Abstract
Syndromic hereditary hearing impairment (HHI) is a clinically and etiologically diverse condition that has a profound influence on affected individuals and their families. As cutaneous findings are more apparent than hearing-related symptoms to clinicians and, more importantly, to caregivers of affected infants and young individuals, establishing a correlation map of skin manifestations and their underlying genetic causes is key to early identification and diagnosis of syndromic HHI. In this article, we performed a comprehensive PubMed database search on syndromic HHI with cutaneous abnormalities, and reviewed a total of 260 relevant publications. Our in-depth analyses revealed that the cutaneous manifestations associated with HHI could be classified into three categories: pigment, hyperkeratosis/nail, and connective tissue disorders, with each category involving distinct molecular pathogenesis mechanisms. This outline could help clinicians and researchers build a clear atlas regarding the phenotypic features and pathogenetic mechanisms of syndromic HHI with cutaneous abnormalities, and facilitate clinical and molecular diagnoses of these conditions.
1. Introduction
Sensorineural hearing impairment (SNHI) is the most common form of inherited sensory defect, which occurs in approximately 1.9/1000 live births [1]. More than 50% of SNHI cases in children can be attributed to genetic causes, and are classified as hereditary hearing impairment (HHI) [2]. Over the past two decades, the genetic causes of HHI have been decoded rapidly, especially with the advent of next-generation sequencing (http://hereditaryhearingloss.org) [3]. Among the deafness genes known, some are associated with syndromic HHI, with symptoms in organ systems outside the auditory pathway. Patients suffering from various forms of syndromic HHI additionally present with skin abnormalities. The goals of this review were to perform a literature survey on comprehensive animal and human studies and to outline the molecular mechanisms underlying HHI with cutaneous abnormalities.
2. Materials and Methods
Our search strategy was based on using Online Mendelian Inheritance in Man (OMIM) and PubMed databases for retrieval of suitable articles relevant to our topic of interest. A collection of these publications was stored and managed on EndNote X9 (Thomson Reuters, New York City, NY, USA). Publications were eligible only if they were relevant to HHI associated with cutaneous abnormalities. Affected patients included in case reports or series were considered to be of interest only if relevant phenotypes, including abnormal cutaneous, hair, or nail findings, as well as SNHI were observed. Publications focusing on individuals with HHI and developmental disorders (e.g., distinctive facial characteristics, congenital heart defect, developmental delay, kyphosis, among others), who did not present with abnormal skin, hair, or nail findings, were not included for discussion in the present review. Studies in which the subjects discussed presented with abnormal cutaneous findings due to other proven diseases (e.g., acanthosis nigricans due to diabetes mellitus) were also excluded. A flowchart of the search strategy is shown in Figure 1.
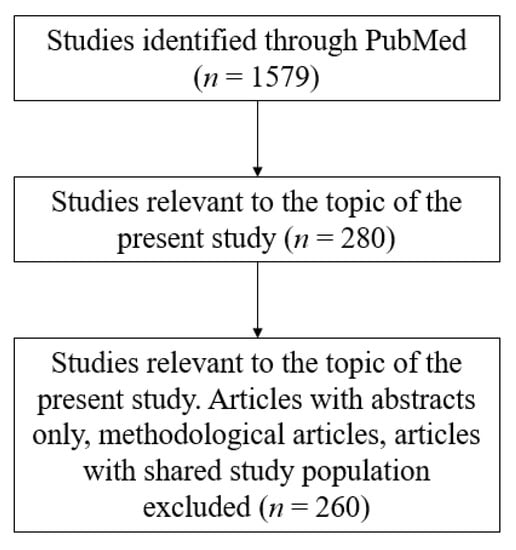
Figure 1.
Article selection.
3. Various Types of HHI Present with Cutaneous Abnormalities
3.1. Search Results
Forty-eight entries in the OMIM database with distinct “MIM (Mendelian Inheritance in Man) numbers” were selected, and a total of 260 publications were retrieved from the PubMed database, including original articles (n = 154), case reports (n = 74), and literature reviews (n = 32), to perform the analysis. The quality of the articles included was meticulously evaluated based on the degree of relevance to the topic of this review. A detailed list sorted by phenotypes (Table 1) is included in the following paragraph. The pathogenesis of these syndromes is covered separately by the fourth section of this article.

Table 1.
Summary of syndromic hereditary hearing impairment (HHI) with cutaneous abnormalities.
3.2. HHI with Pigment Disorders
3.2.1. Waardenburg Syndrome (WS)
With an estimated prevalence of 1/42,000, WS is a rare, heterogeneous condition, the features of which include white forelock, depigmented patches of the skin, and SNHI [4,5,97]. These features are characteristic of type 2 WS, while additional clinical symptoms define other types of WS [98]. Patients with type 1 WS present with dystopia canthorum; patients with type 3 WS, a more severe form than type 1 WS, present with dystopia canthorum and musculoskeletal abnormalities of the arms and hands [99,100]. In contrast, patients with type 4 WS present with Hirschsprung disease [101].
WS types 2 and 4 can be further classified into subtypes according to the genetic origins. A summary of the subtypes of WS and the genes affected are shown in Table 2. Among the different subtypes of WS, types 2B and 2C are linked to pathogenic variants in unidentified genes mapping to 1p21–p13.3 and 8p23, respectively [98,101,102,103,104,105,106].

Table 2.
Subtypes of Waardenburg syndrome (WS) and affected genes.
WS types 2A and 2 with ocular albinism (WS2-OA) both result from pathogenic variants in the microphthalmia-associated transcription factor gene (MITF), and present with SNHI and pigment disorders. WS2-OA also results from pathogenic variants in the TYR gene, the main function of the protein product of which is converting tyrosine into melanin [107,108]. Upstream to MITF, pathogenic variants in KITLG have been found to cause WS type 2 [8,9].
Other pathogenic variants resulting in HHI with pigment disorders include those in PAX3, SOX10, EDNRB, EDN3, and SNAI2 genes. Pathogenic variants in PAX3 lead to WS types 1 and 3, and those in SOX10 to WS types 2E and 4C [109]. Patients with a defective EDNRB signaling pathway develop either WS types 4Aand 4B, or ABCD syndrome (albinism, black lock of hair, cell migration disorder of gut neurocytes, and sensorineural deafness) [110,111]. Manifestations of these syndromes include Hirschsprung disease, depigmented patches of the skin, white eyelashes, pale blue iridis, and white forelock [103,112]. Homozygous deletions of SNAI2 have been detected in patients with WS type 2D [106].
3.2.2. Tietz Albinism-Deafness Syndrome (TADS)
TADS is a rare autosomal-dominant disease featuring SNHI, generalized pigment loss, and lack of retinal pigmentation [113]. Premature graying of hair during adolescence was observed in a patient [10,107]. Pathogenic variants in MITF, including 3-bp del (p.Arg217del), and missense variant c.630C>G (p.Asn210Lys) identified respectively in two families, result in TADS [107,114,115]. Hypopigmentation stems from disrupted transfer of melanosomes from melanocytes to keratinocytes [10]. Although TADS results from alterations in a gene linked to WS type 2, patients do not present with heterochromia or pigmented patches [4,10,114].
3.2.3. COMMAD Syndrome
COMMAD syndrome encompasses coloboma, osteopetrosis, microphthalmia, macrocephaly, albinism, and deafness. Compound heterozygous MITF mutations have been detected in two unrelated families with COMMAD syndrome [11]. In contrast to WS type 2A and TADS, which are associated with autosomal-dominant MITF mutations, COMMAD syndrome seems to be associated with an autosomal recessive inheritance of MITF, suggesting a crucial role for MITF in ocular morphogenesis and bone homeostasis [11].
3.2.4. Histiocytosis-Lymphadenopathy Plus Syndrome
The “histiocytosis-lymphadenopathy plus syndrome” family is a generic term for the H syndrome, Faisalabad histiocytosis (FHC), sinus histiocytosis with massive lymphadenopathy (SHML), and pigmented hypertrichosis with insulin-dependent diabetes mellitus syndrome (PHID) [12]. In the literature, clinical reports and molecular studies are sparse, since it was only recently discovered. The patients had severe SNHI and extensive hyperpigmentation with dark, long hairs. Histologically, polyclonal perivascular lymphohistiocytic infiltrations of the dermis and subcutis were found in hypertrichotic lesions [12,116]. This group of diseases is caused by pathogenic variants in SLC29A3, which encodes ENT3, equilibrative nucleoside transporter 3 [12,116,117,118]. This enzyme is in intracellular membranes and mediates cross-membrane nucleoside transportation [119]. Defective ENT3 impairs mitochondrial and lysosomal functions, as well as macrophage homeostasis [12].
3.2.5. Noonan Syndrome with Multiple Lentigines (NSML)
NSML is a rare autosomal-dominant disease without a credible record of global or regional prevalence to date [15]. As its former name “LEOPARD syndrome” indicates, the syndrome features a myriad of clinical manifestations, including multiple lentigines, conduction abnormalities on electrocardiogram, ocular hypertelorism, pulmonic stenosis, abnormal genitalia, retardation of growth, and SNHI [13,15,16]. Pathogenic variants in PTPN11, RAF1, and BRAF genes, encoding parts of the RAS-MAPK (Mitogen-activated protein kinase) signaling cascade, result in NSML types 1, 2, and 3, respectively [14,120,121]. The larger entity, Noonan syndrome (NS), is an autosomal-dominant disease featuring short stature, facial dysmorphia, congenital heart disease, pulmonary valve stenosis, and SNHI, but without multiple lentigines [122]. NS is caused by RAS-MAPK pathway-debilitating variants in PTPN11, RAF1, BRAF, SOS1, KRAS, among others [122,123].
3.2.6. Vitiligo-Associated Multiple Autoimmune Disease Susceptibility 1 (VAMAS1)
With an unknown prevalence and unclarified mode of inheritance, VAMAS1 features patchy depigmentation of the hair and skin due to the loss of melanocytes, SNHI in certain cases, and a propensity of developing autoimmune thyroid disease, rheumatoid arthritis, and systemic lupus erythematosus [17,124]. The pathogenic variant p.L155H of NLRP1 has been identified to cause VAMAS1 [125]. NLRP1 encodes the sensor component of the NLRP1 inflammasome. In response to pathogens, drugs, or damage-associated signals, this protein is recruited, possibly along with PYCARD (PYD And CARD Domain Containing) protein, to assemble the NLRP1 inflammasome and facilitates innate immunity and inflammation [17,126]. Autoimmune response has also been identified in Vogt–Koyanagi–Harada disease (VKHD), another rare multisystem inflammatory disease characterized by pan-uveitis, SNHI, vitiligo, and neurological deficits. However, current studies suggest a melanocyte-specific Th1 cytokine response in VKHD [127,128].
3.2.7. Genophotodermatoses
Xeroderma pigmentosum (XP) and Cockayne syndrome (CS) are autosomal recessive genophotodermatoses resulting from variants in genes involved in DNA repair [22,129,130]. The prevalence of XP is 1/1,000,000 in Europe and the United States (US), and higher in Japan, the Middle East, and North Africa, whereas CS holds a prevalence of 2–3/1,000,000 in the US and Europe [131,132].
Photosensitivity, SNHI, and neurologic dysfunction are shared cardinal features of XP and CS [21,22,129,133,134]. Lentiginous macules and poikiloderma are more severe in XP, while loss of subcutaneous orbital fat is distinctive of CS [24,135]. Manifestations of these genophotodermatoses can be attributed to accumulated unrepaired DNA damage following defects in key components of the DNA nucleotide excision repair (NER) pathway. Pathogenic variants in ERCC6 and ERCC8 lead to CS types B and A, respectively. Pathogenic variants in XPA, XPC, RAD2, DDB1, ERCC2, ERCC3, ERCC4, ERCC5, and ERCC6 lead to XP groups A-G. Pathogenic variants in POLH result in a variant type of XP, which is called XPV [19,20,130,131,134,136].
3.3. HHI with Hyperkeratosis
Gap junction-related hyperkeratosis syndromes include palmoplantar keratoderma (PPK) with deafness, Vohwinkel syndrome, Bart-Pumphrey syndrome, hystrix-like ichthyosis with deafness (HID), and keratitis-ichthyosis-deafness syndrome (KID). This is a subgroup of the more generic condition PPKs, but epidemiological studies are lacking due to its rarity [34,137,138,139].
SNHI is a shared manifestation among PPK with deafness, Bart-Pumphrey syndrome, HID, KID, and the classic form of Vohwinkel syndrome. By contrast, patients with the variant form of Vohwinkel syndrome do not suffer from SNHI. As for cutaneous manifestation, generalized spiky hyperkeratotic skin is characteristic of HID and KID, while hyperkeratosis is mostly limited to the fingers, palms, and soles in PPK with deafness, Vohwinkel syndrome, and Bart-Pumphrey syndrome [139]. Leukonychia and thickening of the nails have also been reported in cases with Bart-Pumphrey syndrome [31,32,140,141,142].
The five conditions listed in this subgroup share a common genetic cause, i.e., pathogenic variants in GJB2 [31,143,144,145,146,147,148]. In addition, pathogenic variants in the GJB6 gene that encodes connexin 30 (Cx30) have also been identified in a family clinically diagnosed with KID [149].
GJB2 and GJB6 variants cause both syndromic and non-syndromic HHI. The causal relationship of non-syndromic HHI and pathogenic variants in GJB2 and GJB6 have been well-established. Pathogenic variants in GJB2 serve as the most common cause of autosomal recessive HHI and 20% of non-syndromic hearing loss overall [150,151]. GJB6 variants are less prevalent than GJB2 variants but have been identified in 8% of patients with known GJB2 variants [152]. Whether variants in specific domains of GJB2 or GJB6 genes cause syndromic or non-syndromic HHI remains to be elucidated.
3.4. Nail Disorders
3.4.1. Autosomal-Dominant Deafness-Onychodystrophy (DDOD) Syndrome
With a prevalence of less than 1/1,000,000, DDOD features severe SNHI, hypoplastic or dystrophic nails, and occasionally, hypoplastic teeth [37,38]. DDOD is associated with pathogenic variants in the ATP6V1B2 gene [39,40].
3.4.2. Deafness, Onychodystrophy, Osteodystrophy, Mental Retardation, and Seizures (DOORS) Syndrome
With an estimated prevalence of less than 1/1,000,000, the autosomal recessively inherited DOORS differs from DDOD regarding neurological symptoms, including mental retardation and seizures [41,153,154,155]. Pathogenic variants in TBC1D24 are the causative genetic alterations associated with DOORS [42,43,156,157,158,159].
