An Ensemble Approach to Predict the Pathogenicity of Synonymous Variants
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Feature Extraction
2.3. Feature Selection and Ranking
2.4. Classification Model Selection and Evaluation
3. Results
3.1. Selection of Classification Algorithm
3.2. Feature Selection
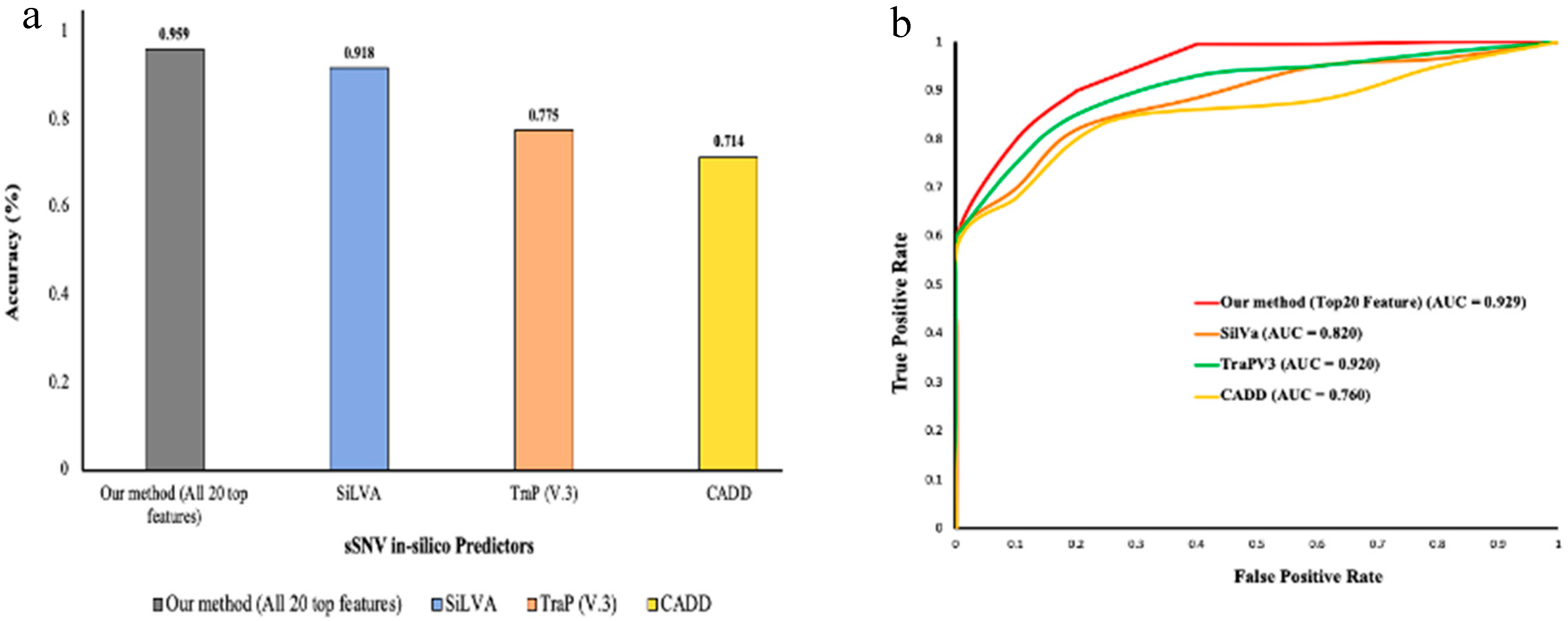
3.3. Benchmarking against other Methods Using Test Datasets
3.4. Reclassification of Uncategorized sSNVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thusberg, J.; Vihinen, M. Pathogenic or not? And if so, then how? Studying the effects of missense mutations using bioinformatics methods. Hum. Mutat. 2009, 30, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Kucukkal, T.G.; Petukh, M.; Li, L.; Alexov, E. Structural and physico-chemical effects of disease and non-disease nsSNPs on proteins. Curr. Opin. Struct. Biol. 2015, 32, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Petukh, M.; Kucukkal, T.G.; Alexov, E. On human disease-causing amino acid variants: Statistical study of sequence and structural patterns. Hum. Mutat. 2015, 36, 524–534. [Google Scholar] [CrossRef]
- Shen, H.; Li, J.; Zhang, J.; Xu, C.; Jiang, Y.; Wu, Z.; Zhao, F.; Liao, L.; Chen, J.; Lin, Y.; et al. Comprehensive Characterization of Human Genome Variation by High Coverage Whole-Genome Sequencing of Forty Four Caucasians. PLoS ONE 2013, 8, e59494. [Google Scholar] [CrossRef] [PubMed]
- Stefl, S.; Nishi, H.; Petukh, M.; Panchenko, A.R.; Alexov, E. Molecular mechanisms of disease-causing missense mutations. J. Mol. Biol. 2013, 425, 3919–3936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zheng, L.; Goncearenco, A.; Panchenko, A.R.; Li, M. Computational Approaches to Prioritize Cancer Driver Missense Mutations. Int. J. Mol. Sci. 2018, 19, 2113. [Google Scholar] [CrossRef]
- Zeng, Z.; Bromberg, Y. Predicting Functional Effects of Synonymous Variants: A Systematic Review and Perspectives. Front. Genet. 2019, 10, 914. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Kimchi-Sarfaty, C. Understanding the contribution of synonymous mutations to human disease. Nat. Rev. Genet. 2011, 12, 683–691. [Google Scholar] [CrossRef]
- Ng, P.C. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Choi, Y.; Sims, G.E.; Murphy, S.; Miller, J.; Chan, A.P. Predicting the Functional Effect of Amino Acid Substitutions and Indels. PLoS ONE 2012, 7, e46688. [Google Scholar] [CrossRef]
- Ioannidis, N.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2018, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Ionita-Laza, I.; McCallum, K.; Xu, B.; Buxbaum, J.D. A spectral approach integrating functional genomic annotations for coding and noncoding variants. Nat. Genet. 2016, 48, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ganakammal, S.R.; Koirala, M.; Wu, B.; Alexov, E. In-silico analysis to identify the role of MEN1 missense mutations in breast cancer. J. Theor. Comput. Chem. 2020, 19, 2041002. [Google Scholar] [CrossRef]
- Peng, Y.; Alexov, E. Investigating the linkage between disease-causing amino acid variants and their effect on protein stability and binding. Proteins Struct. Funct. Bioinform. 2016, 84, 232–239. [Google Scholar] [CrossRef]
- Peng, Y.; Alexov, E.; Basu, S. Structural Perspective on Revealing and Altering Molecular Functions of Genetic Variants Linked with Diseases. Int. J. Mol. Sci. 2019, 20, 548. [Google Scholar] [CrossRef]
- Buske, O.J.; Manickaraj, A.K.; Mital, S.; Ray, P.N.; Brudno, M. Identification of deleterious synonymous variants in human genomes. Bioinformatics 2013, 29, 1843–1850. [Google Scholar] [CrossRef]
