Suicide Related Phenotypes in a Bipolar Sample: Genetic Underpinnings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Sample
2.2. Participants
2.3. Original Genetic Sample
2.4. Outcomes
2.5. Clinical Covariates
2.6. Statistical Model and Flow of Analysis
2.7. Analysis of Clinical Data
2.8. Analysis of Genetic Data
3. Results
3.1. Main Analysis
3.2. Exploratory Analysis
4. Discussion
4.1. Analysis of Single SNPs
4.2. Molecular Pathway Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Definitions of Suicide—Related Phenotypes
Appendix B. Clinical Risk Factors and Prevention Measures for Suicide Behavior in BD
References
- Miller, J.; Black, D.W. Bipolar disorder and suicide: A review. Curr. Psychiatry Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Hansen, P.S.; Laursen, M.F.; Grøntved, S.; Straszek, S.P.V.; Licht, R.W.; Nielsen, R.E. Increasing mortality gap for patients diagnosed with bipolar disorder-A nationwide study with 20 years of follow-up. Bipolar Disord. 2019, 21, 270–275. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, K.; Winkleby, M.A.; Sundquist, J. Comorbidities and mortality in bipolar disorder: A Swedish national cohort study. JAMA Psychiatry 2013, 70, 931–939. [Google Scholar] [CrossRef]
- Pinto, J.V.; Saraf, G.; Kozicky, J.; Beaulieu, S.; Sharma, V.; Parikh, S.V.; Yatham, L.N. Remission and recurrence in bipolar disorder: The data from health outcomes and patient eval-uations in bipolar disorder (HOPE-BD) study. J Affect. Disord. 2020, 268, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Pfennig, A. Epidemiology of Bipolar Disorders. Epilepsia 2005, 46, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Gonda, X.; Pompili, M.; Serafini, G.; Montebovi, F.; Campi, S.; Dome, P.; Duleba, T.; Girardi, P.; Rihmer, Z. Suicidal behavior in bipolar disorder: Epidemiology, characteristics and major risk factors. J. Affect. Disord. 2012, 143, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Voracek, M.; Loibl, L.M. Genetics of suicide: A systematic review of twin studies. Wien. Klin. Wochenschr. 2007, 119, 463–475. [Google Scholar] [CrossRef]
- Brent, D.A.; Mann, J.J. Family genetic studies, suicide, and suicidal behavior. Am. J. Med. Genet. Part C Semin. Med. Genet. 2005, 133C, 13–24. [Google Scholar] [CrossRef]
- Mullins, N.; Bigdeli, T.B.; Børglum, A.; Coleman, J.; Demontis, D.; Mehta, D.D.; Power, R.A.; Ripke, S.; Stahl, E.A.; Starnawska, A.; et al. GWAS of suicide attempt in psychiatric disorders and association with major depression polygenic risk scores. Am. J. Psychiatry 2019, 176, 651–660. [Google Scholar] [CrossRef]
- Ruderfer, D.M.; Walsh, C.G.; Aguirre, M.W.; Tanigawa, Y.; Ribeiro, J.D.; Franklin, J.C.; Rivas, M.A. Significant shared heritability underlies suicide attempt and clinically predicted probability of attempting suicide. Mol. Psychiatry 2020, 25, 2422–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.B.; Ware, E.B.; Mitchell, C.; Chen, C.-Y.; Borja, S.; Cai, T.; Dempsey, C.L.; Fullerton, C.S.; Gelernter, J.; Heeringa, S.G.; et al. Genomewide association studies of suicide attempts in US soldiers. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 786–797. [Google Scholar] [CrossRef]