3.4.3. Heimler Syndrome and Other Peroxisomal Biogenesis Disorders (PBDs)
PBDs are a spectrum of autosomal recessive disorders of different severity, of which Zellweger syndrome (ZS) is the most severe form; neonatal adrenoleukodystrophy (NALD) presents with milder symptoms, and infantile Refsum disease (IRD) and Heimler syndrome constitute the mildest forms. The prevalence of PBDs is 1/50,000 and 1/500,000 in North America and Japan, respectively, while epidemiological figures on Heimler syndrome are to be determined [51,160,161]. PBDs result from pathogenic variants in peroxin-encoding genes, i.e., PEX1, PEX2, PEX3, PEX5, PEX6, PEX10, PEX11β, PEX12, PEX13, PEX14, PEX16, PEX19, and PEX26 [50]. Heimler syndromes 1 and 2 are at the mildest end of the PBD spectrum, and are caused by pathogenic variants in PEX1 and PEX6, respectively [47,51,54]. Errors in the production of peroxins result in impaired myelin sheath formation and neurological deficits, including neonatal seizures, hypotonia, and developmental delays. Decreased peroxisome functionality in the liver and kidneys gives rise to the associated symptoms, including hepatomegaly, intrahepatic biliary dysgenesis, and hydronephrosis. SNHI and distinctive craniofacial features are also cardinal features of PBDs. Nail abnormalities, including Beau lines and leukonychia, have been reported in patients with Heimler syndrome [46,51,53,54,55].
3.4.4. Nail-Patella Syndrome (NPS)
NPS is an autosomal dominantly inherited syndrome with a prevalence of 1/50,000 live births. Nail dysplasia is the cardinal dermatologic manifestation of NPS. Nail changes include partially exposed and/or narrow nail beds, median or partial median clefts, dystrophic nail surfaces, and absence of nails. Fifth finger clinodactyly, hyperextensibility of the proximal interphalangeal joint, loss of creases over the distal interphalangeal joint and triangular lunulae have been reported in NPS patients. Other key features include malformation of dorsal mesenchyme-derivatives, including muscles, tendons, and the patella, along with ocular or renal involvement [162,163,164,165]. Hearing loss has also been reported in patients with NPS [59]. Genetically, pathogenic variants in LMX1B are considered to be causative of NPS [57,59,166,167].
3.4.5. Nephropathy with Pretibial Epidermolysis Bullosa and Deafness (NPEBD)
The only three cases with NPEBD feature nail dystrophy, blisters in the lower extremities, SNHI, and proteinuria in the nephrotic range [65]. Single-nucleotide insertion (383_384insG) in CD151, a gene encoding a component of hemidesmosomes, has been found in all cases. This result implies a role for CD151 in the maintenance of the normal structure and function of the skin, inner ear, and the glomeruli and tubules in the kidney [62,63,64].
3.5. HHI with Connective Tissue Disorders
3.5.1. Hyperelasticity of the Skin, Excess Skin, or Hypermobility of the Joints
Brittle cornea syndrome (BCS), Ehlers-Danlos syndrome musculocontractural type 1 (EDSMC1), congenital symmetric circumferential skin creases (CSCSC) types 1 and 2, and microphthalmia with linear skin defects syndrome (MLS) are connective tissue disorders that present with distinct cutaneous findings and hearing impairment. Epidemiological data are scant due to the rarity of these conditions.
BCS1 and BCS2 are characterized by hyperelasticity of the skin, hypermobility of the joints, blue sclerae, keratoconus, and keratoglobus. Mixed conductive and sensorineural hearing impairments have been reported in cases of BCS, with frequent manifestations that are milder and of later onset than the ophthalmic symptoms. BCS1 and BCS2 result from pathogenic variants in ZNF469 and PRDM5, respectively [66,69,168,169,170,171].
EDSMC1 is characterized by dysmorphisms throughout the musculoskeletal system, easy bruisability, joint hypermobility, and hearing impairment, in certain cases. EDSMC1 can be attributed to pathogenic variants in CHST14 [67,72,74,172,173].
Patients with CSCSC1 and CSCSC2 feature excess skin and ringed creases, as well as hearing impairment [77,174]. CSCSC is considered a tubulinopathy. Accordingly, pathogenic variants in TUBB and MAPRE2 are the causative genetic alterations associated with CSCSC1 and CSCSC2, respectively [77,79,174].
Microphthalmia with linear skin defects syndrome (MLS), or linear skin defects with multiple congenital anomalies 1 (LSDMCA1), also features linear skin defects and hearing impairment, and is caused by pathogenic variants in the holocytochrome c-type synthase-encoding HCCS gene [175,176,177,178,179].
3.5.2. Cryopyrin-Related Autoinflammatory Syndromes (CAPS)
A spectrum of autosomal-dominant autoinflammatory syndromes of different severities, including Muckle-Wells syndrome (MWS), familial cold autoinflammatory syndromes 1 and 2 (FCAS1, FCAS2), and chronic infantile neurologic cutaneous and articular (CINCA) syndrome, are related to cryopyrin. The prevalence of CAPS in France and the USA is estimated to be 1/360,000 and 1–2/1,000,000 individuals, respectively [180,181]. Patients present with urticaria, rash, or limb swelling, aggravated by cold temperature [182,183]. SNHI may result from inflammatory processes in the cochlea [180,184,185,186]. FCAS2 arises from pathogenic variants in NLRP12, while MWS, FCAS1, and CINCA syndrome can be attributed to gain-of-function pathogenic variants in the NLRP3 gene [90,185].
3.6. Others
Cornelia de Lange syndrome, with an overall prevalence of 1.6–2.2/100,000, is a mostly sporadic condition characterized by multiple organ-system defects [187]. Patients present with dysmorphic face and upper extremities, and growth and mental retardation. Hearing impairment, either sensorineural or conductive, is nearly ubiquitous [96,188,189,190]. The skin is mostly spared, but cavernous hemangiomas have been observed in a case with Cornelia de Lange syndrome 1 [190,191,192,193,194]. NIPBL, SMC1A, SMC3, RAD21, and HDAC8 are the five genes associated with Cornelia de Lange syndrome [191,192,193,194,195].
3.7. Frequency of SNHI in HHI with Cutaneous Abnormalities
The frequency of SNHI differs among various syndromic HHI with cutaneous abnormalities. For instance, SNHI has been found in over 70% of cases with WS, TADS, COMMAD, or NSML syndromes [11,15,97,114]; and in approximately half of patients with other syndromes such as histiocytosis-lymphadenopathy plus syndrome [196]. On the contrary, the frequency of SNHI is difficult to estimate in rarer conditions such as NPS, DOORS, or DDOD.
4. Molecular Mechanisms Underlying Various Types of HHI with Cutaneous Abnormalities
The pathogenesis behind some of the syndromes discussed in the present study has been documented in the literature. Generally, cutaneous manifestations associated with HHI can be classified into three categories: pigment, hyperkeratosis/nail, and connective tissue disorders (Table 3). We herein summarize the molecular mechanisms underlying syndromic HHI with different cutaneous involvements.

Table 3.
Molecular mechanisms underlying syndromic hereditary hearing impairment (HHI) with cutaneous abnormalities and expression of the affected genes in the inner ear and epidermis.
4.1. HHI with Pigment Disorders
Syndromic HHI with pigmentary disorders was found associated with diverse molecular mechanisms, including differentiation and migration of melanocytes, RAS-MAPK signaling, and DNA repair.
4.1.1. Differentiation and Migration of Melanocytes
As mentioned above, certain subtypes of WS type 2, TADS, and COMMAD syndrome can be attributed to pathogenic variants in MITF, while other types of WS are linked to pathogenic variants in PAX3, SNAI2, SOX10, EDNRB, EDN3, and KITLG. These genes are crucial for the differentiation and migration of melanocytes.
The MITF gene on chromosome 3p14.1–p12.3 encodes the protein MITF, which is a basic helix-loop-helix (hHLH)-leucine zipper and plays a role in the development of various cell types, including neural crest-derived melanocytes, optic cup-derived retinal pigment epithelial cells, and melanocytes [199]. In melanocyte differentiation, MITF transactivates the promoter activity of the tyrosinase gene TYR [200,201,202,203]. Thus, pathogenic variants in the MITF gene might lead to absence of melanocytes in the skin, hair, eyes, and stria vascularis of the cochlea. PAX3 and SOX10 encode transcription factors that synergistically regulate the expression of MITF, and pathogenic variants in these two genes also result in pigmentary abnormalities of the hair, skin, and eyes, as well as in SNHI. Specifically, SOX10 activates the MITF pathway by binding onto the MITF promoter. Loss-of-function variants including a 1076delGA in exon 5, a 6-bp insertion in exon 4, along with a tyr83-to-ter variant and a glu189-to-ter variant were found to cause WS type 4C [101]. On the other hand, a ser135-to-thr variant was identified in a patient with WS type 2E [109]. The activation of KITLG-KIT signaling pathway leads to the activation of downstream MITF, and defective KITLG has been linked to WS type 2 [8,9].
Pathogenic variants in EDNRB, the gene encoding the endothelin-B receptor, and those in the gene for its ligand endothelin-3 (EDN3) also result in a lack of melanocytes. EDNRB and EDN3 take part in the migration and proliferation of neural crest-derived cells including melanocytes [204]. SNAI2 encodes a zinc finger protein essential to the development of neural crest-derived cells [205]. A pathogenic variant in Slugh, the murine homolog of the human SNAI2 gene, causes pigmentary disorders in mice including white forelock and patchy depigmentation over the ventral body, tail, and feet. Hyperactivity and circling behavior observed in Slugh-deficient mice implied the presence of auditory and vestibular dysfunctions. These findings implicate a role for SNAI2 in the development and/or migration of neural crest-derived cells [98,106].
4.1.2. RAS-MAPK Signaling
NSML types 1, 2, and 3 result from pathogenic variants in PTPN11, RAF1, and BRAF genes, respectively, products of which all participate in the RAS-MAPK signaling cascade. The tyrosine phosphatase encoded by PTPN11 relays signals from cell membrane receptors to cytoplasmic tyrosine kinases and up-regulates the MAPK signaling pathway [206]. The serine/threonine-protein kinase encoded by RAF1 links Ras GTPases to the MAPK/ERK (extracellular signal-regulated kinases) cascade and serves as a decision point leading cells to proliferate, differentiate, or undergo apoptosis. The serine/threonine-protein kinase B-raf, encoded by BRAF, facilitates cell membrane-nucleus signaling through phosphorylation of MAP2K1 [207,208]. It may further contribute to postsynaptic responses of hippocampal neurons [209].
Histological specimens of lentiginous lesions of NSML cases with pathogenic PTPN11 variants revealed increased numbers of melanocytes and pigments throughout the epidermis, while immunohistochemical studies revealed increased expression levels of endothelin-1 (ET-1), phosphorylated Akt, mTOR, and STAT3 in lentiginous epidermis compared with non-lentiginous skin areas. Higher melanin synthesis rates of human melanoma cells expressing tyrosine-protein phosphatase non-receptor type 11 have been observed in vitro, supporting the link between PTPN11 and hyperpigmentation in NSML patients [210]. Vestibulocochlear anomalies and atrophic cochlear neurons have been observed in patients with pathogenic PTPN11 variants [211].
4.1.3. DNA Repair
XP and CS are caused by defective DNA repair pathways. Defects in XPC and XPE, factors in charge of global genome nucleotide excision repair (GG-NER), in XPA, XPG, XPB, and XPD, which oversee DNA unwinding, as well as in XPF and XPG, mediating excision of the damaged nucleotides, lead to hyper- and hypopigmented macules in sun-exposed areas and an increased risk of skin malignancies [212]. Defects in POLH lead to XPV, a rare subtype of XP.
Increased numbers of melanocytes and elevated melanin levels have been found in skin specimens of freckles from XPC patients. Hyperpigmentation in XP results from increased proliferation and early differentiation of melanocytes due to the mutagenic tendency of cells with impaired GG-NER [21]. UV(ultraviolet)-induced oxidative stress could also induce hyperpigmentation. Melanogenesis is regulated through the ERK signaling pathway activated by mitochondrial reactive oxidative species [213]. The production of UV-induced protective pigments is up-regulated by the mitochondrial protein prohibitin [214,215]. Defective repair mechanisms and UV-induced changes in microenvironment spark apoptotic pathways in XP melanocytes, resulting in hypopigmented areas. Apoptosis of cells in XP patients is triggered by lower doses of UV than needed to induce apoptosis in normal cells [216,217,218,219]. Compared to XP, the phenotype of CS includes progeroid appearance, generally without pigmentary changes [220]. XP and CS are associated with SNHI of cochlear origin on audiological assessments. Temporal bone histology at autopsy revealed atrophy of the sensory epithelium and neurons in the cochlea. Atrophies of the stria vascularis, hair cells, or Scarpa’s ganglion have been observed in different cases of XP [133,221].
4.2. HHI with Hyperkeratosis Or Nail Disorders
4.2.1. HHI with Hyperkeratosis
Syndromic HHI with hyperkeratosis are caused by pathogenic variants in two gap junction genes, GJB2 and GJB6, which encode connexins that are key to intercellular signaling [222]. The ectoderm-derived epithelia of the inner ear and the epidermis share the expression of Cx26 and Cx30 [223,224]. In the skin, Cx26 is mainly expressed in the palmoplantar epidermis and the inner and outer root sheaths of the human hair follicle, while Cx30 is predominantly expressed in the differentiated layers of the interfollicular epidermis [225,226,227]. Defective connexins result in leaky hemichannels and impaired intercellular communication [139,228]. Cx26 plays a role in wound healing and is also involved in the normal differentiation and proliferation of keratinocytes, which may explain the hyperkeratosis observed in individuals with defective Cx26 [228,229].
In the inner ear, connexins are abundantly expressed in the cochlear sensory epithelium, and are key factors in maintaining the potassium levels of the endolymph [20]. Immunochemical stainings have revealed that Cx26 and Cx30 are expressed in the spiral limbus, spiral ligament, stria vascularis, and supporting cells of the organ of Corti. Cx26 contributes to normal development of the cochlear sensory epithelium, and compromised inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) permeability of Cx26 has been implicated as a cause of SNHI [230,231]. Additionally, the endocochlear potential generated by the stria vascularis is remarkably disturbed in Cx30-deficient mice [232].
