Strawberry FaWRKY25 Transcription Factor Negatively Regulated the Resistance of Strawberry Fruits to Botrytis cinerea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Inoculating B. cinerea in the Fruits That Received Different Treatments
2.3. Measuring JA Contents
2.4. FaWRKY25 Gene Characteristics, Cloning, and Vector Construction
2.5. Transient Gene Transformation Methods
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
3.1. FaWRKY25 Protein Sequence Alignment and Phylogenic Tree Analysis
3.2. Effect of JA Concentration on B. cinerea in Strawberries
3.3. Analysis of the FaWRKY25 Expression and JA Concentration in Strawberry Fruit alongside Maturation and in Response to B. cinerea
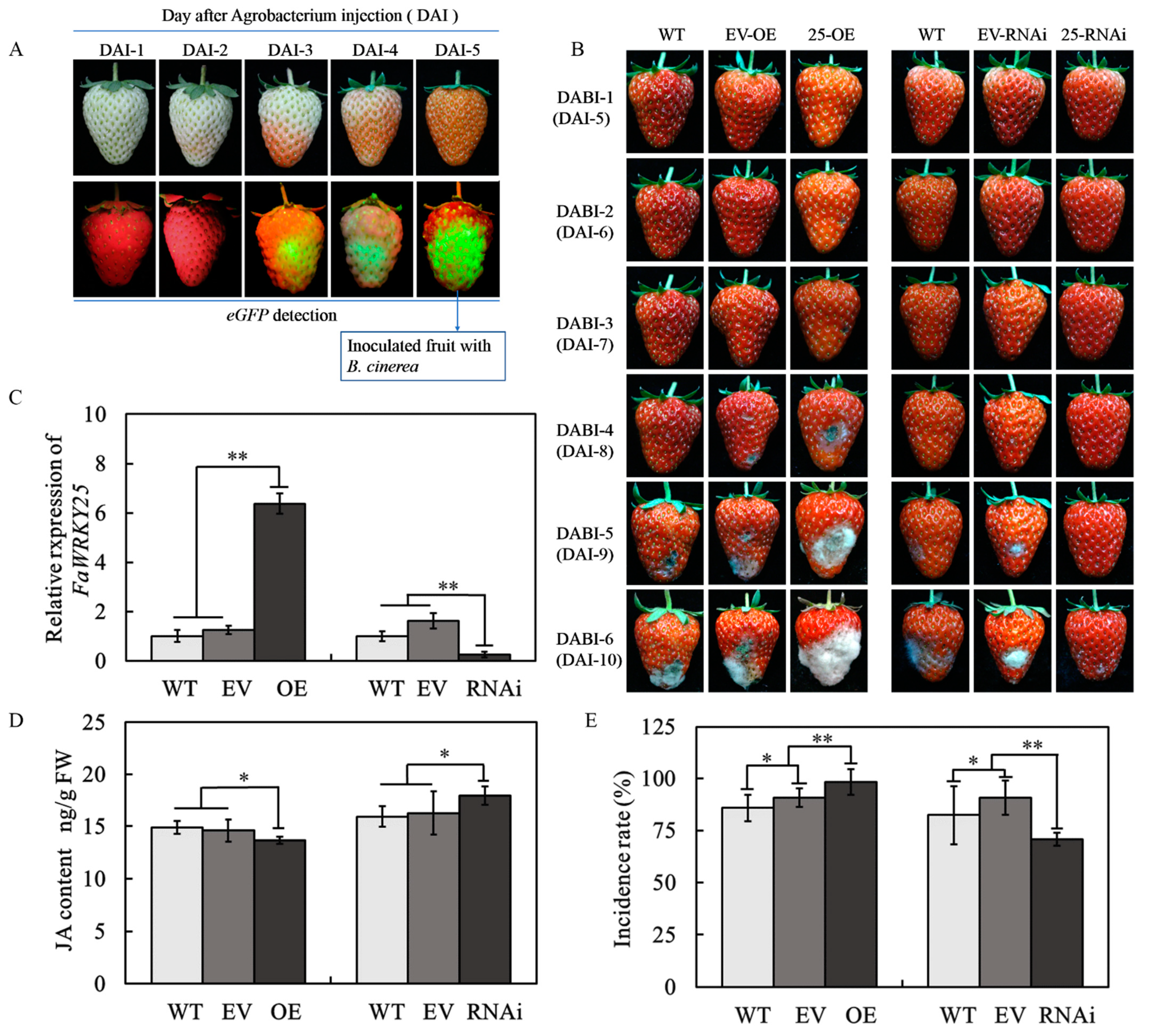
3.4. Regulatory Effect of Transient FaWRKY25 Expression in Strawberries on B. cinerea
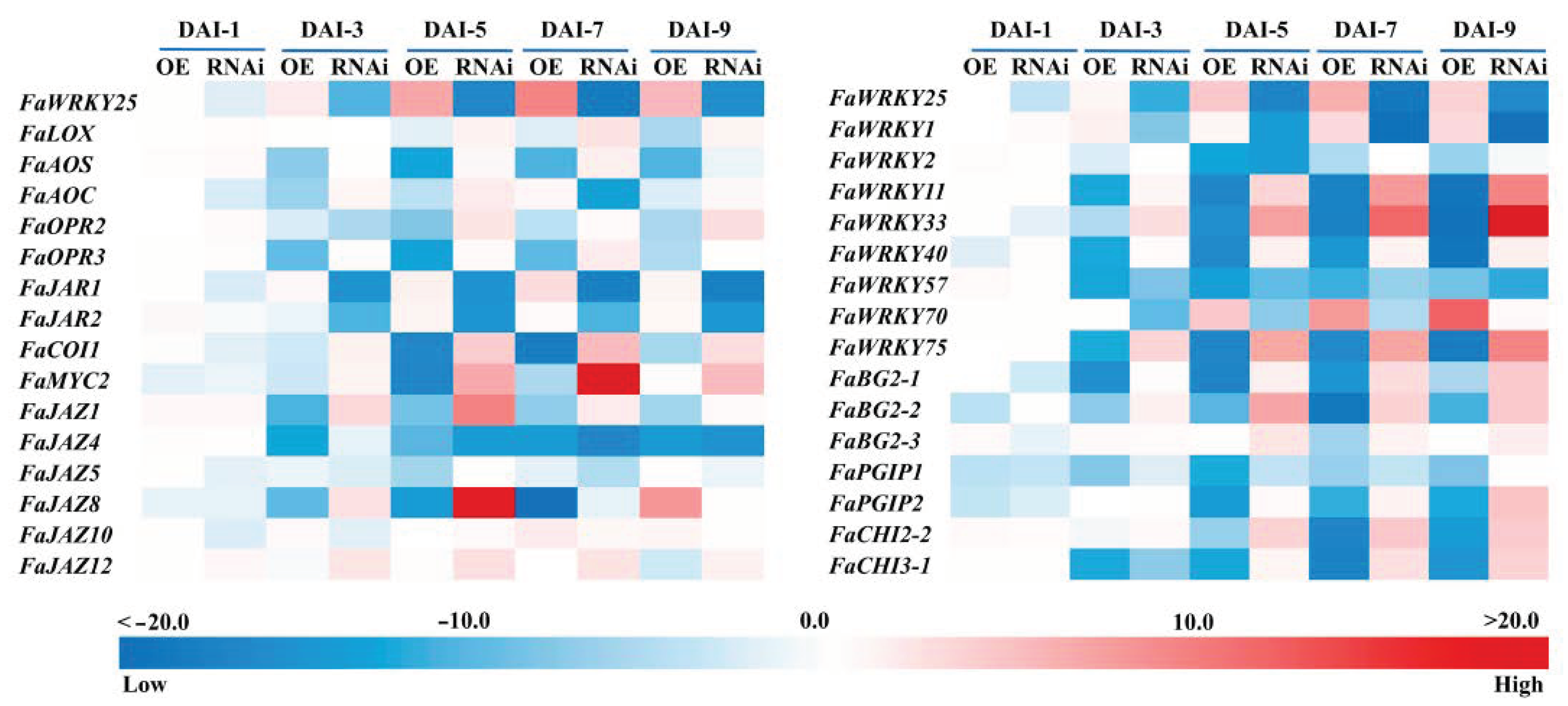
3.5. Effect of FaWRKY25 on the Genes Related to B. cinerea Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sturzeanu, M.; Coman, M.; Stanciu, C. Commercial value of strawberry cultivars in the climatic conditions of central Romania. Acta Hortic. 2013, 981, 127–130. [Google Scholar] [CrossRef]
- Bestfleisch, M.; Luderer-Pflimpf, M.; Hofer, M.; Schulte, E.; Flachowsky, H. Evaluation of strawberry (Fragaria, L.) genetic resources for resistance to Botrytis cinerea. Plant Pathol. 2015, 64, 396–405. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco, B.-U. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela-Riffo, F.; Zúñiga, P.E.; Morales-Quintana, L.; Lolas, M.; Cáceres, M.; Figueroa, C.R. Priming of defense systems and upregulation of MYC2 and JAZ1 genes after Botrytis cinerea inoculation in methyl jasmonate-T reated strawberry fruits. Plants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Chang, X.; Dai, T.; Li, L.; Liu, P.Q.; Wang, G.Z.H.; Liu, P.F.; Huang, Z.Q.; Liu, X.L. Metabolic profiling to identify the latent infection of strawberry by Botrytis cinerea. Evol. Bioinform. 2019, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Haile, Z.M.; Nagpala-De Guzman, E.G.; Moretto, M.; Sonego, P.; Engelen, K.; Zoli, L.; Moser, C.; Baraldi, S.E. Transcriptome profiles of strawberry (Fragaria vesca) fruit interacting with Botrytis cinerea at different ripening stages. Front. Plant Sci. 2019, 10, 1131–1148. [Google Scholar] [CrossRef]