- Mullins, N.; Perroud, N.; Uher, R.; Butler, A.W.; Cohen-Woods, S.; Rivera, M.; Malki, K.; Euesden, J.; Power, R.; Tansey, K.; et al. Genetic relationships between suicide attempts, suicidal ideation and major psychiatric disorders: A genome-wide association and polygenic scoring study. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Galfalvy, H.; Haghighi, F.; Hodgkinson, C.; Goldman, D.; Oquendo, M.A.; Burke, A.; Huang, Y.-Y.; Giegling, I.; Rujescu, D.; Bureau, A.; et al. A genome-wide association study of suicidal behavior. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Erlangsen, A.; Appadurai, V.; Wang, Y.; Turecki, G.; Mors, O.; Werge, T.; Mortensen, P.B.; Starnawska, A.; Børglum, A.; Schork, A.; et al. Genetics of suicide attempts in individuals with and without mental disorders: A population-based genome-wide association study. Mol. Psychiatry 2020, 25, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.S.; Thase, M.E.; Otto, M.; Bauer, M.; Miklowitz, D.; Wisniewski, S.; Lavori, P.; Lebowitz, B.; Rudorfer, M.; Frank, E.; et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol. Psychiatry 2003, 53, 1028–1042. [Google Scholar] [CrossRef]
- Bellivier, F.; Laplanche, J.-L.; Leboyer, M.; Feingold, J.; Bottos, C.; Allilaire, J.-F.; Launay, J.-M. Serotonin transporter gene and manic depressive illness: An association study. Biol. Psychiatry 1997, 41, 750–752. [Google Scholar] [CrossRef]
- Ohara, K.; Nagai, M.; Tsukamoto, T.; Tani, K.; Suzuki, Y.; Ohara, K. 5-HT2A receptor gene promoter polymor-phism--1438G/A and mood disorders. Neuroreport 1998, 9, 1139–1141. [Google Scholar] [CrossRef]
- Ohara, K.; Nagai, M.; Tsukamoto, T.; Tani, K.; Suzuki, Y.; Ohara, K. Functional polymorphism in the serotonin transporter promoter at the SLC6A4 locus and mood disorders. Biol. Psychiatry 1998, 44, 550–554. [Google Scholar] [CrossRef]
- Rujescu, D.; Giegling, I.; Dahmen, N.; Szegedi, A.; Anghelescu, I.; Gietl, A.; Schäfer, M.; Müller-Siecheneder, F.; Bondy, B.; Möller, H.J. Association study of suicidal behavior and affective disorders with a genetic polymorphism in ABCG1, a positional candidate on chromosome 21q22.3. Neuropsychobiology 2000, 42, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Zubenko, G.S.; Maher, B.S.; Hughes HB 3rd Zubenko, W.N.; Scott Stiffler, J.; Marazita, M.L. Genome-wide linkage survey for genetic loci that affect the risk of suicide attempts in families with recurrent, early-onset, major depression. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004, 129B, 47–54. [Google Scholar] [CrossRef]
- Hesselbrock, V.; Dick, D.; Hesselbrock, M.; Foroud, T.; Schuckit, M.; Edenberg, H.; Bucholz, K.; Kramer, J.; Reich, T.; Goate, A.; et al. The search for genetic risk factors associated with suicidal behavior. Alcohol. Clin. Exp. Res. 2004, 28, 70S–76S. [Google Scholar] [CrossRef]
- De Lara, C.L.; Dumais, A.; Rouleau, G.; Lesage, A.; Dumont, M.; Chawky, N.; Alda, M.; Benkelfat, C.; Turecki, G. STin2 Variant and Family History of Suicide as Significant Predictors of Suicide Completion in Major Depression. Biol. Psychiatry 2006, 59, 114–120. [Google Scholar] [CrossRef]
- Cheng, R.; Juo, S.H.; Loth, J.E.; Nee, J.; Iossifov, I.; Blumenthal, R.; Sharpe, L.; Kanyas, K.; Lerer, B.; Lilliston, B.; et al. Genome-wide linkage scan in a large bipolar disorder sample from the National Institute of Mental Health genetics initiative suggests putative loci for bipolar disorder, psychosis, suicide, and panic disorder. Mol. Psychiatry 2006, 11, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Willour, V.L.; Zandi, P.P.; Badner, J.A.; Steele, J.; Miao, K.; Lopez, V.; MacKinnon, D.F.; Mondimore, F.M.; Schweizer, B.; McInnis, M.G.; et al. Attempted Suicide in Bipolar Disorder Pedigrees: Evidence for Linkage to 2p12. Biol. Psychiatry 2007, 61, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Iga, J.