GJB4 encodes Cx30.3, pathogenic variants in which have been linked to erythrokeratodermia variabilis et progressiva, or EKVP [233]. EKVP is a rare, mostly autosomal-dominant genodermatosis featuring erythema gyratum repens and stable hyperkeratotic plaques [234]. How GJB4 variants induce EKVP remains hypothetical. The link between GJB4 and SNHI has not yet been well-established either; however, GJB4 variants have been identified in 11 patients with non-syndromic hearing loss in Taiwan. These patients suffered from congenital bilateral SNHI but no skin lesion was found [235,236]. GJB4 variants have also been identified in Iranian patients with autosomal recessive non-syndromic hearing loss [237,238]. These pilot genotype-phenotype correlation studies serve as the steppingstone to clarify the link between GJB4 and SNHI.
4.2.2. HHI with Nail Disorders
The molecular underpinnings of syndromic HHI with nail disorders involve a plethora of genes related to proton transportation, vesicle transportation, peroxisome function, and hemidesmosomes.
The DDOD-linked ATP6V1B2 gene encodes a component of the vacuolar ATPase for proton transportation. Impaired lysosomal acidification due to V-ATPase deficiency undermines the Wnt signaling pathway, which is important for normal limb organogenesis. This may explain the dystrophic or atrophic nails present in DDOD patients [239,240,241]. Immunostaining of mouse cochlea showed predominant expression of Atp6v1b2 in the organ of Corti and spiral ganglion neurons. Consistent with histological findings, auditory brainstem response tests showed elevated hearing thresholds in cochlea-specific Atp6v1b2-knockdown mice, supporting the link between ATP6V1B2 and SNHI [39].
The DOORS-linked TBC1D24 encodes a GTPase-activating protein crucial to vesicle transportation [242,243]. TBC1D24 regulates migration of neural crest cells in coordination with ephrinB2 and the scaffold protein Dishevelled (Dsh) [244]. Immunostaining of mouse cochlea showed predominant expression of Tbc1d24 in inner and outer hair cells, and weaker expression in spiral ganglion neurons [245]. Nails and membranous labyrinth are both ectoderm-derived, which underlies the coexistence of nail dystrophy and SNHI [155].
Heimler syndromes 1 and 2 arise from pathogenic variants in PEX1 and PEX6, respectively, which lead to impaired peroxisome biogenesis [49,52]. Decreased metabolism of very long chain fatty acids underpins the cutaneous findings in the PBD spectrum [45,48]. Reduced or defective peroxisomes in Heimler syndrome patients have been found through immunofluorescence microscopy [51,246]. As oxidative stress is linked to hearing loss, this finding consolidates the relationship between peroxisomal dysfunction and SNHI in Heimler syndrome [49,247,248].
The NPS-related gene LMX1B encodes the LIM homeobox transcription factor, defects in which hinder limb and skin development; the dystrophic nails and orthopedic abnormalities may result from altered embryonic dorsoventral patterning [58,60,61]. Strong expression of the mouse homolog Lmx1b in the hindbrain implies that LMX1B variants disturb inner ear development [249].
The NPEBD-linked CD151 encodes a tetraspan protein crucial to hemidesmosome integrity [63]. CD151 facilitates basement membrane formation, migration of keratinocytes, and adhesion and migration of epithelial cells, highlighting its role in skin integrity and wound healing [250]. Hearing loss has been observed in laminin-deficient mice. As CD151 is key to laminin-binding among other tetraspanin-integrin interactions, defective CD151 may impair normal hearing [251,252].
4.3. HHI with Connective Tissue Disorders
Syndromic HHI with connective tissue disorders result from the deregulation of the extracellular matrix (ECM), dermatan-sulfate (DS) biosynthesis, microtubule assembly, mitochondria-mediated cell death, and inflammatory cascades.
The products of BCS1 and BCS2-associated genes, i.e., zinc finger protein 469 encoded by ZNF469, and PR domain-containing protein 5 encoded by PRDM5, regulate and maintain the ECM [169,253]. Pathogenic variants in PRDM5 lead to decreased or disorganized vital ECM components, including collagen I fibers and decorin, which has been shown in patient-derived fibroblast models [253,254]. Disorganized ECM leads to skin fragility and hyperelasticity in BCS patients [171]. SNHI has been documented in both PRDM5- and ZNF469-associated types of BCS [169,253].
The enzyme products of EDSMC1 and EDSMC2-causing genes CHST14 and dermatan-sulfate epimerase (DSE) are dermatan-4-sulfotransferase-1 (D4ST1) and dermatan-sulfate epimerase, respectively. These enzymes facilitate DS biosynthesis [173,255]. D4ST1 dysfunction hinders normal production and assembly of the ECM. Additionally, disrupted ECM components, including fibronectin and fibrillar collagen types I, III, and V, have been found in D4ST1-deficient patients [74,173]. These ECM defects lead to skin hyperextensibility, easy bruising, increased palmar wrinkling, and propensity to subcutaneous hematoma formation in EDSMC patients [71,173]. EDSMC1 patients with high-tone SNHI have been reported in the literature [72,173]. EDSMC2-causing variants in DSE also result in dysfunctional DS and ECM disarray; however, SNHI has not been reported in EDSMC2 patients [256].
Products of CSCSC1 and CSCSC2-associated genes, i.e., tubulin β chain encoded by TUBB and end-binding protein 2 encoded by MAPRE2, are crucial to microtubule assembly and polymerization [77,78]. Altered MAPRE2 expression perturbs branchial arch pattering, explaining the skin and craniofacial anomalies in CSCSC1 patients [77]. In cochlear sensory cells, microtubules form both dynamic and supporting structures of the organ of Corti [257]. Immunohistochemical staining of the inner ear revealed diffuse expression of β-tubulin, an autoantigen targeted in autoimmune inner ear disease [258,259,260,261,262,263,264]. Antibodies recognizing β-tubulin were isolated in the serum of 59% of patients with Meniere’s disease [265]. Taken together, microtubule assembly and dynamics are crucial for maintaining normal hearing.
The product of the MLS gene HCCS is crucial to mitochondrial-mediated apoptosis [175,176,177]. Defects in this synthase results in a shift from apoptosis to necrosis and induces inflammation and damage to neighboring cells, inducing the cutaneous manifestation of MLS [266].
The CAPS-linked NLRP3 and NLPR12 are mainly expressed in neutrophils and chondrocytes, and gain-of-function variants lead to over-activation of the inflammasome, overstimulation of interleukin (IL)-1β receptors, and overproduction and secretion of IL-1β [185,267,268]. Following the constitutive activation of the NLRP3 inflammasome, mast cells in CAPS patients produce IL-1β, induce neutrophil migration, and promote vascular leakage independent of stimuli [269]. Tissue-resident macrophage/monocyte-like cells reside perivascularly throughout the cochlea [185,270]. NLRP3 inflammasome-induced secretion of IL-1β induces cochlear inflammation, and thus SNHI [271,272]. The recombinant IL-1 receptor antagonist (IL-1Ra) Anakinra ameliorates SNHI, consolidating the role of IL-1β in hearing loss [185,268]. IL-1β also causes higher permeability of cytokines between the perilymph and CSF (cerebrospinal fluid) space via the modiolus, prompting spiral ligament fibrocytes to produce inflammatory mediators [182].
5. Conclusions
Listed in this review is a comprehensive array of syndromic HHI with abnormal cutaneous findings. This provides an outline for clinicians and researchers encountering patients with abnormal manifestations, which are evident in the setting of an outpatient clinic appointment (e.g., in a well-baby clinic). The pathogenesis of the skin manifestations and syndromic HHI of certain syndromes has not yet been fully elucidated. Further molecular and functional studies are necessary to unveil the underlying mechanisms.
Author Contributions
Conceptualization, T.-L.L., C.-C.W.; methodology, T.-L.L., C.-C.W.; writing—original draft preparation, T.-L.L.; writing—review and editing, T.-L.L., P.-H.L., J.-B.H., C.-C.W.; supervision, P.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morton, C.C.; Nance, W.E. Newborn hearing screening—A silent revolution. N. Engl. J. Med. 2006, 354, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, N.; Smith, R.J.; Van Camp, G. Forty-six genes causing nonsyndromic hearing impairment: Which ones should be analyzed in DNA diagnostics? Mutat. Res. 2009, 681, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, H.; Booth, K.T.; Ephraim, S.S.; Crone, B.; Black-Ziegelbein, E.A.; Marini, R.J.; Shearer, A.E.; Sloan-Heggen, C.M.; Kolbe, D.; Casavant, T.; et al. Genomic Landscape and Mutational Signatures of Deafness-Associated Genes. Am. J. Hum. Genet. 2018, 103, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Pingault, V.; Ente, D.; Dastot-Le Moal, F.; Goossens, M.; Marlin, S.; Bondurand, N. Review and update of mutations causing Waardenburg syndrome. Hum. Mutat. 2010, 31, 391–406. [Google Scholar] [CrossRef]
- Read, A.P.; Newton, V.E. Waardenburg syndrome. J. Med. Genet. 1997, 34, 656–665. [Google Scholar] [CrossRef]
- Tamayo, M.L.; Gelvez, N.; Rodriguez, M.; Florez, S.; Varon, C.; Medina, D.; Bernal, J.E. Screening program for Waardenburg syndrome in Colombia: Clinical definition and phenotypic variability. Am. J. Med. Genet. A 2008, 146a, 1026–1031. [Google Scholar] [CrossRef]
- Hughes, A.E.; Newton, V.E.; Liu, X.Z.; Read, A.P. A gene for Waardenburg syndrome type 2 maps close to the human homologue of the microphthalmia gene at chromosome 3p12-p14.1. Nat. Genet. 1994, 7, 509–512. [Google Scholar] [CrossRef]
- Xu, C.; Ren, W.; Zhang, Y.; Zheng, F.; Zhao, H.; Shang, H.; Guo, W.; Yang, S. KIT gene mutation causes deafness and hypopigmentation in Bama miniature pigs. Am. J. Transl. Res. 2020, 12, 5095–5107. [Google Scholar]
- Zazo Seco, C.; Serrão de Castro, L.; van Nierop, J.W.; Morín, M.; Jhangiani, S.; Verver, E.J.; Schraders, M.; Maiwald, N.; Wesdorp, M.; Venselaar, H.; et al. Allelic Mutations of KITLG, Encoding KIT Ligand, Cause Asymmetric and Unilateral Hearing Loss and Waardenburg Syndrome Type 2. Am. J. Hum. Genet. 2015, 97, 647–660. [Google Scholar] [CrossRef]
- Izumi, K.; Kohta, T.; Kimura, Y.; Ishida, S.; Takahashi, T.; Ishiko, A.; Kosaki, K. Tietz syndrome: Unique phenotype specific to mutations of MITF nuclear localization signal. Clin. Genet. 2008, 74, 93–95. [Google Scholar] [CrossRef]
- George, A.; Zand, D.J.; Hufnagel, R.B.; Sharma, R.; Sergeev, Y.V.; Legare, J.M.; Rice, G.M.; Scott Schwoerer, J.A.; Rius, M.; Tetri, L.; et al. Biallelic Mutations in MITF Cause Coloboma, Osteopetrosis, Microphthalmia, Macrocephaly, Albinism, and Deafness. Am. J. Hum. Genet. 2016, 99, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.V.; Morris, M.R.; Cangul, H.; Gleeson, D.; Straatman-Iwanowska, A.; Davies, N.; Keenan, S.; Pasha, S.; Rahman, F.; Gentle, D.; et al. Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Gorlin, R.J.; Anderson, R.C.; Blaw, M. Multiple lentigenes syndrome. Am. J. Dis. Child. (1960) 1969, 117, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, A.; Carta, C.; Moretti, S.; Zampino, G.; Digilio, M.C.; Pantaleoni, F.; Scioletti, A.P.; Esposito, G.; Cordeddu, V.; Lepri, F.; et al. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: Molecular diversity and associated phenotypic spectrum. Hum. Mutat. 2009, 30, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, A.; Digilio, M.C.; Dallapiccola, B. Leopard syndrome. Orphanet J. Rare Dis. 2008, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Digilio, M.C.; Sarkozy, A.; de Zorzi, A.; Pacileo, G.; Limongelli, G.; Mingarelli, R.; Calabro, R.; Marino, B.; Dallapiccola, B. LEOPARD syndrome: Clinical diagnosis in the first year of life. Am. J. Med. Genet. A 2006, 140, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Birlea, S.A.; Fain, P.R.; Gowan, K.; Riccardi, S.L.; Holland, P.J.; Mailloux, C.M.; Sufit, A.J.D.; Hutton, S.M.; Amadi-Myers, A.; et al. Variant of TYR and Autoimmunity Susceptibility Loci in Generalized Vitiligo. N. Engl. J. Med. 2010, 362, 1686–1697. [Google Scholar] [CrossRef]