- Chen, P.H.; Chen, R.Y.; Chou, J.Y. Screening and evaluation of yeast antagonists for biological control of Botrytis cinerea on strawberry fruits. Mycobiology 2018, 46, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Yan, C.; Xie, D. Jasmonate in plant defence: Sentinel or double agent? Plant Biotechnol. J. 2015, 13, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.L.; Yang, X.B.; Zhang, Q.; Zhou, M.; Zhao, E.Z.; Tang, Y.X.; Zhu, X.M.; Shao, J.R.; Wu, Y.M. Induction of annexin by heavy metals and jasmonic acid in Zea mays. Funct. Integr. Genom. 2013, 13, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Liao, Y.X.; Kan, J.Q.; Han, L.; Zheng, Y.H. Response of direct or priming defense against Botrytis cinerea to methyl jasmonate treatment at different concentrations in grape berries. Int. J. Food Microbiol. 2015, 194, 32–39. [Google Scholar] [CrossRef]
- Gabriela, S.; Eugenio, S.; Pablo, F.; Carlos, F. Independent preharvest applications of methyl jasmonate and chitosan elicit differential upregulation of defense-related genes with reduced incidence of gray mold decay during postharvest storage of fragaria chiloensis Fruit. Int. J. Mol. Sci. 2017, 18, 1420. [Google Scholar]
- Windram, O.; Madhou, P.; McHattie, S.; Hill, C.; Hickman, R.; Cooke, E.; Jenkins, D.J.; Penfold, C.A.; Baxter, L.; Breeze, E.; et al. Arabidopsis defense against Botrytis cinerea: Chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell 2012, 24, 3530–3557. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D.; Rajendran, S.R.C.K.; Gaur, M.; Sajeesh, P.K.; Kumar, A. Plant defense signaling and responses against necrotrophic fungal pathogens. J. Plant. Growth Regul. 2016, 35, 1159–1174. [Google Scholar] [CrossRef]
- Desveaux, D.; Maréchal, A.; Brisson, N. Whirly transcription factors: Defense gene regulation and beyond. Trends Plant Sci. 2005, 10, 95–102. [Google Scholar] [CrossRef]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and functional analysis of a MYB transcription factorgene that is a key regulator for the development of red coloration inapple skin. Plant Cell Physiol 2007, 48, 958–970. [Google Scholar] [CrossRef]
- Seo, E.; Choi, D. Functional studies of transcription factors involved in plant defenses in the genomics era. Brief. Funct. Genom. 2015, 14, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Schuttenhofer, C.; Yuan, L. Regulation of specialized metabolism by WRKY transcription factors. Plant. Physiol. 2015, 167, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.Y.; Qamar, S.A.; Chen, Z.X.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant Physiol. 2006, 48, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hong, Y.B.; Zhang, Y.F.; Li, X.H.; Huang, L.; Zhang, H.J.; Li, D.Y.; Song, F.M. Tomato WRKY transcriptional factor SlDRW1 is required for disease resistance against Botrytis cinerea and tolerance to oxidative stress. Plant Sci. 2014, 227, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Ishihama, N.; Nakano, T.; Yoshioka, M.; Yoshioka, H. Nicotiana benthamiana MAPK-WRKY pathway confers resistance to a necrotrophic pathogen Botrytis cinerea. Plant Signal Behav. 