- Livingstone, M.; Folkman, L.; Yang, Y.; Zhang, P.; Mort, M.; Cooper, D.N.; Liu, Y.; Stantic, B.; Zhou, Y. Investigating DNA-, RNA-, and protein-based features as a means to discriminate pathogenic synonymous variants. Hum. Mutat. 2017, 38, 1336–1347. [Google Scholar] [CrossRef]
- Gelfman, S.; Wang, Q.; McSweeney, K.M.; Ren, Z.; La Carpia, F.; Halvorsen, M.; Schoch, K.; Ratzon, F.; Heinzen, E.L.; Boland, M.J.; et al. Annotating pathogenic non-coding variants in genic regions. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Shi, F.; Yao, Y.; Bin, Y.; Zheng, C.-H.; Xia, J. Computational identification of deleterious synonymous variants in human genomes using a feature-based approach. BMC Med. Genom. 2019, 12, 81–88. [Google Scholar] [CrossRef]
- Landrum, M.; Chitipiralla, S.; Brown, G.R.; Chen, C.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; Kaur, K.; Liu, C.; et al. ClinVar: Improvements to accessing data. Nucleic Acids Res. 2020, 48, D835–D844. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; Zheng-Bradley, X.; Smith, R.; Kulesha, E.; Xiao, C.; Toneva, I.; Vaughan, B.; Preuss, D.; Leinonen, R.; The 1000 Genomes Project Consortium; et al. The 1000 Genomes Project: Data management and community access. Nat. Methods 2012, 9, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Shaw, K.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Qual. Life Res. 2013, 133, 1–9. [Google Scholar] [CrossRef]
- Wen, P.; Xiao, P.; Xia, J. dbDSM: A manually curated database for deleterious synonymous mutations. Bioinformatics 2016, 32, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G.C.P.; Vihinen, M. VariSNP, A Benchmark Database for Variations From dbSNP. Hum. Mutat. 2015, 36, 161–166. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; ACMG Laboratory Quality Assurance Committee; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Hoover, J. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef]
- Ganakammal, S.R.; Alexov, E. Evaluation of performance of leading algorithms for variant pathogenicity predictions and designing a combinatory predictor method: Application to Rett syndrome variants. PeerJ 2019, 7, e8106. [Google Scholar] [CrossRef]
- Davydov, E.V.; Goode, D.; Sirota, M.; Cooper, G.M.; Sidow, A.; Batzoglou, S. Identifying a High Fraction of the Human Genome to be under Selective Constraint Using GERP++. PLoS Comput. Biol. 2010, 6, e1001025. [Google Scholar] [CrossRef]
- Pollard, K.S.; Hubisz, M.J.; Rosenbloom, K.R.; Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2009, 20, 110–121. [Google Scholar] [CrossRef]
- Hubisz, M.J.; Pollard, K.S.; Siepel, A. PHAST and RPHAST: Phylogenetic analysis with space/time models. Briefings Bioinform. 2010, 12, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Haeussler, M.; Zweig, A.S.; Tyner, C.; Speir, M.L.; Rosenbloom, K.R.; Raney, B.J.; Lee, C.M.; Lee, B.T.; Hinrichs, A.S.; Gonzalez, J.N.; et al. The UCSC Genome Browser database: 2019 update. Nucleic Acids Res. 2019, 47, D853–D858. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Y.; Alipanahi, B.; Lee, L.J.; Bretschneider, H.; Merico, D.; Yuen, R.K.C.; Hua, Y.; Gueroussov, S.; Najafabadi, H.S.; Hughes, T.R.; et al. The human splicing code reveals new insights into the genetic determinants of disease. Science 2014, 347, 1254806. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]

| Feature Class | Feature | Description |
|---|---|---|
| In silico predictors | CADD | It uses a c-score obtained by the integration of multiple variant annotation resources. |
| EIGEN | It uses a supervised approach to derive the aggregate functional score from various annotation resources. | |