- Cleaver, J.E. Do we know the cause of xeroderma pigmentosum? Carcinogenesis 1990, 11, 875–882. [Google Scholar] [CrossRef][Green Version]
- Kashiyama, K.; Nakazawa, Y.; Pilz, D.T.; Guo, C.; Shimada, M.; Sasaki, K.; Fawcett, H.; Wing, J.F.; Lewin, S.O.; Carr, L.; et al. Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia. Am. J. Hum. Genet. 2013, 92, 807–819. [Google Scholar] [CrossRef]
- Soltys, D.T.; Rocha, C.R.; Lerner, L.K.; de Souza, T.A.; Munford, V.; Cabral, F.; Nardo, T.; Stefanini, M.; Sarasin, A.; Cabral-Neto, J.B.; et al. Novel XPG (ERCC5) mutations affect DNA repair and cell survival after ultraviolet but not oxidative stress. Hum. Mutat. 2013, 34, 481–489. [Google Scholar] [CrossRef]
- Kasraian, Z.; Trompezinski, S.; Cario-André, M.; Morice-Picard, F.; Ged, C.; Jullie, M.L.; Taieb, A.; Rezvani, H.R. Pigmentation abnormalities in nucleotide excision repair disorders: Evidence and hypotheses. Pigment. Cell Melanoma Res. 2019, 32, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Nance, M.A.; Berry, S.A. Cockayne syndrome: Review of 140 cases. Am. J. Med. Genet. 1992, 42, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Mallery, D.L.; Tanganelli, B.; Colella, S.; Steingrimsdottir, H.; van Gool, A.J.; Troelstra, C.; Stefanini, M.; Lehmann, A.R. Molecular analysis of mutations in the CSB (ERCC6) gene in patients with Cockayne syndrome. Am. J. Hum. Genet. 1998, 62, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Rapin, I.; Lindenbaum, Y.; Dickson, D.W.; Kraemer, K.H.; Robbins, J.H. Cockayne syndrome and xeroderma pigmentosum. Neurology 2000, 55, 1442–1449. [Google Scholar] [CrossRef]
- Licht, C.L.; Stevnsner, T.; Bohr, V.A. Cockayne syndrome group B cellular and biochemical functions. Am. J. Hum. Genet. 2003, 73, 1217–1239. [Google Scholar] [CrossRef]
- Verbov, J. Palmoplantar keratoderma, deafness and atopy. Br. J. Dermatol. 1987, 116, 881–882. [Google Scholar] [CrossRef]
- Heathcote, K.; Syrris, P.; Carter, N.D.; Patton, M.A. A connexin 26 mutation causes a syndrome of sensorineural hearing loss and palmoplantar hyperkeratosis (MIM 148350). J. Med. Genet. 2000, 37, 50–51. [Google Scholar] [CrossRef]
- Maestrini, E.; Korge, B.P.; Ocaña-Sierra, J.; Calzolari, E.; Cambiaghi, S.; Scudder, P.M.; Hovnanian, A.; Monaco, A.P.; Munro, C.S. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel’s syndrome) in three unrelated families. Hum. Mol. Genet. 1999, 8, 1237–1243. [Google Scholar] [CrossRef]
- Sensi, A.; Bettoli, V.; Zampino, M.R.; Gandini, E.; Calzolari, E. Vohwinkel syndrome (mutilating keratoderma) associated with craniofacial anomalies. Am. J. Med. Genet. 1994, 50, 201–203. [Google Scholar] [CrossRef]
- De Zwart-Storm, E.A.; van Geel, M.; Veysey, E.; Burge, S.; Cooper, S.; Steijlen, P.M.; Martin, P.E.; van Steensel, M.A. A novel missense mutation in GJB2, p.Tyr65His, causes severe Vohwinkel syndrome. Br. J. Dermatol. 2011, 164, 197–199. [Google Scholar] [CrossRef]
- Richard, G.; Brown, N.; Ishida-Yamamoto, A.; Krol, A. Expanding the phenotypic spectrum of Cx26 disorders: Bart-Pumphrey syndrome is caused by a novel missense mutation in GJB2. J. Investig. Dermatol. 2004, 123, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, F.; Sartorato, E.L.; Marques-de-Faria, A.P.; Steiner, C.E. G59S mutation in the GJB2 (connexin 26) gene in a patient with Bart-Pumphrey syndrome. Am. J. Med. Genet. A 2005, 136, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Bart, R.S.; Pumphrey, R.E. Knuckle pads, leukonychia and deafness. A dominantly inherited syndrome. N. Engl. J. Med. 1967, 276, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, M.; van Steensel, M.A.; Küster, W.; Hennies, H.C.; Happle, R.; Steijlen, P.M.; König, A. HID and KID syndromes are associated with the same connexin 26 mutation. Br. J. Dermatol. 2002, 146, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Baden, H.P.; Bronstein, B.R. Ichthyosiform dermatosis and deafness. Report of a case and review of the literature. Arch. Dermatol. 1988, 124, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Grob, J.J.; Breton, A.; Bonafe, J.L.; Sauvan-Ferdani, M.; Bonerandi, J.J. Keratitis, ichthyosis, and deafness (KID) syndrome. Vertical transmission and death from multiple squamous cell carcinomas. Arch. Dermatol. 1987, 123, 777–782. [Google Scholar] [CrossRef]
- Mikaelian, D.O.; Der Kaloustian, V.M.; Shahin, N.A.; Barsoumian, V.M. Congenital Ectodermal Dysplasia with Hearing Loss. Arch. Otolaryngol. 1970, 92, 85–89. [Google Scholar] [CrossRef]
- Kondoh, T.; Tsuru, A.; Matsumoto, T.; Matsuzaka, T.; Tsuji, Y. Autosomal dominant onychodystrophy and congenital sensorineural deafness. J. Hum. Genet. 1999, 44, 60–62. [Google Scholar] [CrossRef][Green Version]
- Yuan, Y.; Zhang, J.; Chang, Q.; Zeng, J.; Xin, F.; Wang, J.; Zhu, Q.; Wu, J.; Lu, J.; Guo, W.; et al. De novo mutation in ATP6V1B2 impairs lysosome acidification and causes dominant deafness-onychodystrophy syndrome. Cell Res. 2014, 24, 1370–1373. [Google Scholar] [CrossRef]
- Menendez, I.; Carranza, C.; Herrera, M.; Marroquin, N.; Foster, J., 2nd; Cengiz, F.B.; Bademci, G.; Tekin, M. Dominant deafness-onychodystrophy syndrome caused by an ATP6V1B2 mutation. Clin. Case Rep. 2017, 5, 376–379. [Google Scholar] [CrossRef]
- James, A.W.; Miranda, S.G.; Culver, K.; Hall, B.D.; Golabi, M. DOOR syndrome: Clinical report, literature review and discussion of natural history. Am. J. Med. Genet. A 2007, 143A, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Afawi, Z.; Mandelstam, S.; Korczyn, A.D.; Kivity, S.; Walid, S.; Shalata, A.; Oliver, K.L.; Corbett, M.; Gecz, J.; Berkovic, S.F.; et al. TBC1D24 mutation associated with focal epilepsy, cognitive impairment and a distinctive cerebro-cerebellar malformation. Epilepsy Res. 2013, 105, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Campeau, P.M.; Kasperaviciute, D.; Lu, J.T.; Burrage, L.C.; Kim, C.; Hori, M.; Powell, B.R.; Stewart, F.; Félix, T.M.; van den Ende, J.; et al. The genetic basis of DOORS syndrome: An exome-sequencing study. Lancet Neurol. 2014, 13, 44–58. [Google Scholar] [CrossRef]
- Lüthy, K.; Mei, D.; Fischer, B.; De Fusco, M.; Swerts, J.; Paesmans, J.; Parrini, E.; Lubarr, N.; Meijer, I.A.; Mackenzie, K.M.; et al. TBC1D24-TLDc-related epilepsy exercise-induced dystonia: Rescue by antioxidants in a disease model. Brain 2019, 142, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Moser, H.W. Genotype-phenotype correlations in disorders of peroxisome biogenesis. Mol. Genet. Metab. 1999, 68, 316–327. [Google Scholar] [CrossRef]
- Crane, D.I.; Maxwell, M.A.; Paton, B.C. PEX1 mutations in the Zellweger spectrum of the peroxisome biogenesis disorders. Hum. Mutat. 2005, 26, 167–175. [Google Scholar] [CrossRef]
- Ong, K.R.; Visram, S.; McKaig, S.; Brueton, L.A. Sensorineural deafness, enamel abnormalities and nail abnormalities: A case report of Heimler syndrome in identical twin girls. Eur. J. Med. Genet. 2006, 49, 187–193. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Huang, X. Drosophila models of peroxisomal biogenesis disorder: Peroxins are required for spermatogenesis and very-long-chain fatty acid metabolism. Human Mol. Genet. 2009, 19, 494–505. [Google Scholar] [CrossRef]
- Smith, J.J.; Aitchison, J.D. Peroxisomes take shape. Nat. Rev. Mol. Cell Biol. 2013, 14, 803–817. [Google Scholar] [CrossRef]
- Fujiki, Y.; Okumoto, K.; Mukai, S.; Honsho, M.; Tamura, S. Peroxisome biogenesis in mammalian cells. Front. Physiol. 2014, 5, 307. [Google Scholar] [CrossRef]
- Ratbi, I.; Falkenberg, K.D.; Sommen, M.; Al-Sheqaih, N.; Guaoua, S.; Vandeweyer, G.; Urquhart, J.E.; Chandler, K.E.; Williams, S.G.; Roberts, N.A.; et al. Heimler Syndrome Is Caused by Hypomorphic Mutations in the Peroxisome-Biogenesis Genes PEX1 and PEX6. Am. J. Hum. Genet. 2015, 97, 535–545. [Google Scholar] [CrossRef]
- Grimm, I.; Erdmann, R.; Girzalsky, W. Role of AAA+-proteins in peroxisome biogenesis and function. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, K.D.; Braverman, N.E.; Moser, A.B.; Steinberg, S.J.; Klouwer, F.C.C.; Schlüter, A.; Ruiz, M.; Pujol, A.; Engvall, M.; Naess, K.; et al. Allelic Expression Imbalance Promoting a Mutant PEX6 Allele Causes Zellweger Spectrum Disorder. Am. J. Hum. Genet. 2017, 101, 965–976. [Google Scholar] [CrossRef]
- Schieferdecker, A.; Wendler, P. Structural Mapping of Missense Mutations in the Pex1/Pex6 Complex. Int. J. Mol. Sci. 2019, 20, 3756. [Google Scholar] [CrossRef]
- Yu, H.-L.; Shen, Y.; Sun, Y.-M.; Zhang, Y. Two novel mutations of PEX6 in one Chinese Zellweger spectrum disorder and their clinical characteristics. Ann Transl. Med. 2019, 7, 5. [Google Scholar] [CrossRef]
- Hawkins, C.F.; Smith, O.E. Renal dysplasia in a family with multiple hereditary abnormalities including iliac horns. Lancet (Lond. Engl.) 1950, 1, 803–808. [Google Scholar] [CrossRef]
- Dreyer, S.D.; Zhou, G.; Baldini, A.; Winterpacht, A.; Zabel, B.; Cole, W.; Johnson, R.L.; Lee, B. Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat. Genet. 1998, 19, 47–50. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Yin, J.; Kania, A.; Zhao, Z.Q.; Johnson, R.L.; Chen, Z.F. Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord. Development 2004, 131, 3693–3703. [Google Scholar] [CrossRef]
- Bongers, E.M.; Huysmans, F.T.; Levtchenko, E.; de Rooy, J.W.; Blickman, J.G.; Admiraal, R.J.; Huygen, P.L.; Cruysberg, J.R.; Toolens, P.A.; Prins, J.B.; et al. Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur. J. Hum. Genet. 2005, 13, 935–946. [Google Scholar] [CrossRef]
- Dai, J.X.; Johnson, R.L.; Ding, Y.Q. Manifold functions of the Nail-Patella Syndrome gene Lmx1b in vertebrate development. Dev. Growth Differ. 2009, 51, 241–250. [Google Scholar] [CrossRef]
- Feenstra, J.M.; Kanaya, K.; Pira, C.U.; Hoffman, S.E.; Eppey, R.J.; Oberg, K.C. Detection of genes regulated by Lmx1b during limb dorsalization. Dev. Growth Differ. 2012, 54, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Kagan, A.; Feld, S.; Chemke, J.; Bar-Khayim, Y. Occurrence of hereditary nephritis, pretibial epidermolysis bullosa and β-thalassemia minor in two siblings with end-stage renal disease. Nephron 1988, 49, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Sterk, L.M.; Geuijen, C.A.; Oomen, L.C.; Calafat, J.; Janssen, H.; Sonnenberg, A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J. Cell Biol. 2000, 149, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Karamatic Crew, V.; Burton, N.; Kagan, A.; Green, C.A.; Levene, C.; Flinter, F.; Brady, R.L.; Daniels, G.; Anstee, D.J. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 2004, 104, 2217–2223. [Google Scholar] [CrossRef]
- Reimer, A.; He, Y.; Has, C. Update on Genetic Conditions Affecting the Skin and the Kidneys. Front. Pediatrics 2018, 6, 43. [Google Scholar] [CrossRef]
- Al-Hussain, H.; Zeisberger, S.M.; Huber, P.R.; Giunta, C.; Steinmann, B. Brittle cornea syndrome and its delineation from the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI): Report on 23 patients and review of the literature. Am. J. Med. Genet. A 2004, 124, 28–34. [Google Scholar] [CrossRef]
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef]
- Bertelsen, T.I. Dysgenesis mesodermalis corneae et sclerae. Rupture of both corneae in a patient with blue sclerae. Acta Ophthalmol (Copenh) 1968, 46, 486–491. [Google Scholar] [CrossRef]
- Porter, L.F.; Galli, G.G.; Williamson, S.; Selley, J.; Knight, D.; Elcioglu, N.; Aydin, A.; Elcioglu, M.; Venselaar, H.; Lund, A.H.; et al. A role for repressive complexes and H3K9 di-methylation in PRDM5-associated brittle cornea syndrome. Hum. Mol. Genet. 2015, 24, 6565–6579. [Google Scholar] [CrossRef]
- Cameron, J.A. Corneal abnormalities in Ehlers-Danlos syndrome type VI. Cornea 1993, 12, 54–59. [Google Scholar] [CrossRef]
- Steinmann, B.; Gitzelmann, R.; Vogel, A.; Grant, M.E.; Harwood, R.; Sear, C.H. Ehlers-Danlos syndrome in two siblings with deficient lysyl hydroxylase activity in cultured skin fibroblasts but only mild hydroxylysine deficit in skin. Helv. Paediatr. Acta 1975, 30, 255–274. [Google Scholar] [PubMed]