2016, 11, e1183085. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.P.; Somssich, I.E. The Role of WRKY transcription factors in plant immunity. Plant Physiol. 2009, 150, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.; Vinod, K.M.; Zheng, Z.; Fan, B.; Chen, Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol. 2008, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.P.; Chen, C.H.; Fan, B.F.; Chen, Z.X. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell 2006, 18, 1310–1326. [Google Scholar] [CrossRef] [Green Version]
- Lai, Z.B.; Li, Y.; Wang, F.; Cheng, Y.; Fan, B.F.; Yu, J.Q.; Chen, Z.X. Arabidopsis sigma factor binding proteins are activators of the WRKY33 transcription factor in plant defense. Plant Cell 2011, 23, 3824–3841. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.M.; Venugopal, S.; Navarre, D.; Kachroo, A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 2011, 155, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.Y.; Li, Y.X.; Zhang, Q.; Ren, S.; Shen, Y.Y.; Qin, L.; Xing, Y. Genome-wide analysis of the expression of WRKY family genes in different developmental stages of wild strawberry (Fragaria vesca) fruit. PLoS ONE 2016, 11, e0154312. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, Y.; Han, Y.T.; Zhang, K.; Zhao, F.L.; Feng, J.Y. The WRKY transcription factors in the diploid woodland strawberry Fragaria vesca: Identification and expression analysis under biotic and abiotic stresses. Plant Physiol. Biochem. 2016, 105, 29–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, Q.Z. Genome-wide characterization of the WRKY gene family in cultivated strawberry (Fragaria × ananassa Duch.) and the importance of several group III members in continuous cropping. Sci. Rep. 2019, 9, 8423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Encinas-Villarejo, S.; Maldonado, A.M.; Amil-Ruiz, F.; de los Santos, B.; Romero, F.; Pliego-Alfaro, F. Evidence for a positive regulatory role of strawberry (Fragaria × ananassa) FaWRKY1 and Arabidopsis AtWRKY75 proteins in resistance. J. Exp. Bot. 2009, 60, 3043–3065. [Google Scholar] [CrossRef] [Green Version]
- Higuera, J.J.; Garrido-Gala, J.; Lekhbou, A.; Arjona-Girona, I.; Amil-Ruiz, F.; Mercado, J.A.; Pliego-Alfaro, F.; Muñoz-Blanco, J.; López-Herrera, C.J.; Caballero, J.L.; et al. The strawberry FaWRKY1 franscription factor negatively regulates resistance to Colletotrichum acutatum in fruit upon infection. Front Plant Sci. 2019, 10, 480–497. [Google Scholar] [CrossRef]