-I.; Ueno, S.-I.; Yamauchi, K.; Numata, S.; Tayoshi-Shibuya, S.; Kinouchi, S.; Nakataki, M.; Song, H.; Hokoishi, K.; Tanabe, H.; et al. The Val66Met polymorphism of the brain-derived neurotrophic factor gene is associated with psychotic feature and suicidal behavior in Japanese major depressive patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Huang, J.; Purcell, S.; Fava, M.; Rush, A.J.; Sullivan, P.F.; Hamilton, S.P.; McMahon, F.J.; Schulze, T.; Potash, J.B.; et al. Genome-wide association study of suicide attempts in mood disorder patients. Am. J. Psychiatry 2010, 167, 1499–1507. [Google Scholar] [CrossRef]
- Schosser, A.; Butler, A.W.; Ising, M.; Perroud, N.; Uher, R.; Ng, M.Y.; Cohen-Woods, S.; Craddock, N.; Owen, M.J.; Korszun, A.; et al. Genomewide Association Scan of Suicidal Thoughts and Behaviour in Major Depression. PLoS ONE 2011, 6, e20690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willour, V.L.; Seifuddin, F.; Mahon, P.B.; Jancic, D.; Pirooznia, M.; Steele, J.; Schweizer, B.; Goes, F.S.; Mondimore, F.M.; Mackinnon, D.F.; et al. A genome-wide association study of attempted suicide. Mol. Psychiatry 2011, 17, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Schosser, A.; Calati, R.; Serretti, A.; Massat, I.; Kocabas, N.A.; Papageorgiou, K.; Linotte, S.; Mendlewicz, J.; Souery, D.; Zohar, J.; et al. The impact of COMT gene polymorphisms on suicidality in treatment resistant major depressive disorder—A European Multicenter Study. Eur. Neuropsychopharmacol. 2012, 22, 259–266. [Google Scholar] [CrossRef]
- Zai, C.C.; Gonçalves, V.F.; Tiwari, A.K.; Gagliano, S.A.; Hosang, G.; de Luca, V.; Shaikh, S.A.; King, N.; Chen, Q.; Xu, W.; et al. A genome-wide association study of suicide severity scores in bipolar disorder. J. Psychiatr. Res. 2015, 65, 23–29. [Google Scholar] [CrossRef]
- Gross, J.A.; Bureau, A.; Croteau, J.; Galfalvy, H.; Oquendo, M.A.; Haghighi, F.; Turecki, G. A genome-wide copy number variant study of suicidal behavior. PLoS ONE 2015, 10, e0128369. [Google Scholar] [CrossRef]
- Sokolowski, M.; Wasserman, J. Polygenic associations of neurodevelopmental genes in suicide attempt. Mol. Psychiatry 2016, 21, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Trabjerg, B.B.; Mors, O.; Børglum, A.; Hougaard, D.M.; Mattheisen, M.; Meier, S.M.; Byrne, E.; Mortensen, P.B.; Munk-Olsen, T.; et al. Association of the polygenic risk score for schizophrenia with mortality and suicidal behavior—A Danish population-based study. Schizophr. Res. 2017, 184, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Levey, D.F.; Polimanti, R.; Cheng, Z.; Zhou, H.; Nuñez, Y.Z.; Jain, S.; He, F.; Sun, X.; Ursano, R.J.; Kessler, R.C.; et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl. Psychiatry 2019, 9, 22. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- Bowden, C.; Perlis, R.; Thase, M.; Ketter, T.; Ostacher, M.; Calabrese, J.; Reilly-Harrington, N.; Gonzalez, J.; Singh, V.; Nierenberg, A.; et al. Aims and Results of the NIMH Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). CNS Neurosci. Ther. 2011, 18, 243–249. [Google Scholar] [CrossRef]