- Kosho, T.; Miyake, N.; Hatamochi, A.; Takahashi, J.; Kato, H.; Miyahara, T.; Igawa, Y.; Yasui, H.; Ishida, T.; Ono, K.; et al. A new Ehlers-Danlos syndrome with craniofacial characteristics, multiple congenital contractures, progressive joint and skin laxity, and multisystem fragility-related manifestations. Am. J. Med. Genet. A 2010, 152, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Syx, D.; Vlummens, P.; Symoens, S.; Nampoothiri, S.; Hermanns-Lê, T.; Van Laer, L.; De Paepe, A. Musculocontractural Ehlers-Danlos Syndrome (former EDS type VIB) and adducted thumb clubfoot syndrome (ATCS) represent a single clinical entity caused by mutations in the dermatan-4-sulfotransferase 1 encoding CHST14 gene. Hum. Mutat. 2010, 31, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Janecke, A.R.; Li, B.; Boehm, M.; Krabichler, B.; Rohrbach, M.; Müller, T.; Fuchs, I.; Golas, G.; Katagiri, Y.; Ziegler, S.G.; et al. The phenotype of the musculocontractural type of Ehlers-Danlos syndrome due to CHST14 mutations. Am. J. Med. Genet. A 2016, 170a, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; De Paepe, A.; Steinmann, B.; Tsipouras, P.; Wenstrup, R.J. Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am. J. Med. Genet. 1998, 77, 31–37. [Google Scholar] [CrossRef]
- Kunze, J.; Riehm, H. A new genetic disorder: Autosomal-dominant multiple benign ring-shaped skin creases. Eur. J. Pediatrics 1982, 138, 301–303. [Google Scholar] [CrossRef]
- Isrie, M.; Breuss, M.; Tian, G.; Hansen, A.H.; Cristofoli, F.; Morandell, J.; Kupchinsky, Z.A.; Sifrim, A.; Rodriguez-Rodriguez, C.M.; Dapena, E.P.; et al. Mutations in Either TUBB or MAPRE2 Cause Circumferential Skin Creases Kunze Type. Am. J. Hum. Genet. 2015, 97, 790–800. [Google Scholar] [CrossRef]
- Tinsa, F.; Aissa, K.; Meddeb, M.; Bousnina, D.; Boussetta, K.; Bousnina, S. Multiple congenital anomalies/mental retardation syndrome with multiple circumferential skin creases: A new syndrome? J. Child. Neurol. 2009, 24, 224–227. [Google Scholar] [CrossRef]
- Wouters, L.; Rodriguez Rodriguez, C.M.; Dapena, E.P.; Poorten, V.V.; Devriendt, K.; Van Esch, H. Circumferential skin creases, cleft palate, typical face, intellectual disability and growth delay: “circumferential skin creases Kunze type”. Eur. J. Med. Genet. 2011, 54, 236–240. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Grillo, A.; Ferrero, G.B.; Roth, E.J.; Magenis, E.; Grompe, M.; Hultén, M.; Gould, C.; Baldini, A.; Zoghbi, H.Y.; et al. Microphthalmia with linear skin defects (MLS) syndrome: Clinical, cytogenetic, and molecular characterization. Am. J. Med. Genet. 1994, 49, 229–234. [Google Scholar] [CrossRef]
- Zumwalt, J.; Moorhead, C.; Golkar, L. Fourteen-month-old girl with facial skin thinning. Pediatric Dermatol. 2012, 29, 217–218. [Google Scholar] [CrossRef] [PubMed]
- al-Gazali, L.I.; Mueller, R.F.; Caine, A.; Antoniou, A.; McCartney, A.; Fitchett, M.; Dennis, N.R. Two 46,XX,t(X;Y) females with linear skin defects and congenital microphthalmia: A new syndrome at Xp22.3. J. Med. Genet. 1990, 27, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Happle, R.; Daniëls, O.; Koopman, R.J. MIDAS syndrome (microphthalmia, dermal aplasia, and sclerocornea): An X-linked phenotype distinct from Goltz syndrome. Am. J. Med. Genet. 1993, 47, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Kile, R.L.; Rusk, H.A. A Case of Cold Urticaria with an Unusual Family History. J. Am. Med. Assoc. 1940, 114, 1067–1068. [Google Scholar] [CrossRef]
- Zip, C.M.; Ross, J.B.; Greaves, M.W.; Scriver, C.R.; Mitchell, J.J.; Zoar, S. Familial cold urticaria. Clin. Exp. Dermatol. 1993, 18, 338–341. [Google Scholar] [CrossRef]
- Jéru, I.; Duquesnoy, P.; Fernandes-Alnemri, T.; Cochet, E.; Yu, J.W.; Lackmy-Port-Lis, M.; Grimprel, E.; Landman-Parker, J.; Hentgen, V.; Marlin, S.; et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl. Acad. Sci. USA 2008, 105, 1614–1619. [Google Scholar] [CrossRef]
- Borghini, S.; Tassi, S.; Chiesa, S.; Caroli, F.; Carta, S.; Caorsi, R.; Fiore, M.; Delfino, L.; Lasigliè, D.; Ferraris, C.; et al. Clinical presentation and pathogenesis of cold-induced autoinflammatory disease in a family with recurrence of an NLRP12 mutation. Arthritis Rheum 2011, 63, 830–839. [Google Scholar] [CrossRef]
- Shen, M.; Tang, L.; Shi, X.; Zeng, X.; Yao, Q. NLRP12 autoinflammatory disease: A Chinese case series and literature review. Clin. Rheumatol. 2017, 36, 1661–1667. [Google Scholar] [CrossRef]
- Muckle, T.J.; Wells, M. Urticaria, deafness, and amyloidosis: A new heredo-familial syndrome. Q. J. Med. 1962, 31, 235–248. [Google Scholar]
- Hoffman, H.M.; Mueller, J.L.; Broide, D.H.; Wanderer, A.A.; Kolodner, R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001, 29, 301–305. [Google Scholar] [CrossRef]
- Dodé, C.; Le Dû, N.; Cuisset, L.; Letourneur, F.; Berthelot, J.M.; Vaudour, G.; Meyrier, A.; Watts, R.A.; Scott, D.G.; Nicholls, A.; et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: A novel mutation underlies both syndromes. Am. J. Hum. Genet. 2002, 70, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Prieur, A.M. A recently recognised chronic inflammatory disease of early onset characterised by the triad of rash, central nervous system involvement and arthropathy. Clin. Exp. Rheumatol. 2001, 19, 103–106. [Google Scholar] [PubMed]
- Boschan, C.; Witt, O.; Lohse, P.; Foeldvari, I.; Zappel, H.; Schweigerer, L. Neonatal-onset multisystem inflammatory disease (NOMID) due to a novel S331R mutation of the CIAS1 gene and response to interleukin-1 receptor antagonist treatment. Am. J. Med. Genet. A 2006, 140, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Aksentijevich, I.; Nowak, M.; Mallah, M.; Chae, J.J.; Watford, W.T.; Hofmann, S.R.; Stein, L.; Russo, R.; Goldsmith, D.; Dent, P.; et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): A new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum 2002, 46, 3340–3348. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, J.; Prieur, A.M.; Quartier, P.; Berquin, P.; Certain, S.; Cortis, E.; Teillac-Hamel, D.; Fischer, A.; de Saint Basile, G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 2002, 71, 198–203. [Google Scholar] [CrossRef]
- Boyle, M.I.; Jespersgaard, C.; Brondum-Nielsen, K.; Bisgaard, A.M.; Tumer, Z. Cornelia de Lange syndrome. Clin. Genet. 2015, 88, 1–12. [Google Scholar] [CrossRef]
- Song, J.; Feng, Y.; Acke, F.R.; Coucke, P.; Vleminckx, K.; Dhooge, I.J. Hearing loss in Waardenburg syndrome: A systematic review. Clin. Genet. 2016, 89, 416–425. [Google Scholar] [CrossRef]
- Tassabehji, M.; Newton, V.E.; Read, A.P. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat. Genet. 1994, 8, 251–255. [Google Scholar] [CrossRef]
- Klein, D. Historical background and evidence for dominant inheritance of the Klein-Waardenburg syndrome (type III). Am. J. Med. Genet. 1983, 14, 231–239. [Google Scholar] [CrossRef]
- Waardenburg, P.J. A new syndrome combining developmental anomalies of the eyelids, eyebrows and nose root with pigmentary defects of the iris and head hair and with congenital deafness. Am. J. Hum. Genet. 1951, 3, 195–253. [Google Scholar]
- Pingault, V.; Bondurand, N.; Kuhlbrodt, K.; Goerich, D.E.; Préhu, M.O.; Puliti, A.; Herbarth, B.; Hermans-Borgmeyer, I.; Legius, E.; Matthijs, G.; et al. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat. Genet. 1998, 18, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Bondurand, N.; Dastot-Le Moal, F.; Stanchina, L.; Collot, N.; Baral, V.; Marlin, S.; Attie-Bitach, T.; Giurgea, I.; Skopinski, L.; Reardon, W.; et al. Deletions at the SOX10 gene locus cause Waardenburg syndrome types 2 and 4. Am. J. Hum. Genet. 2007, 81, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Edery, P.; Attié, T.; Amiel, J.; Pelet, A.; Eng, C.; Hofstra, R.M.; Martelli, H.; Bidaud, C.; Munnich, A.; Lyonnet, S. Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease (Shah-Waardenburg syndrome). Nat. Genet. 1996, 12, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, R.M.W.; Osinga, J.; Tan-Sindhunata, G.; Wu, Y.; Kamsteeg, E.-J.; Stulp, R.P.; van Ravenswaaij-Arts, C.; Majoor-Krakauer, D.; Angrist, M.; Chakravarti, A.; et al. A homozygous mutation in the endothelin-3 gene associated with a combined Waardenburg type 2 and Hirschsprung phenotype (Shah-Waardenburg syndrome). Nat. Genet. 1996, 12, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Khajavi, M.; Ohyama, T.; Hirabayashi, S.; Wilson, J.; Reggin, J.D.; Mancias, P.; Butler, I.J.; Wilkinson, M.F.; Wegner, M.; et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat. Genet. 2004, 36, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, M.; Rodríguez-García, A.; Pérez-Losada, J.; Sagrera, A.; Read, A.P.; Sánchez-García, I. SLUG (SNAI2) deletions in patients with Waardenburg disease. Hum. Mol. Genet. 2002, 11, 3231–3236. [Google Scholar] [CrossRef]
- Tachibana, M. MITF: A Stream Flowing for Pigment Cells. Pigment. Cell Res. 2000, 13, 230–240. [Google Scholar] [CrossRef]
- Bard, L.A. Heterogeneity in Waardenburg’s syndrome. Report of a family with ocular albinism. Arch. Ophthalmol. (Chic. Ill. 1960) 1978, 96, 1193–1198. [Google Scholar] [CrossRef]
- Bondurand, N.; Pingault, V.; Goerich, D.E.; Lemort, N.; Sock, E.; Caignec, C.L.; Wegner, M.; Goossens, M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Human Mol. Genet. 2000, 9, 1907–1917. [Google Scholar] [CrossRef]
- Gross, A.; Kunze, J.; Maier, R.F.; Stoltenburg-Didinger, G.; Grimmer, I.; Obladen, M. Autosomal-recessive neural crest syndrome with albinism, black lock, cell migration disorder of the neurocytes of the gut, and deafness: ABCD syndrome. Am. J. Med. Genet. 1995, 56, 322–326. [Google Scholar] [CrossRef]
- Verheij, J.B.; Kunze, J.; Osinga, J.; van Essen, A.J.; Hofstra, R.M. ABCD syndrome is caused by a homozygous mutation in the EDNRB gene. Am. J. Med. Genet. 2002, 108, 223–225. [Google Scholar] [CrossRef]
- Bondurand, N.; Southard-Smith, E.M. Mouse models of Hirschsprung disease and other developmental disorders of the enteric nervous system: Old and new players. Dev. Biol. 2016, 417, 139–157. [Google Scholar] [CrossRef]
- Tietz, W. A syndrome of deaf-mutism associated with albinism showing dominant autosomal inheritance. Am. J. Hum. Genet. 1963, 15, 259–264. [Google Scholar] [PubMed]
- Smith, S.D.; Kelley, P.M.; Kenyon, J.B.; Hoover, D. Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J. Med. Genet. 2000, 37, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, K.; Chida, K.; Tsukamoto, I.; Morii, E.; Munakata, H.; Arnheiter, H.; Kuroki, T.; Kitamura, Y.; Nomura, S. The recessive phenotype displayed by a dominant negative microphthalmia-associated transcription factor mutant is a result of impaired nucleation potential. Mol. Cell. Biol. 1996, 16, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Molho-Pessach, V.; Agha, Z.; Aamar, S.; Glaser, B.; Doviner, V.; Hiller, N.; Zangen, D.H.; Raas-Rothschild, A.; Ben-Neriah, Z.; Shweiki, S.; et al. The H syndrome: A genodermatosis characterized by indurated, hyperpigmented, and hypertrichotic skin with systemic manifestations. J. Am. Acad. Dermatol. 2008, 59, 79–85. [Google Scholar] [CrossRef]
- Jaouadi, H.; Zaouak, A.; Sellami, K.; Messaoud, O.; Chargui, M.; Hammami, H.; Jones, M.; Jouini, R.; Chadli Debbiche, A.; Chraiet, K.; et al. H syndrome: Clinical, histological and genetic investigation in Tunisian patients. J. Dermatol. 2018, 45, 978–985. [Google Scholar] [CrossRef]
- Jonard, L.; Couloigner, V.; Pierrot, S.; Louha, M.; Gherbi, S.; Denoyelle, F.; Marlin, S. Progressive hearing loss associated with a unique cervical node due to a homozygous SLC29A3 mutation: A very mild phenotype. Eur. J. Med. Genet. 2012, 55, 56–58. [Google Scholar] [CrossRef]
- Baldwin, S.A.; Yao, S.Y.; Hyde, R.J.; Ng, A.M.; Foppolo, S.; Barnes, K.; Ritzel, M.W.; Cass, C.E.; Young, J.D. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J. Biol. Chem. 2005, 280, 15880–15887. [Google Scholar] [CrossRef]