- Amil-Ruiz, F.; Garrido-Gala, J.; Gadea, J.; Blanco-Portales, R.; Muñoz-Mérida, A.; Trelles, O. Partial activation of SA- and JA-defensive pathways instrawberry upon colletotrichum acutatum interaction. Front Plant Sci. 2016, 7, 1036. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Cui, M.Y.; Hu, Y.; Gao, K.; Xie, Y.G.; Jiang, Y.; Feng, J.Y. Ectopic expression of FvWRKY42, a WRKY transcription factor from the diploid woodland strawberry (Fragaria vesca), enhances resistance to powdery mildew, improves osmotic stress resistance, and increases abscisic acid sensitivity in Arabidopsis. Plant Sci. 2018, 275, 60–74. [Google Scholar] [CrossRef]
- Wu, T.Y.; Krishnamoorthi, S.; Goh, H.; Leong, R.; Sanson, A.C.; Urano, D. Crosstalk between heterotrimeric G protein-coupled signaling pathways and WRKY transcription factors modulating plant responses to suboptimal micronutrient conditions. J. Exp. Bot. 2020, 71, 3227–3239. [Google Scholar] [CrossRef]
- Doll, J.; Muth, M.; Riester, L.; Nebel, S.; Bresson, J.; Lee, H.C.; Zentgraf, U. Arabidopsis thaliana WRKY25 transcription factor mediates oxidative stress tolerance and regulates senescence in a redox-dependent manner. Front Plant Sci. 2019, 10, 1734. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.Y.; Mosher, S.L.; Fan, B.F.; Klessig, D.F.; Chen, Z.X. Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol. 2007, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.F.; Song, Y.Z.; Xing, F.Y.; Wang, N.; Wen, F.J.; Zhu, C.X. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2016, 253, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Fu, Q.T.; Chen, L.G.; Huang, W.D.; Yu, D.Q. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 2011, 233, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.N.; Martin, G.B.; Pombo, M.A.; Rosli, H.G. WRKY22 and WRKY25 transcription factors are positive regulators of defense responses in Nicotiana benthamiana. Plant Mol. Biol. 2020, 105, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jia, S.Z.; Yan, Z.M.; Wang, Y.H.; Zhao, F.X.; Sun, Y.F. A strawberry mitogen-activated protein kinase gene, FaMAPK19, is involved in disease resistance against Botrytis cinerea. Sci. Hortic. 2020, 265, 109259. [Google Scholar] [CrossRef]
- Jia, H.F.; Wang, Y.H.; Sun, M.Z.; Li, B.B.; Han, Y.; Zhao, Y.X.; Li, X.L.; Ding, N.; Li, C.; Ji, W.L.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef]