- Team R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.D.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Howie, B.N.; Donnelly, P.; Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef] [Green Version]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; He, Q.-Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. BioSyst. 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Corfitsen, H.T.; Drago, A. Enriched developmental biology molecular pathways impact on antipsychotics-induced weight gain. Pharm. Genom. 2020, 30, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Corfitsen, H.T.; Krantz, B.; Larsen, A.; Drago, A. Molecular pathway analysis associates alterations in obesity-related genes and antipsychotic-induced weight gain. Acta Neuropsychiatr. 2020, 32, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Drago, A.; Fischer, E.K. A molecular pathway analysis informs the genetic risk for arrhythmias during antipsychotic treatment. Int. Clin. Psychopharmacol. 2018, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.K.; Drago, A. A molecular pathway analysis stresses the role of inflammation and oxidative stress towards cognition in schizophrenia. J. Neural Transm. 2017, 124, 765–774. [Google Scholar] [CrossRef]
- O’Dushlaine, C.; Kenny, E.; Heron, E.; Donohoe, G.; Gill, M.; Morris, D.; International Schizophrenia Consortium; Corvin, A. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol. Psychiatry 2011, 16, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, M.; Konoshita, T.; Makino, Y.; Suzuki, J.; Ishizuka, T.; Nakamura, H. An association study of C9orf3, a novel component of the renin-angiotensin system, and hypertension in diabetes. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Hennig, E.E. Genetic susceptibility to joint occurrence of polycystic ovary syndrome and hashimoto’s thyroiditis: How far is our understanding? Front. Immunol. 2021, 12, 606620. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.G.; Urbanek, M.; Ehrmann, D.A.; Armstrong, L.L.; Lee, J.Y.; Sisk, R.; Karaderi, T.; Barber, T.M.; McCarthy, M.I.; Franks, S.; et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015, 6, 7502. [Google Scholar] [CrossRef] [Green Version]
- Louwers, Y.V.; Stolk, L.; Uitterlinden, A.G.; Laven, J.S. Cross-ethnic meta-analysis of genetic variants for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E2006–E2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, F.R.; Hinds, D.A.; Tung, J.Y.; Stolk, L.; Styrkarsdottir, U.; Saxena, R.; Bjonnes, A.; Broer, L.; Dunger, D.B.; Halldórsson, B.V.; et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat. Commun. 2015, 6, 8464. [Google Scholar] [CrossRef] [Green Version]
- Brower, M.A.; Jones, M.R.; Rotter, J.I.; Krauss, R.M.; Legro, R.S.; Azziz, R.; Goodarzi, M.O. Further investigation in europeans of susceptibility variants for polycystic ovary syndrome discovered in genome-wide association studies of Chinese individuals. J. Clin. Endocrinol. Metab. 2015, 100, E182–E186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, F.; Karaderi, T.; Jones, M.R.; Meun, C.; He, C.; Drong, A.; Kraft, P.; Lin, N.; Huang, H.; Broer, L.; et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018, 14, e1007813. [Google Scholar] [CrossRef] [Green Version]
- Nishida, K.; Kuwano, Y.; Rokutan, K. The MicroRNA-23b/27b/24 Cluster Facilitates Colon Cancer Cell Migration by Targeting FOXP2. Cancers 2020, 12, 174. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.-H.; Man, Y.-Y.; Liu, Y.; Yin, C.-J.; Li, J.-L.; Shi, H.-C.; Zhao, H.; Zhao, S.-G. Loss of miR-23b/27b/24-1 Cluster Impairs Glucose Tolerance via Glycolysis Pathway in Mice. Int. J. Mol. Sci. 2021, 22, 550. [Google Scholar] [CrossRef] [PubMed]