- Digilio, M.C.; Conti, E.; Sarkozy, A.; Mingarelli, R.; Dottorini, T.; Marino, B.; Pizzuti, A.; Dallapiccola, B. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am. J. Hum. Genet. 2002, 71, 389–394. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.R.; Kim, H.J.; Lee, K.A.; Lee, M.G. LEOPARD Syndrome with PTPN11 Gene Mutation Showing Six Cardinal Symptoms of LEOPARD. Ann. Dermatol. 2011, 23, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet (Lond. Engl.) 2013, 381, 333–342. [Google Scholar] [CrossRef]
- El Bouchikhi, I.; Belhassan, K.; Moufid, F.Z.; Iraqui Houssaini, M.; Bouguenouch, L.; Samri, I.; Atmani, S.; Ouldim, K. Noonan syndrome-causing genes: Molecular update and an assessment of the mutation rate. Int. J. Pediatrics Adolesc. Med. 2016, 3, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Bader, P.I.; Biegel, A.; Epinette, W.W.; Nance, W.E. Vitiligo and dysgammaglobulinemia. A case report and family study. Clin. Genet. 1975, 7, 62–76. [Google Scholar] [CrossRef]
- Jin, Y.; Mailloux, C.M.; Gowan, K.; Riccardi, S.L.; LaBerge, G.; Bennett, D.C.; Fain, P.R.; Spritz, R.A. NALP1 in vitiligo-associated multiple autoimmune disease. N. Engl. J. Med. 2007, 356, 1216–1225. [Google Scholar] [CrossRef]
- Goudie, R.B.; Goudie, D.R.; Dick, H.M.; Ferguson-Smith, M.A. Unstable mutations in vitiligo, organ-specific autoimmune diseases, and multiple endocrine adenoma/peptic-ulcer syndrome. Lancet (Lond. Engl.) 1980, 2, 285–287. [Google Scholar] [CrossRef]
- Damico, F.M.; Cunha-Neto, E.; Goldberg, A.C.; Iwai, L.K.; Marin, M.L.; Hammer, J.; Kalil, J.; Yamamoto, J.H. T-cell recognition and cytokine profile induced by melanocyte epitopes in patients with HLA-DRB1*0405-positive and -negative Vogt-Koyanagi-Harada uveitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2465–2471. [Google Scholar] [CrossRef]
- Sugita, S.; Takase, H.; Taguchi, C.; Imai, Y.; Kamoi, K.; Kawaguchi, T.; Sugamoto, Y.; Futagami, Y.; Itoh, K.; Mochizuki, M. Ocular infiltrating CD4+ T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2547–2554. [Google Scholar] [CrossRef]
- Karikkineth, A.C.; Scheibye-Knudsen, M.; Fivenson, E.; Croteau, D.L.; Bohr, V.A. Cockayne syndrome: Clinical features, model systems and pathways. Ageing Res. Rev. 2017, 33, 3–17. [Google Scholar] [CrossRef]
- Oh, K.S.; Khan, S.G.; Jaspers, N.G.; Raams, A.; Ueda, T.; Lehmann, A.; Friedmann, P.S.; Emmert, S.; Gratchev, A.; Lachlan, K.; et al. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): Xeroderma pigmentosum without and with Cockayne syndrome. Hum. Mutat. 2006, 27, 1092–1103. [Google Scholar] [CrossRef]
- Hirai, Y.; Kodama, Y.; Moriwaki, S.; Noda, A.; Cullings, H.M.; Macphee, D.G.; Kodama, K.; Mabuchi, K.; Kraemer, K.H.; Land, C.E.; et al. Heterozygous individuals bearing a founder mutation in the XPA DNA repair gene comprise nearly 1% of the Japanese population. Mutat. Res. 2006, 601, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kleijer, W.J.; Laugel, V.; Berneburg, M.; Nardo, T.; Fawcett, H.; Gratchev, A.; Jaspers, N.G.; Sarasin, A.; Stefanini, M.; Lehmann, A.R. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair 2008, 7, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Totonchy, M.B.; Tamura, D.; Pantell, M.S.; Zalewski, C.; Bradford, P.T.; Merchant, S.N.; Nadol, J.; Khan, S.G.; Schiffmann, R.; Pierson, T.M.; et al. Auditory analysis of xeroderma pigmentosum 1971–2012: Hearing function, sun sensitivity and DNA repair predict neurological degeneration. Brain 2013, 136, 194–208. [Google Scholar] [CrossRef]
- Vermeulen, W.; Scott, R.J.; Rodgers, S.; Müller, H.J.; Cole, J.; Arlett, C.F.; Kleijer, W.J.; Bootsma, D.; Hoeijmakers, J.H.; Weeda, G. Clinical heterogeneity within xeroderma pigmentosum associated with mutations in the DNA repair and transcription gene ERCC3. Am. J. Hum. Genet. 1994, 54, 191–200. [Google Scholar] [PubMed]
- Frouin, E.; Laugel, V.; Durand, M.; Dollfus, H.; Lipsker, D. Dermatologic Findings in 16 Patients with Cockayne Syndrome and Cerebro-Oculo-Facial-Skeletal Syndrome. JAMA Dermatol. 2013, 149, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Calmels, N.; Botta, E.; Jia, N.; Fawcett, H.; Nardo, T.; Nakazawa, Y.; Lanzafame, M.; Moriwaki, S.; Sugita, K.; Kubota, M.; et al. Functional and clinical relevance of novel mutations in a large cohort of patients with Cockayne syndrome. J. Med. Genet. 2018, 55, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Iossa, S.; Marciano, E.; Franzé, A. GJB2 Gene Mutations in Syndromic Skin Diseases with Sensorineural Hearing Loss. Curr. Genom. 2011, 12, 475–785. [Google Scholar] [CrossRef]
- Janecke, A.R.; Hennies, H.C.; Gunther, B.; Gansl, G.; Smolle, J.; Messmer, E.M.; Utermann, G.; Rittinger, O. GJB2 mutations in keratitis-ichthyosis-deafness syndrome including its fatal form. Am. J. Med. Genet. A 2005, 133A, 128–131. [Google Scholar] [CrossRef]
- Shuja, Z.; Li, L.; Gupta, S.; Mese, G.; White, T.W. Connexin26 Mutations Causing Palmoplantar Keratoderma and Deafness Interact with Connexin43, Modifying Gap Junction and Hemichannel Properties. J. Investig. Dermatol. 2016, 136, 225–235. [Google Scholar] [CrossRef]
- Balighi, K.; Moeineddin, F.; Lajevardi, V.; Ahmadreza, R. A family with leukonychia totalis. Indian J. Dermatol. 2010, 55, 102–104. [Google Scholar] [CrossRef]
- Ramer, J.C.; Vasily, D.B.; Ladda, R.L. Familial leuconychia, knuckle pads, hearing loss, and palmoplantar hyperkeratosis: An additional family with Bart-Pumphrey syndrome. J. Med. Genet. 1994, 31, 68–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, J.; Nicholson, B.J. The role of connexins in ear and skin physiology—Functional insights from disease-associated mutations. Biochim. Biophys. Acta 2013, 1828, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, D.; Denoyelle, F.; Blons, H.; Lyonnet, S.; Loundon, N.; Rouillon, I.; Hadj-Rabia, S.; Petit, C.; Couderc, R.; Garabédian, E.N.; et al. The GJB2 mutation R75Q can cause nonsyndromic hearing loss DFNA3 or hereditary palmoplantar keratoderma with deafness. Am. J. Med. Genet. A 2005, 137, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Lazic, T.; Li, Q.; Frank, M.; Uitto, J.; Zhou, L.H. Extending the phenotypic spectrum of keratitis-ichthyosis-deafness syndrome: Report of a patient with GJB2 (G12R) Connexin 26 mutation and unusual clinical findings. Pediatric Dermatol. 2012, 29, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Markova, T.G.; Brazhkina, N.B.; Bliznech, E.A.; Bakhshinyan, V.V.; Polyakov, A.V.; Tavartkiladze, G.A. Phenotype in a patient with p.D50N mutation in GJB2 gene resemble both KID and Clouston syndromes. Int. J. Pediatric Otorhinolaryngol. 2016, 81, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Mazereeuw-Hautier, J.; Bitoun, E.; Chevrant-Breton, J.; Man, S.Y.; Bodemer, C.; Prins, C.; Antille, C.; Saurat, J.H.; Atherton, D.; Harper, J.I.; et al. Keratitis-ichthyosis-deafness syndrome: Disease expression and spectrum of connexin 26 (GJB2) mutations in 14 patients. Br. J. Dermatol. 2007, 156, 1015–1019. [Google Scholar] [CrossRef]
- Serrano Castro, P.J.; Naranjo Fernandez, C.; Quiroga Subirana, P.; Payan Ortiz, M. Vohwinkel Syndrome secondary to missense mutation D66H in GJB2 gene (connexin 26) can include epileptic manifestations. Seizure 2010, 19, 129–131. [Google Scholar] [CrossRef][Green Version]
- Uyguner, O.; Tukel, T.; Baykal, C.; Eris, H.; Emiroglu, M.; Hafiz, G.; Ghanbari, A.; Baserer, N.; Yuksel-Apak, M.; Wollnik, B. The novel R75Q mutation in the GJB2 gene causes autosomal dominant hearing loss and palmoplantar keratoderma in a Turkish family. Clin. Genet. 2002, 62, 306–309. [Google Scholar] [CrossRef]
- Jan, A.Y.; Amin, S.; Ratajczak, P.; Richard, G.; Sybert, V.P. Genetic heterogeneity of KID syndrome: Identification of a Cx30 gene (GJB6) mutation in a patient with KID syndrome and congenital atrichia. J. Investig. Dermatol. 2004, 122, 1108–1113. [Google Scholar] [CrossRef]
- Venkatesh, M.D.; Moorchung, N.; Puri, B. Genetics of non syndromic hearing loss. Med. J. Armed. Forces India 2015, 71, 363–368. [Google Scholar] [CrossRef]
- Estivill, X.; Fortina, P.; Surrey, S.; Rabionet, R.; Melchionda, S.; D’Agruma, L.; Mansfield, E.; Rappaport, E.; Govea, N.; Milà, M.; et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet (Lond. Engl.) 1998, 351, 394–398. [Google Scholar] [CrossRef]
- Pandya, A.; Xia, X.J.; Erdenetungalag, R.; Amendola, M.; Landa, B.; Radnaabazar, J.; Dangaasuren, B.; Van Tuyle, G.; Nance, W.E. Heterogenous point mutations in the mitochondrial tRNA Ser(UCN) precursor coexisting with the A1555G mutation in deaf students from Mongolia. Am. J. Hum. Genet. 1999, 65, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.O.; Pecotte, J.K. Additional case report of the DOOR syndrome. Am. J. Med. Genet. 1984, 19, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, O.; Mazas, J.J.; Ortíz, I.; de DeMatos, F. The deafness, onycho-osteo-dystrophy, mental retardation syndrome. Two new cases. Human Genet. 1981, 58, 228–230. [Google Scholar] [CrossRef]
- Santos, M.; Reis-Rego, Â.; Coutinho, M.; Almeida, E.S.C. DOOR syndrome: A case report and its embryological basis. Int. J. Pediatric Otorhinolaryngol. 2019, 117, 57–60. [Google Scholar] [CrossRef]
- Bakhchane, A.; Charif, M.; Salime, S.; Boulouiz, R.; Nahili, H.; Roky, R.; Lenaers, G.; Barakat, A. Recessive TBC1D24 Mutations Are Frequent in Moroccan Non-Syndromic Hearing Loss Pedigrees. PLoS ONE 2015, 10, e0138072. [Google Scholar] [CrossRef]
- Balestrini, S.; Milh, M.; Castiglioni, C.; Lüthy, K.; Finelli, M.J.; Verstreken, P.; Cardon, A.; Stražišar, B.G.; Holder, J.L., Jr.; Lesca, G.; et al. TBC1D24 genotype-phenotype correlation: Epilepsies and other neurologic features. Neurology 2016, 87, 77–85. [Google Scholar] [CrossRef]
- Banuelos, E.; Ramsey, K.; Belnap, N.; Krishnan, M.; Balak, C.; Szelinger, S.; Siniard, A.L.; Russell, M.; Richholt, R.; De Both, M.; et al. Case Report: Novel mutations in TBC1D24 are associated with autosomal dominant tonic-clonic and myoclonic epilepsy and recessive Parkinsonism, psychosis, and intellectual disability. F1000Research 2017, 6, 553. [Google Scholar] [CrossRef]
- Rehman, A.U.; Friedman, T.B.; Griffith, A.J. Unresolved questions regarding human hereditary deafness. Oral Dis. 2017, 23, 551–558. [Google Scholar] [CrossRef]
- Gao, F.-J.; Hu, F.-Y.; Xu, P.; Qi, Y.-H.; Li, J.-K.; Zhang, Y.-J.; Chen, F.; Chang, Q.; Song, F.; Shen, S.-M.; et al. Expanding the clinical and genetic spectrum of Heimler syndrome. Orphanet J. Rare Dis. 2019, 14, 290. [Google Scholar] [CrossRef]
- Levesque, S.; Morin, C.; Guay, S.P.; Villeneuve, J.; Marquis, P.; Yik, W.Y.; Jiralerspong, S.; Bouchard, L.; Steinberg, S.; Hacia, J.G.; et al. A founder mutation in the PEX6 gene is responsible for increased incidence of Zellweger syndrome in a French Canadian population. BMC Med. Genet. 2012, 13, 72. [Google Scholar] [CrossRef]
- Fong, E.E. Iliac horns (symmetrical bilateral central posterior iliac processes). Radiology 1946, 47, 517. [Google Scholar] [CrossRef]
- Haras, B.; Vulpoi, F.; Onose, G. A case of nail-patella syndrome associated with thyrotoxicosis. J. Med. life 2012, 5, 126–129. [Google Scholar]
- O’Neill, S.; Murphy, C.; McElwain, J. A hypoplastic patella fracture in nail patella syndrome: A case report. J. Med. Case Rep. 2012, 6, 196. [Google Scholar] [CrossRef]
- Towers, A.L.; Clay, C.A.; Sereika, S.M.; McIntosh, I.; Greenspan, S.L. Skeletal integrity in patients with nail patella syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1961–1965. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dunston, J.A.; Lin, S.; Park, J.W.; Malbroux, M.; McIntosh, I. Phenotype severity and genetic variation at the disease locus: An investigation of nail dysplasia in the nail patella syndrome. Ann. Hum. Genet. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dunston, J.A.; Reimschisel, T.; Ding, Y.Q.; Sweeney, E.; Johnson, R.L.; Chen, Z.F.; McIntosh, I. A neurological phenotype in nail patella syndrome (NPS) patients illuminated by studies of murine Lmx1b expression. Eur. J. Hum. Genet. 2005, 13, 330–335. [Google Scholar] [CrossRef]
- Aldahmesh, M.A.; Mohamed, J.Y.; Alkuraya, F.S. A novel mutation in PRDM5 in brittle cornea syndrome. Clin. Genet. 2012, 81, 198–199. [Google Scholar] [CrossRef]