- Fait, A.; Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachev, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 2008, 148, 730–750. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Wang, Y.H.; Zhang, G.; Feng, Y.N.; Yan, Z.M.; Wu, J.H.; Chen, X.H. Genome-wide identification and expression analysis of the dtrawberry FvbZIP gene family and the role of key gene FabZIP46 in fruit resistance to gray mold. Plants 2020, 9, 1199. [Google Scholar] [CrossRef]
- Kilam, D.; Saifi, M.; Agnihotri, A.M.; Abdin, Z. Development of an efficient high-performance thin layer chromatography method for determination of jasmonic acid in leaf tissue of Stevia rebaudiana (Bertoni) Bertoni. Nat. Prod. Res. 2017, 31, 1713–1716. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, L.J. The WRKY transcription factor superfamily: Its origin in eukaryotes and expansion in plants. BMC Evol. Biol. 2005, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Satapathy, L.; Kumar, D.; Mukhopadhyay, K. WRKY transcription factors: Involvement in plant–pathogen interactions. Adv. Appl. Microbiol. 2017, 11, 229–246. [Google Scholar]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant. Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis wrky33 is a key transcriptional regulator of hormonal and metabolic responses toward botrytis cinerea infection. Plant. Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate asnegative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Guidarelli, M.; Baraldi, E. Transient transformation meets gene function discovery: The strawberry fruit case. Front Plant Sci. 2015, 6, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, R.F.; Carvalho, S.D.; O’grady, K.; Folta, K.M. Agroinfiltration of strawberry fruit -a powerful transient expression system for gene validation. Curr. Plant Biol. 2016, 6, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Kombrink, E. Jasmonates: Structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem. Biol. 2010, 5, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Gimenez-Ibanez, S.; Chini, A.; Solano, R. How microbes twist jasmonate signaling around their little fingers. Plants 2016, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Staswick, P.E.; Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Chico, J.M.; Chini, A.; Fonseca, S.; Solano, R. JAZ repressors set the rhythm in jasmonate signaling. Curr. Opin. Plant Biol. 2008, 11, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Boter, M.; Solano, R. Plant oxylipins: Coi1/jazs/myc2 as the core jasmonic acid-signalling module. FEBS J. 2009, 276, 4682–4692. [Google Scholar] [CrossRef] [PubMed]