- McAlinden, A.; Varghese, N.; Wirthlin, L.; Chang, L.-W. Differentially Expressed MicroRNAs in Chondrocytes from Distinct Regions of Developing Human Cartilage. PLoS ONE 2013, 8, e75012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.-C.; Zhu, J.-G.; Chen, X.-B.; Chen, S.-M.; Han, Z.-D.; Dai, Q.-S.; Ling, X.-H.; Fu, X.; Lin, Z.-Y.; Deng, Y.-H.; et al. MicroRNA-23b downregulates peroxiredoxin III in human prostate cancer. FEBS Lett. 2012, 586, 2451–2458. [Google Scholar] [CrossRef]
- Bang, C.; Fiedler, J.; Thum, T. Cardiovascular importance of the microRNA-23/27/24 family. Microcirculation 2012, 19, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Pan, W.; Song, X.; Liu, Y.; Shao, X.; Tang, Y.; Liang, D.; He, D.; Wang, H.; Liu, W.; et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat. Med. 2012, 18, 1077–1086. [Google Scholar] [CrossRef]
- Boon, R.A.; Hergenreider, E.; Dimmeler, S. Atheroprotective mechanisms of shear stress-regulated microRNAs. Thromb. Haemost. 2012, 108, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, J.; Li, L.; Li, H.; Mao, S.; Zhang, F.; Zen, K.; Zhang, C.-Y.; Zhang, Q. MicroRNA-23a/b and microRNA-27a/b suppress Apaf-1 protein and alleviate hypoxia-induced neuronal apoptosis. Cell Death Dis. 2014, 5, e1132. [Google Scholar] [CrossRef]
- Johnson, C.E.; Huang, Y.Y.; Parrish, A.B.; Smith, M.I.; Vaughn, A.E.; Zhang, Q.; Wright, K.; Van Dyke, T.; Wechsler-Reya, R.J.; Kornbluth, S.; et al. Differential Apaf-1 levels allow cytochrome c to induce apoptosis in brain tumors but not in normal neural tissues. Proc. Natl. Acad. Sci. USA 2007, 104, 20820–20825. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.P.; Fiori, L.M.; Gross, J.A.; LaBonte, B.; Yerko, V.; Mechawar, N.; Turecki, G. Regulatory role of miRNAs in polyamine gene expression in the prefrontal cortex of depressed suicide completers. Int. J. Neuropsychopharmacol. 2013, 17, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Kouter, K.; Paska, A.V. Biomarkers for suicidal behavior: miRNAs and their potential for diagnostics through liquid biopsy—A systematic review. Epigenomics 2020, 12, 2219–2235. [Google Scholar]
- Cox, J.J.; Reimann, F.; Nicholas, A.K.; Thornton, G.; Roberts, E.; Springell, K.; Karbani, G.; Jafri, H.; Mannan, J.; Raashid, Y.; et al. An SCN9A channelopathy causes congenital inability to experience pain. Nat. Cell Biol. 2006, 444, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Fertleman, C.R.; Baker, M.D.; Parker, K.A.; Moffatt, S.; Elmslie, F.V.; Abrahamsen, B.; Ostman, J.; Klugbauer, N.; Wood, J.N.; Gardiner, R.M.; et al. SCN9A mutations in paroxysmal extreme pain disorder: Allelic variants underlie distinct channel defects and phenotypes. Neuron 2006, 52, 767–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Dib-Hajj, S.D.; Lin, Z.; Li, Y.; Eastman, E.M.; Tyrrell, L.; Cao, X.; Yang, Y.; Waxman, S.G. Early- and late-onset inherited erythromelalgia: Genotype–phenotype correlation. Brain 2009, 132, 1711–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, M.; Nakajima, J.; Klinger, A.B.; Neacsu, C.; Hühne, K.; O’Reilly, A.O.; Kist, A.M.; Lampe, A.K.; Fischer, K.; Gibson, J. Inherited pain: Sodium channel Nav1.7 A1632T mutation causes erythromelalgia due to a shift of fast inactivation. J. Biol. Chem. 2014, 289, 1971–1980. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.; Lari, H.; Saffy, S.; Klonsky, E.D. Examining the Three-Step Theory (3ST) of Suicide in a Prospective Study of Adult Psychiatric Inpatients. Behav. Ther. 2021, 52, 673–685. [Google Scholar] [CrossRef]