- Burkitt Wright, E.M.M.; Spencer, H.L.; Daly, S.B.; Manson, F.D.C.; Zeef, L.A.H.; Urquhart, J.; Zoppi, N.; Bonshek, R.; Tosounidis, I.; Mohan, M.; et al. Mutations in PRDM5 in brittle cornea syndrome identify a pathway regulating extracellular matrix development and maintenance. Am. J. Hum. Genet. 2011, 88, 767–777. [Google Scholar] [CrossRef]
- Porter, L.F.; Gallego-Pinazo, R.; Keeling, C.L.; Kamieniorz, M.; Zoppi, N.; Colombi, M.; Giunta, C.; Bonshek, R.; Manson, F.D.; Black, G.C. Bruch’s membrane abnormalities in PRDM5-related brittle cornea syndrome. Orphanet J. Rare Dis. 2015, 10, 145. [Google Scholar] [CrossRef]
- Wan, Q.; Tang, J.; Han, Y.; Xiao, Q.; Deng, Y. Brittle cornea syndrome: A case report and review of the literature. BMC Ophthalmol. 2018, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Malfait, F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin. Genet. 2012, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Syx, D.; Van Damme, T.; Symoens, S.; Maiburg, M.C.; van de Laar, I.; Morton, J.; Suri, M.; Del Campo, M.; Hausser, I.; Hermanns-Lê, T.; et al. Genetic heterogeneity and clinical variability in musculocontractural Ehlers-Danlos syndrome caused by impaired dermatan sulfate biosynthesis. Hum. Mutat. 2015, 36, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, T.; Eguchi, J.; Hamasaki, Y.; Doi, T.; Kinoshita, E.; Matsumoto, T.; Abe, K.; Ohtani, Y.; Moriuchi, H. Hearing impairment, undescended testis, circumferential skin creases, and mental handicap (HITCH) syndrome: A case report. Am. J. Med. Genet. A 2004, 125A, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Babbitt, S.E.; Sutherland, M.C.; San Francisco, B.; Mendez, D.L.; Kranz, R.G. Mitochondrial cytochrome c biogenesis: No longer an enigma. Trends Biochem. Sci. 2015, 40, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Margari, L.; Colonna, A.; Craig, F.; Gentile, M.; Giannella, G.; Lamanna, A.L.; Legrottaglie, A.R. Microphthalmia with Linear Skin Defects (MLS) associated with Autism Spectrum Disorder (ASD) in a patient with Familial 12.9Mb Terminal Xp deletion. BMC Pediatrics 2014, 14, 220. [Google Scholar] [CrossRef]
- Sharma, V.M.; Ruiz de Luzuriaga, A.M.; Waggoner, D.; Greenwald, M.; Stein, S.L. Microphthalmia with linear skin defects: A case report and review. Pediatric Dermatol. 2008, 25, 548–552. [Google Scholar] [CrossRef]
- Van Rahden, V.A.; Fernandez-Vizarra, E.; Alawi, M.; Brand, K.; Fellmann, F.; Horn, D.; Zeviani, M.; Kutsche, K. Mutations in NDUFB11, encoding a complex I component of the mitochondrial respiratory chain, cause microphthalmia with linear skin defects syndrome. Am. J. Hum. Genet. 2015, 96, 640–650. [Google Scholar] [CrossRef]
- Wimplinger, I.; Morleo, M.; Rosenberger, G.; Iaconis, D.; Orth, U.; Meinecke, P.; Lerer, I.; Ballabio, A.; Gal, A.; Franco, B.; et al. Mutations of the mitochondrial holocytochrome c-type synthase in X-linked dominant microphthalmia with linear skin defects syndrome. Am. J. Hum. Genet. 2006, 79, 878–889. [Google Scholar] [CrossRef]
- Cuisset, L.; Jeru, I.; Dumont, B.; Fabre, A.; Cochet, E.; Le Bozec, J.; Delpech, M.; Amselem, S.; Touitou, I. Mutations in the autoinflammatory cryopyrin-associated periodic syndrome gene: Epidemiological study and lessons from eight years of genetic analysis in France. Ann. Rheum. Dis. 2011, 70, 495–499. [Google Scholar] [CrossRef]
- Kümmerle-Deschner, J.B. Cryopyrin-associated periodic syndrome. Z. Rheumatol. 2012, 71, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Brewer, C.C.; Zalewski, C.; King, K.A.; Butman, J.A.; Plass, N.; Henderson, C.; Goldbach-Mansky, R.; Kim, H.J. Cryopyrin-associated periodic syndromes: Otolaryngologic and audiologic manifestations. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol. Head Neck Surg. 2011, 145, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle-Deschner, J.B.; Ozen, S.; Tyrrell, P.N.; Kone-Paut, I.; Goldbach-Mansky, R.; Lachmann, H.; Blank, N.; Hoffman, H.M.; Weissbarth-Riedel, E.; Hugle, B.; et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann. Rheum. Dis. 2017, 76, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; He, L.; Pang, X.; Wang, X.; Yang, T.; Wu, H. NLRP3 Is Expressed in the Spiral Ganglion Neurons and Associated with Both Syndromic and Nonsyndromic Sensorineural Deafness. Neural Plast. 2016, 2016, 3018132. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Kawashima, Y.; Kurima, K.; Chae, J.J.; Ross, A.M.; Pinto-Patarroyo, G.; Patel, S.K.; Muskett, J.A.; Ratay, J.S.; Chattaraj, P.; et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E7766–e7775. [Google Scholar] [CrossRef]
- Nakanishi, H.; Prakash, P.; Ito, T.; Kim, H.J.; Brewer, C.C.; Harrow, D.; Roux, I.; Hosokawa, S.; Griffith, A.J. Genetic Hearing Loss Associated with Autoinflammation. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Barisic, I.; Tokic, V.; Loane, M.; Bianchi, F.; Calzolari, E.; Garne, E.; Wellesley, D.; Dolk, H. Descriptive epidemiology of Cornelia de Lange syndrome in Europe. Am. J. Med. Genet. A 2008, 146a, 51–59. [Google Scholar] [CrossRef]
- Borck, G.; Zarhrate, M.; Bonnefont, J.P.; Munnich, A.; Cormier-Daire, V.; Colleaux, L. Incidence and clinical features of X-linked Cornelia de Lange syndrome due to SMC1L1 mutations. Hum. Mutat. 2007, 28, 205–206. [Google Scholar] [CrossRef]
- Harris, C.M.; Shawkat, F.; Russell-Eggitt, I.; Wilson, J.; Taylor, D. Intermittent horizontal saccade failure (‘ocular motor apraxia’) in children. Br. J. Ophthalmol. 1996, 80, 151–158. [Google Scholar] [CrossRef]
- Tonkin, E.T.; Wang, T.J.; Lisgo, S.; Bamshad, M.J.; Strachan, T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat. Genet. 2004, 36, 636–641. [Google Scholar] [CrossRef]
- Deardorff, M.A.; Bando, M.; Nakato, R.; Watrin, E.; Itoh, T.; Minamino, M.; Saitoh, K.; Komata, M.; Katou, Y.; Clark, D.; et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 2012, 489, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, M.A.; Kaur, M.; Yaeger, D.; Rampuria, A.; Korolev, S.; Pie, J.; Gil-Rodriguez, C.; Arnedo, M.; Loeys, B.; Kline, A.D.; et al. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de Lange syndrome with predominant mental retardation. Am. J. Hum. Genet. 2007, 80, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, M.A.; Wilde, J.J.; Albrecht, M.; Dickinson, E.; Tennstedt, S.; Braunholz, D.; Monnich, M.; Yan, Y.; Xu, W.; Gil-Rodriguez, M.C.; et al. RAD21 mutations cause a human cohesinopathy. Am. J. Hum. Genet. 2012, 90, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Krantz, I.D.; McCallum, J.; DeScipio, C.; Kaur, M.; Gillis, L.A.; Yaeger, D.; Jukofsky, L.; Wasserman, N.; Bottani, A.; Morris, C.A.; et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 2004, 36, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, M.A.; Porter, N.J.; Christianson, D.W. Structural aspects of HDAC8 mechanism and dysfunction in Cornelia de Lange syndrome spectrum disorders. Protein Sci. 2016, 25, 1965–1976. [Google Scholar] [CrossRef]
- Kismet, E.; Köseoglu, V.; Atay, A.A.; Deveci, S.; Demirkaya, E.; Tuncer, K. Sinus histiocytosis with massive lymphadenopathy in three brothers. Pediatrics Int. 2005, 47, 473–476. [Google Scholar] [CrossRef]
- Shen, J.; Scheffer, D.I.; Kwan, K.Y.; Corey, D.P. SHIELD: An integrative gene expression database for inner ear research. Database (Oxf.) 2015, 2015, bav071. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Motavaf, M.; Soveizi, M.; Maleki, M.; Mahdieh, N. MYO15A splicing mutations in hearing loss: A review literature and report of a novel mutation. Int. J. Pediatric Otorhinolaryngol. 2017, 96, 35–38. [Google Scholar] [CrossRef]
- Fang, D.; Tsuji, Y.; Setaluri, V. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 2002, 30, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Fisher, D.E. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab. Investig. 2017, 97, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Murisier, F.; Guichard, S.; Beermann, F. The tyrosinase enhancer is activated by Sox10 and Mitf in mouse melanocytes. Pigment. Cell Res. 2007, 20, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, H.; Chen, H.; Mei, L.; He, C.; Jiang, L.; Li, J.-D.; Feng, Y. Functional analysis of MITF gene mutations associated with Waardenburg syndrome type 2. FEBS Lett. 2012, 586, 4126–4131. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-O.; Levorse, J.M.; Shin, M.K. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev. Biol. 2003, 259, 162–175. [Google Scholar] [CrossRef]
- Ichihashi, M.; Fujiwara, Y.; Uehara, Y.; Matsumoto, A. A mild form of xeroderma pigmentosum assigned to complementation group G and its repair heterogeneity. J. Investig. Dermatol 1985, 85, 284–287. [Google Scholar] [CrossRef]
- Pannone, L.; Bocchinfuso, G.; Flex, E.; Rossi, C.; Baldassarre, G.; Lissewski, C.; Pantaleoni, F.; Consoli, F.; Lepri, F.; Magliozzi, M.; et al. Structural, Functional, and Clinical Characterization of a Novel PTPN11 Mutation Cluster Underlying Noonan Syndrome. Hum. Mutat. 2017, 38, 451–459. [Google Scholar] [CrossRef]
- Brennan, D.F.; Dar, A.C.; Hertz, N.T.; Chao, W.C.; Burlingame, A.L.; Shokat, K.M.; Barford, D. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 2011, 472, 366–369. [Google Scholar] [CrossRef]
- Lavoie, H.; Sahmi, M.; Maisonneuve, P.; Marullo, S.A.; Thevakumaran, N.; Jin, T.; Kurinov, I.; Sicheri, F.; Therrien, M. MEK drives BRAF activation through allosteric control of KSR proteins. Nature 2018, 554, 549–553. [Google Scholar] [CrossRef]
- Stephens, R.M.; Sithanandam, G.; Copeland, T.D.; Kaplan, D.R.; Rapp, U.R.; Morrison, D.K. 95-kilodalton B-Raf serine/threonine kinase: Identification of the protein and its major autophosphorylation site. Mol. Cell. Biol. 1992, 12, 3733–3742. [Google Scholar] [CrossRef][Green Version]
- Motegi, S.I.; Yokoyama, Y.; Ogino, S.; Yamada, K.; Uchiyama, A.; Perera, B.; Takeuchi, Y.; Ohnishi, H.; Ishikawa, O. New insights into the pathogenesis of multiple lentigines in LEOPARD syndrome with PTPN11 gene mutation. J. Dermatol. Sci. 2016, 84, e142. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Ahn, J.; Hong, S.H.; Kim, E.; Chung, W.-H.; Cho, Y.-S.; Moon, I.J. Case Series Outcomes of Cochlear Implantation in Children with Noonan Syndrome. Korean J. Otorhinolaryngol. -Head Neck Surg. 2019, 62. [Google Scholar] [CrossRef]
- Moriwaki, S.; Kanda, F.; Hayashi, M.; Yamashita, D.; Sakai, Y.; Nishigori, C.; Xeroderma Pigmentosum Clinical Practice Guidelines Revision Committee. Xeroderma pigmentosum clinical practice guidelines. J. Dermatol. 2017, 44, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, K.Y.; Park, S.G.; Yu, S.N.; Kim, Y.W.; Nam, H.W.; An, H.H.; Kim, Y.W.; Ahn, S.C. Mitochondrial ROS activates ERK/autophagy pathway as a protected mechanism against deoxypodophyllotoxin-induced apoptosis. Oncotarget 2017, 8, 111581–111596. [Google Scholar] [CrossRef] [PubMed]
- Rosania, G.R. Mitochondria Give Cells a Tan. Chem. Biol. 2005, 12, 412–413. [Google Scholar] [CrossRef]
- Snyder, J.R.; Hall, A.; Ni-Komatsu, L.; Khersonsky, S.M.; Chang, Y.-T.; Orlow, S.J. Dissection of Melanogenesis with Small Molecules Identifies Prohibitin as a Regulator. Chem. Biol. 2005, 12, 477–484. [Google Scholar] [CrossRef]
- Andera, L.; Wasylyk, B. Transcription Abnormalities Potentiate Apoptosis of Normal Human Fibroblasts. Mol. Med. 1997, 3, 852–863. [Google Scholar] [CrossRef]
- Dumaz, N.; van Kranen, H.J.; de Vries, A.; Berg, R.J.; Wester, P.W.; van Kreijl, C.F.; Sarasin, A.; Daya-Grosjean, L.; de Gruijl, F.R. The role of UV-B light in skin carcinogenesis through the analysis of p53 mutations in squamous cell carcinomas of hairless mice. Carcinogenesis 1997, 18, 897–904. [Google Scholar] [CrossRef]
- Ljungman, M.; Zhang, F.; Chen, F.; Rainbow, A.J.; McKay, B.C. Inhibition of RNA polymerase II as a trigger for the p53 response. Oncogene 1999, 18, 583–592. [Google Scholar] [CrossRef]
- McKay, B.C.; Becerril, C.; Ljungman, M. P53 plays a protective role against UV- and cisplatin-induced apoptosis in transcription-coupled repair proficient fibroblasts. Oncogene 2001, 20, 6805–6808. [Google Scholar] [CrossRef]
- Wilson, B.T.; Stark, Z.; Sutton, R.E.; Danda, S.; Ekbote, A.V.; Elsayed, S.M.; Gibson, L.; Goodship, J.A.; Jackson, A.P.; Keng, W.T.; et al. The Cockayne Syndrome Natural History (CoSyNH) study: Clinical findings in 102 individuals and recommendations for care. Genet. Med. 2016, 18, 483–493. [Google Scholar] [CrossRef]