| TraP (V3) | It evaluates the ability of a variant to cause disease by damaging the final transcript. | |
| Conservation Score | GERP++ | GERP++ score is used to measure the conservation at the mutation position |
| Phylop (100 ways) | It computes P-values for conservation-based specific lineage | |
| PHAST Cons | Scores based on conserved element | |
| Codon Usage | dRSCU | Change in RSCU caused by mutation |
| RSCU | RSCU (Relative synonymous codon usage) of new codon | |
| Splicing Properties | MES | Max splice site score |
| MES-KM | Has a value of 1 if site changes most or 0 if not | |
| dMES | Max change in splice site score | |
| MES- | Max splice site score decrease | |
| MES+ | Max splice site score increase | |
| dpsi | The delta PSI is the predicted change in percent-inclusion due to the variant | |
| dpsiz | The z-score of the dPSI relative | |
| FAS6+ | Hexamer splice suppressor motifs gained | |
| FAS6- | Hexamer splice suppressor motifs lost | |
| MEC-MC | Has a value of 1 if strongest site change or 0 if not | |
| MEC-CS | Has a value of 1 if a cryptic site now strongest or 0 if not | |
| PESS- | Octamer splice suppressor motifs lost | |
| PESS+ | Octamer splice suppressor motifs gained | |
| PESE- | Octamer splice enhancer motifs lost | |
| PESE+ | Octamer splice suppressor motifs gained | |
| SR- | SR-protein motifs lost | |
| SR+ | SR-protein motifs gained | |
| Sequence Properties | CpG_exon | Observed/expected CpG content of exon |
| CpG | Has a value of 1 if mutation change a CpG or 0 if not | |
| f_premrna | Relative distance to end of pre-mRNA | |
| f_mrna | Relative distance to end of mature mRNA |
| Statistics | Formula |
|---|---|
| Precision | |
| Recall | |
| F-measure | |
| MCC | |
| Accuracy | |
| Receiver operating characteristic (ROC) curve | Plotted between TP rate to FP rate |
| Area under the ROC Curve (AUC) | Area Under the ROC curve, it measures the capability of a model to distinguish between classes. |
| Classification Algorithm | Precision | Recall | F-Measure | MCC | Accuracy | AUC | |
|---|---|---|---|---|---|---|---|
| Training Set 1 | Random forest | 0.886 | 0.802 | 0.842 | 0.703 | 0.849 | 0.929 |
| Naive Bayes | 0.862 | 0.744 | 0.799 | 0.631 | 0.812 | 0.888 | |
| Training Set 2 | Random forest | 0.928 | 0.852 | 0.888 | 0.789 | 0.893 | 0.959 |
| Naive Bayes | 0.873 | 0.761 | 0.813 | 0.656 | 0.825 | 0.898 | |
| Training Set 3 | Random forest | 0.948 | 0.831 | 0.886 | 0.792 | 0.893 | 0.941 |
| Naive Bayes | 0.872 | 0.757 | 0.811 | 0.652 | 0.823 | 0.894 | |
| Training Set 4 | Random forest | 0.928 | 0.844 | 0.884 | 0.781 | 0.889 | 0.953 |
| Naive Bayes | 0.868 | 0.786 | 0.825 | 0.67 | 0.833 | 0.912 | |
| Training Set 5 | Random forest | 0.923 | 0.844 | 0.882 | 0.777 | 0.886 | 0.948 |
| Naive Bayes | 0.877 | 0.761 | 0.815 | 0.66 | 0.827 | 0.905 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranganathan Ganakammal, S.; Alexov, E. An Ensemble Approach to Predict the Pathogenicity of Synonymous Variants. Genes 2020, 11, 1102. https://doi.org/10.3390/genes11091102
Ranganathan Ganakammal S, Alexov E. An Ensemble Approach to Predict the Pathogenicity of Synonymous Variants. Genes. 2020; 11(9):1102. https://doi.org/10.3390/genes11091102
Chicago/Turabian StyleRanganathan Ganakammal, Satishkumar, and Emil Alexov. 2020. "An Ensemble Approach to Predict the Pathogenicity of Synonymous Variants" Genes 11, no. 9: 1102. https://doi.org/10.3390/genes11091102
APA StyleRanganathan Ganakammal, S., & Alexov, E. (2020). An Ensemble Approach to Predict the Pathogenicity of Synonymous Variants. Genes, 11(9), 1102. https://doi.org/10.3390/genes11091102