- Klempan, T.A.; Sequeira, A.; Canetti, L.; Lalovic, A.; Ernst, C.; Ffrench-Mullen, J.; Turecki, G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol. Psychiatry 2007, 14, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Pantazatos, S.P.; Huang, Y.-Y.; Rosoklija, G.B.; Dwork, A.J.; Arango, V.; Mann, J.J. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: Evidence for altered glial, endothelial and ATPase activity. Mol. Psychiatry 2017, 22, 760–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Levinson, A.J.; Fitzgerald, P.; Favalli, G.; Blumberger, D.M.; Daigle, M.; Daskalakis, Z.J. Evidence of cortical inhibitory deficits in major depressive disorder. Biol. Psychiatry 2010, 67, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Verwer, R.; Gao, S.-F.; Qi, X.-R.; Lucassen, P.J.; Kessels, H.; Swaab, D. Prefrontal alterations in GABAergic and glutamatergic gene expression in relation to depression and suicide. J. Psychiatr. Res. 2018, 102, 261–274. [Google Scholar] [CrossRef]
- Ghosal, S.; Bang, E.; Yue, W.; Hare, B.D.; Lepack, A.E.; Girgenti, M.J.; Duman, R.S. Activity-dependent brain-derived neu-rotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol. Psychiatry 2018, 83, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lener, M.S.; Niciu, M.J.; Ballard, E.D.; Park, M.; Park, L.T.; Nugent, A.C.; Zarate, C.A., Jr. Glutamate and γ-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol. Psychiatry 2016, 81, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.C.; Sibille, E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol. Psychiatry 2015, 20, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.E.M.; Gardner, A.C.; Kwon, S.; Chea, W.; Muthukumaraswamy, S.D. Differences in excitatory and inhibitory neu-rotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 105, 33–44. [Google Scholar] [CrossRef]
- Fee, C.; Banasr, M.; Sibille, E. Somatostatin-positive γ-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol. Psychiatry 2017, 82, 549–559. [Google Scholar] [CrossRef]
- Radhu, N.; de Jesus, D.R.; Ravindran, L.N.; Zanjani, A.; Fitzgerald, P.; Daskalakis, Z.J. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin. Neurophysiol. 2013, 124, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Tanti, A.; Lutz, P.-E.; Kim, J.; O’Leary, L.; Théroux, J.-F.; Turecki, G.; Mechawar, N. Evidence of decreased gap junction coupling between astrocytes and oligodendrocytes in the anterior cingulate cortex of depressed suicides. Neuropsychopharmacology 2019, 44, 2099–2111. [Google Scholar] [CrossRef]
- Monsalve, E.M.; García-Gutiérrez, M.S.; Navarrete, F.; Giner, S.; Laborda, J.; Manzanares, J. Abnormal expression pattern of notch receptors, ligands, and downstream effectors in the dorsolateral prefrontal cortex and amygdala of suicidal victims. Mol. Neurobiol. 2013, 49, 957–965. [Google Scholar] [CrossRef]