- Viana, L.M.; Seyyedi, M.; Brewer, C.C.; Zalewski, C.; DiGiovanna, J.J.; Tamura, D.; Totonchy, M.; Kraemer, K.H.; Nadol, J.B., Jr. Histopathology of the inner ear in patients with xeroderma pigmentosum and neurologic degeneration. Otol. Neurotol. 2013, 34, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Hervé, J.C.; Derangeon, M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013, 352, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, F.J.; Rodríguez-Ballesteros, M.; Alvarez, A.; Hutchin, T.; Leonardi, E.; de Oliveira, C.A.; Azaiez, H.; Brownstein, Z.; Avenarius, M.R.; Marlin, S.; et al. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J. Med. Genet. 2005, 42, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.M.; Goodenough, D.A. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998, 8, 477–483. [Google Scholar] [CrossRef]
- Coutinho, P.; Qiu, C.; Frank, S.; Tamber, K.; Becker, D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol. Int. 2003, 27, 525–541. [Google Scholar] [CrossRef]
- Lucke, T.; Choudhry, R.; Thom, R.; Selmer, I.S.; Burden, A.D.; Hodgins, M.B. Upregulation of connexin 26 is a feature of keratinocyte differentiation in hyperproliferative epidermis, vaginal epithelium, and buccal epithelium. J. Investig. Dermatol. 1999, 112, 354–361. [Google Scholar] [CrossRef]
- Rouan, F.; White, T.W.; Brown, N.; Taylor, A.M.; Lucke, T.W.; Paul, D.L.; Munro, C.S.; Uitto, J.; Hodgins, M.B.; Richard, G. trans-dominant inhibition of connexin-43 by mutant connexin-26: Implications for dominant connexin disorders affecting epidermal differentiation. J. Cell Sci. 2001, 114, 2105–2113. [Google Scholar]
- Lilly, E.; Sellitto, C.; Milstone, L.M.; White, T.W. Connexin channels in congenital skin disorders. Semin. Cell Dev. Biol. 2016, 50, 4–12. [Google Scholar] [CrossRef]
- Wang, C.M.; Lincoln, J.; Cook, J.E.; Becker, D.L. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes 2007, 56, 2809–2817. [Google Scholar] [CrossRef]
- Beltramello, M.; Piazza, V.; Bukauskas, F.F.; Pozzan, T.; Mammano, F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell Biol. 2005, 7, 63–69. [Google Scholar] [CrossRef]
- Jagger, D.J.; Forge, A. Connexins and gap junctions in the inner ear--it’s not just about K⁺ recycling. Cell Tissue Res. 2015, 360, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Salmon, M.; Regnault, B.; Cayet, N.; Caille, D.; Demuth, K.; Hardelin, J.P.; Janel, N.; Meda, P.; Petit, C. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc. Natl. Acad. Sci. USA 2007, 104, 6229–6234. [Google Scholar] [CrossRef] [PubMed]
- Common, J.E.; O’Toole, E.A.; Leigh, I.M.; Thomas, A.; Griffiths, W.A.; Venning, V.; Grabczynska, S.; Peris, Z.; Kansky, A.; Kelsell, D.P. Clinical and genetic heterogeneity of erythrokeratoderma variabilis. J. Investig. Dermatol. 2005, 125, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Chouk, C.; Litaiem, N. Erythrokeratodermia Variabilis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Li, T.C.; Wang, W.H.; Li, C.; Yang, J.J. Association between mutations in the gap junction β4 gene and nonsyndromic hearing loss: Genotype-phenotype correlation patterns. Mol. Med. Rep. 2015, 11, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Wang, W.H.; Lin, Y.C.; Weng, H.H.; Yang, J.T.; Hwang, C.F.; Wu, C.M.; Li, S.Y. Prospective variants screening of connexin genes in children with hearing impairment: Genotype/phenotype correlation. Human Genet. 2010, 128, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kooshavar, D.; Tabatabaiefar, M.A.; Farrokhi, E.; Abolhasani, M.; Noori-Daloii, M.R.; Hashemzadeh-Chaleshtori, M. Digenic inheritance in autosomal recessive non-syndromic hearing loss cases carrying GJB2 heterozygote mutations: Assessment of GJB4, GJA1, and GJC3. Int. J. Pediatric Otorhinolaryngol. 2013, 77, 189–193. [Google Scholar] [CrossRef]
- Laleh, M.A.; Naseri, M.; Zonouzi, A.A.P.; Zonouzi, A.P.; Masoudi, M.; Ahangari, N.; Shams, L.; Nejatizadeh, A. Diverse pattern of gap junction β-2 and gap junction β-4 genes mutations and lack of contribution of DFNB21, DFNB24, DFNB29, and DFNB42 loci in autosomal recessive nonsyndromic hearing loss patients in Hormozgan, Iran. J. Res. Med. Sci. 2017, 22, 99. [Google Scholar] [CrossRef]
- Church, V.L.; Francis-West, P. Wnt signalling during limb development. Int. J. Dev. Biol. 2002, 46, 927–936. [Google Scholar]
- Dobrowolski, R.; Vick, P.; Ploper, D.; Gumper, I.; Snitkin, H.; Sabatini, D.D.; De Robertis, E.M. Presenilin deficiency or lysosomal inhibition enhances Wnt signaling through relocalization of GSK3 to the late-endosomal compartment. Cell Rep. 2012, 2, 1316–1328. [Google Scholar] [CrossRef]
- Geetha-Loganathan, P.; Nimmagadda, S.; Scaal, M. Wnt signaling in limb organogenesis. Organogenesis 2008, 4, 109–115. [Google Scholar] [CrossRef]
- Corbett, M.A.; Bahlo, M.; Jolly, L.; Afawi, Z.; Gardner, A.E.; Oliver, K.L.; Tan, S.; Coffey, A.; Mulley, J.C.; Dibbens, L.M.; et al. A focal epilepsy and intellectual disability syndrome is due to a mutation in TBC1D24. Am. J. Hum. Genet. 2010, 87, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Falace, A.; Filipello, F.; La Padula, V.; Vanni, N.; Madia, F.; De Pietri Tonelli, D.; de Falco, F.A.; Striano, P.; Dagna Bricarelli, F.; Minetti, C.; et al. TBC1D24, an ARF6-interacting protein, is mutated in familial infantile myoclonic epilepsy. Am. J. Hum. Genet. 2010, 87, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Hwang, Y.-S.; Lee, M.; Sun, J.; Cho, H.J.; Knapik, L.; Daar, I.O. TBC1d24-ephrinB2 interaction regulates contact inhibition of locomotion in neural crest cell migration. Nat. Commun. 2018, 9, 3491. [Google Scholar] [CrossRef] [PubMed]
- Azaiez, H.; Booth, K.T.; Bu, F.; Huygen, P.; Shibata, S.B.; Shearer, A.E.; Kolbe, D.; Meyer, N.; Black-Ziegelbein, E.A.; Smith, R.J. TBC1D24 mutation causes autosomal-dominant nonsyndromic hearing loss. Hum. Mutat. 2014, 35, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Imamura, A.; Shimozawa, N.; Suzuki, Y.; Zhang, Z.; Tsukamoto, T.; Fujiki, Y.; Orii, T.; Osumi, T.; Kondo, N. Restoration of biochemical function of the peroxisome in the temperature-sensitive mild forms of peroxisome biogenesis disorder in humans. Brain Dev. 2000, 22, 8–12. [Google Scholar] [CrossRef]
- Mardones, P.; Hetz, C. Peroxisomes Get Loud: A Redox Antidote to Hearing Loss. Cell 2015, 163, 790–791. [Google Scholar] [CrossRef]
- Wong, A.C.; Ryan, A.F. Mechanisms of sensorineural cell damage, death and survival in the cochlea. Front. Aging Neurosci. 2015, 7, 58. [Google Scholar] [CrossRef]
- Nichols, D.H.; Pauley, S.; Jahan, I.; Beisel, K.W.; Millen, K.J.; Fritzsch, B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008, 334, 339–358. [Google Scholar] [CrossRef]
- Cowin, A.J.; Adams, D.; Geary, S.M.; Wright, M.D.; Jones, J.C.; Ashman, L.K. Wound healing is defective in mice lacking tetraspanin CD151. J. Investig. Dermatol. 2006, 126, 680–689. [Google Scholar] [CrossRef]
- Liu, W.; Atturo, F.; Aldaya, R.; Santi, P.; Cureoglu, S.; Obwegeser, S.; Glueckert, R.; Pfaller, K.; Schrott-Fischer, A.; Rask-Andersen, H. Macromolecular organization and fine structure of the human basilar membrane—RELEVANCE for cochlear implantation. Cell Tissue Res. 2015, 360, 245–262. [Google Scholar] [CrossRef]
- Pillers, D.-A.M.; Kempton, J.B.; Duncan, N.M.; Pang, J.; Dwinnell, S.J.; Trune, D.R. Hearing loss in the laminin-deficient dy mouse model of congenital muscular dystrophy. Mol. Genet. Metab. 2002, 76, 217–224. [Google Scholar] [CrossRef]
- Rohrbach, M.; Spencer, H.L.; Porter, L.F.; Burkitt-Wright, E.M.; Bürer, C.; Janecke, A.; Bakshi, M.; Sillence, D.; Al-Hussain, H.; Baumgartner, M.; et al. ZNF469 frequently mutated in the brittle cornea syndrome (BCS) is a single exon gene possibly regulating the expression of several extracellular matrix components. Mol. Genet. Metab. 2013, 109, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.G.; Honnens de Lichtenberg, K.; Carrara, M.; Hans, W.; Wuelling, M.; Mentz, B.; Multhaupt, H.A.; Fog, C.K.; Jensen, K.T.; Rappsilber, J.; et al. Prdm5 regulates collagen gene transcription by association with RNA polymerase II in developing bone. PLoS Genet. 2012, 8, e1002711. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, S.; Kosho, T.; Yamada, S.; Sugahara, K. Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders. Pharmaceuticals 2017, 10, 34. [Google Scholar] [CrossRef]
- Müller, T.; Mizumoto, S.; Suresh, I.; Komatsu, Y.; Vodopiutz, J.; Dundar, M.; Straub, V.; Lingenhel, A.; Melmer, A.; Lechner, S.; et al. Loss of dermatan sulfate epimerase (DSE) function results in musculocontractural Ehlers-Danlos syndrome. Hum. Mol. Genet. 2013, 22, 3761–3772. [Google Scholar] [CrossRef]
- Slepecky, N.B.; Henderson, C.G.; Saha, S. Post-translational modifications of tubulin suggest that dynamic microtubules are present in sensory cells and stable microtubules are present in supporting cells of the mammalian cochlea. Hear. Res. 1995, 91, 136–147. [Google Scholar] [CrossRef]
- Cai, Q.; Du, X.; Zhou, B.; Cai, C.; Kermany, M.H.; Yoo, T. Induction of tolerance by oral administration of β-tubulin in an animal model of autoimmune inner ear disease. ORL J. Otorhinolaryngol. Relat. Spec. 2009, 71, 135–141. [Google Scholar] [CrossRef]
- Du, X.; Yoo, T.; Mora, R. Distribution of β-tubulin in guinea pig inner ear. ORL J. Otorhinolaryngol. Relat. Spec. 2003, 65, 7–16. [Google Scholar] [CrossRef]
- Riente, L.; Bongiorni, F.; Nacci, A.; Migliorini, P.; Segnini, G.; Delle Sedie, A.; Ursino, F.; Tommasi, S.; Fattori, B. Antibodies to inner ear antigens in Meniere’s disease. Clin. Exp. Immunol. 2004, 135, 159–163. [Google Scholar] [CrossRef]
- Yoo, T.J.; Du, X.; Kwon, S.S. Molecular mechanism of autoimmune hearing loss. Acta Otolaryngol. Suppl. 2002, 122, 3–9. [Google Scholar] [CrossRef]
- Yoo, T.J.; Shea, J., Jr.; Ge, X.; Kwon, S.S.; Yazawa, Y.; Sener, O.; Mora, F.; Mora, R.; Mora, M.; Barbieri, M.; et al. Presence of autoantibodies in the sera of Meniere’s disease. Ann. Otol. Rhinol. Laryngol. 2001, 110, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Kermany, M.H.; Cai, Q.; Cai, C.; Zhou, Y.; Nair, U.; Liu, W.; Yoo, T.J. Experimental autoimmune hearing loss is exacerbated in IL-10-deficient mice and reversed by IL-10 gene transfer. Gene Ther. 2012, 19, 228–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, B.; Kermany, M.H.; Glickstein, J.; Cai, Q.; Cai, C.; Zhou, Y.; Nair, U.; Kim, J.W.; Kim, P.; Liu, W.; et al. Murine autoimmune hearing loss mediated by CD4+ T cells specific for β-tubulin. Clin. Immunol. 2011, 138, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.J.; Tanaka, H.; Kwon, S.S.; Mora, F.; Mora, M.; Yazawa, Y.; Suzuki, M.; Kitajima, K. β-Tubulin as an autoantigen for autoimmune inner ear disease. Int. Congr. Ser. 2003, 1240, 1207–1210. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol Pathol 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Scheffel, J.; Mahnke, N.A.; Hofman, Z.L.M.; Maat, S.D.; Wu, J.; Bonnekoh, H.; Pengelly, R.J.; Ennis, S.; Holloway, J.W.; Kirchner, M.; et al. Cold-induced urticarial autoinflammatory syndrome related to factor XII activation. Nat. Commun. 2020, 11, 179. [Google Scholar] [CrossRef]
- Vambutas, A.; Lesser, M.; Mullooly, V.; Pathak, S.; Zahtz, G.; Rosen, L.; Goldofsky, E. Early efficacy trial of anakinra in corticosteroid-resistant autoimmune inner ear disease. J. Clin. Investig. 2014, 124, 4115–4122. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kambe, N.; Saito, M.; Nishikomori, R.; Nishikomiri, R.; Kim, Y.-G.; Murakami, M.; Nunez, G.; Matsue, H. Mast cells mediate neutrophil recruitment and vascular leakage through the NLRP3 inflammasome in histamine-independent urticaria. J. Exp. Med. 2009, 206, 1037–1046. [Google Scholar] [CrossRef]
- Hirose, K.; Discolo, C.M.; Keasler, J.R.; Ransohoff, R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005, 489, 180–194. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Galán, J.E.; Askenase, P.W.; et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).