper" Genes 11, no. 9: 1074. https://doi.org/10.3390/genes11091074
APA StyleChen, X., Li, Z.-D., Dai, Y.-T., Jiang, M.-X., & Zhang, C.-X. (2020). Identification and Characterization of Three Heat Shock Protein 90 (Hsp90) Homologs in the Brown Planthopper. Genes, 11(9), 1074. https://doi.org/10.3390/genes11091074