- Alberi, L.; Liu, S.; Wang, Y.; Badie, R.; Smith-Hicks, C.; Wu, J.; Pierfelice, T.J.; Abazyan, B.; Mattson, M.P.; Kuhl, D.; et al. Activity-induced notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 2011, 69, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Torres-Platas, S.G.; Hercher, C.; Davoli, M.A.; Maussion, G.; LaBonte, B.; Turecki, G.; Mechawar, N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 2011, 36, 2650–2658. [Google Scholar] [CrossRef] [Green Version]
- Bagheri-Mohammadi, S. Adult neurogenesis and the molecular signalling pathways in brain: The role of stem cells in adult hippocampal neurogenesis. Int. J. Neurosci. 2021, 1–13. [Google Scholar] [CrossRef]
- Snyder, J.L.; Kearns, C.A.; Appel, B. Fbxw7 regulates Notch to control specification of neural precursors for oligodendrocyte fate. Neural Dev. 2012, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, Y. Brain-derived neurotrophic factor in suicide pathophysiology. In The Primate Visual System; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Soloff, P.H.; Pruitt, P.; Sharma, M.; Radwan, J.; White, R.; Diwadkar, V.A. Structural brain abnormalities and suicidal behavior in borderline personality disorder. J. Psychiatr. Res. 2012, 46, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.-P.; Lee, T.-W.; Tsai, S.-J.; Chen, T.-J.; Yang, C.-H.; Lirng, J.-F.; Tsai, C.-F. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and Voxel-based morphometry. J. Geriatr. Psychiatry Neurol. 2010, 23, 171–184. [Google Scholar] [CrossRef]
- Ventorp, F.; Barzilay, R.; Erhardt, S.; Samuelsson, M.; Träskman-Bendz, L.; Janelidze, S.; Weizman, A.; Offen, D.; Brundin, L. The CD44 ligand hyaluronic acid is elevated in the cerebrospinal fluid of suicide attempters and is associated with increased blood–brain barrier permeability. J. Affect. Disord. 2016, 193, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Dityatev, A.; Schachner, M.; Sonderegger, P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci. 2010, 11, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Gundelfinger, E.D.; Frischknecht, R.; Choquet, D.; Heine, M. Converting juvenile into adult plasticity: A role for the brain’s extracellular matrix. Eur. J. Neurosci. 2010, 31, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Ishikawa, Y.; Shiosaka, S. Does extracellular proteolysis control mammalian cognition? Rev. Neurosci. 2013, 24, 365–374. [Google Scholar] [CrossRef]
- Glezer, I.; Bittencourt, J.C.; Rivest, S. Neuronal expression of Cd36, Cd44, and Cd83 antigen transcripts maps to distinct and specific murine brain circuits. J. Comp. Neurol. 2009, 517, 906–924. [Google Scholar] [CrossRef] [PubMed]
- Kaaijk, P.; Pals, S.T.; Morsink, F.; Bosch, D.; Troost, D. Differential expression of CD44 splice variants in the normal human central nervous system. J. Neuroimmunol. 1997, 73, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.L.; Liu, Z.; Shen, J.; Werner, A.; Kreutzberg, G.W.; Raivich, G. Regulation of the cell adhesion molecule CD44 after nerve transection and direct trauma to the mouse brain. J. Comp. Neurol. 2000, 426, 468–492. [Google Scholar] [CrossRef]