| Cultivars | MeJA Concentration (μM) | Treatment | Hours Post Inoculation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | 144 | |||
| Benihoppe | 0(CK,H2O) | +Bc | - | - | + | + | ++ | +++ | ++++ |
| 100 | - | - | + | + | ++ | +++ | +++ | ||
| 150 | - | - | + | + | ++ | ++ | +++ | ||
| 200 | - | - | - | + | + | ++ | ++ | ||
| 250 | - | - | - | - | - | + | + | ||
| 300 | - | - | - | - | + | + | + | ||
| 350 | - | - | - | - | + | + | ++ | ||
| MeJA Concentration (μM) | Incidence (%) of Strawberry Fruit After Inoculation with Botrytis cinerea | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 h | 72 h | 96 h | 120 h | 144 h | ||||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 0(CK,H2O) | 10.0 a | 10.2 | - | - | 9.1 a | 10.9 a | 4.0 * | - | 20.7 a | 14.0 a | 8.9 a | 3.3 | 18.4 b | 15.3 a | 18.4 a | 21.8 a | 18.2 b | 11.3 ab | 26.2 a | 36.7 a |
| 100 | 6.2 b | - | - | - | 7.8 b | 8.4 a | 0.7 | - | 15.8 b | 12.9 a | 5.3 b | 3.1 | 22.0 a | 12.7 b | 15.8 ab | 20.9 a | 12.9 cd | 13.3 a | 24.2 a | 24.9 b |
| 150 | 3.3 c | - | - | - | 5.8 c | 3.6 b | - | - | 11.3 c | 12.2 a | 2.7 c | - | 15.1 c | 12.9 b | 13.8 b | 2.9 b | 13.1 cd | 14.9 a | 15.3 b | 19.3 c |
| 200 | - | - | - | - | 2.7 d | - | - | - | 7.6 d | 4.4 b | 0.4 d | - | 16.0 bc | 9.8 c | 4.0 c | - | 24.7 a | 9.1 cd | 4.9 c | 4.4 d |
| 250 | - | - | - | - | - | - | - | - | - | - | - | - | 3.1 e | - | - | - | 6.9 e | - | - | - |
| 300 | - | - | - | - | - | - | - | - | 4.2 e | - | - | - | 9.6 d | 3.6 d | - | - | 9.3 de | 8.9 cd | 0.7 d | - |
| 350 | - | - | - | - | - | - | - | - | 6.4 de | - | - | - | 10.2 d | 4.0 d | - | - | 14.7 bc | 6.4 d | 2.2 cd | 0.9 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, S.; Wang, Y.; Zhang, G.; Yan, Z.; Cai, Q. Strawberry FaWRKY25 Transcription Factor Negatively Regulated the Resistance of Strawberry Fruits to Botrytis cinerea. Genes 2021, 12, 56. https://doi.org/10.3390/genes12010056
Jia S, Wang Y, Zhang G, Yan Z, Cai Q. Strawberry FaWRKY25 Transcription Factor Negatively Regulated the Resistance of Strawberry Fruits to Botrytis cinerea. Genes. 2021; 12(1):56. https://doi.org/10.3390/genes12010056
Chicago/Turabian StyleJia, Sizhen, Yuanhua Wang, Geng Zhang, Zhiming Yan, and Qingsheng Cai. 2021. "Strawberry FaWRKY25 Transcription Factor Negatively Regulated the Resistance of Strawberry Fruits to Botrytis cinerea" Genes 12, no. 1: 56. https://doi.org/10.3390/genes12010056
APA StyleJia, S., Wang, Y., Zhang, G., Yan, Z., & Cai, Q. (2021). Strawberry FaWRKY25 Transcription Factor Negatively Regulated the Resistance of Strawberry Fruits to Botrytis cinerea. Genes, 12(1), 56. https://doi.org/10.3390/genes12010056