- Thalmeier, A.; Dickmann, M.; Giegling, I.; Schneider, B.; Hartmann, A.M.; Maurer, K.; Schnabel, A.; Kauert, G.; Möller, H.-J.; Rujescu, D. Gene expression profiling of post-mortem orbitofrontal cortex in violent suicide victims. Int. J. Neuropsychopharmacol. 2007, 11, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galfalvy, H.; Zalsman, G.; Huang, Y.-Y.; Murphy, L.; Rosoklija, G.; Dwork, A.J.; Haghighi, F.; Arango, V.; Mann, J.J. A pilot genome wide association and gene expression array study of suicide with and without major depression. World J. Biol. Psychiatry 2011, 14, 574–582. [Google Scholar] [CrossRef] [Green Version]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A Specific inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zetterberg, H.; Jakobsson, J.; Redsäter, M.; Andreasson, U.; Pålsson, E.; Ekman, C.J.; Sellgren, C.; Johansson, A.G.; Blennow, K.; Landén, M. Blood–cerebrospinal fluid barrier dysfunction in patients with bipolar disorder in relation to antipsychotic treatment. Psychiatry Res. 2014, 217, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, B.R.; Smit, A.B.; Spijker, S.; Oever, M.C.V.D. Neural ECM in addiction, schizophrenia, and mood disorder. Prog. Brain Res. 2014, 214, 263–284. [Google Scholar] [PubMed]
- Robinson, A. Living history: An autobiography of Arthur Robinson. Am. J. Med. Genet. 1990, 35, 475–480. [Google Scholar] [CrossRef]
- Zimmermann, D.R.; Dours-Zimmermann, M.T. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem. Cell Biol. 2008, 130, 635–653. [Google Scholar] [CrossRef] [Green Version]
- Abou-Abbass, H.; Abou-El-Hassan, H.; Bahmad, H.; Zibara, K.; Zebian, A.; Youssef, R.; Ismail, J.; Zhu, R.; Zhou, S.; Dong, X.; et al. Glycosylation and other PTMs alterations in neurodegenerative diseases: Current status and future role in neurotrauma. Electrophoresis 2016, 37, 1549–1561. [Google Scholar] [CrossRef]
- Wei, X.; Li, L. Comparative glycoproteomics: Approaches and applications. Briefings Funct. Genom. Proteom. 2009, 8, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.-H.; Katagiri, Y.; Susarla, B.; Figge, D.; Symes, A.; Geller, H.M. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J. Comp. Neurol. 2012, 520, 3295–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myer, D.J.; Gurkoff, G.G.; Lee, S.M.; Hovda, D.A.; Sofroniew, M.V. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 2006, 129, 2761–2772. [Google Scholar] [CrossRef]
- Desplats, P.A.; Denny, C.A.; Kass, K.E.; Gilmartin, T.; Head, S.R.; Sutcliffe, J.G.; Seyfried, T.N.; Thomas, E.A. Glycolipid and ganglioside metabolism imbalances in Huntington’s disease. Neurobiol. Dis. 2007, 27, 265–277. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, N.B. Chronic neurodegenerative consequences of traumatic brain injury. Restor. Neurol. Neurosci. 2014, 32, 337–365. [Google Scholar] [CrossRef]
- Silveyra, M.-X.; Cuadrado-Corrales, N.; Marcos, A.; Barquero, M.-S.; Rabano, A.; Calero, M.; Saez-Valero, J. Altered glycosylation of acetylcholinesterase in Creutzfeldt-Jakob disease. J. Neurochem. 2006, 96, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Goodyear, C. Glycolipid antigens and autoantibodies in autoimmune neuropathies. Trends Immunol. 2013, 34, 453–459. [Google Scholar] [CrossRef]
- Karis, K.; Eskla, K.-L.; Kaare, M.; Täht, K.; Tuusov, J.; Visnapuu, T.; Innos, J.; Jayaram, M.; Timmusk, T.; Weickert, C.S.; et al. Altered expression profile of IgLON family of neural cell adhesion molecules in the dorsolateral prefrontal cortex of schizophrenic patients. Front. Mol. Neurosci. 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhou, L.; Yu, Y.; Fan, H.; Fan, F.; Tan, S.; Wang, Z.; Boz, Z.; Shi, J.; Yang, F.; et al. Serum NCAM levels and cognitive deficits in first episode schizophrenia patients versus health controls. Schizophr. Res. 2018, 192, 457–458. [Google Scholar] [CrossRef] [Green Version]
- Pivac, N.; Knežević, A.; Gornik, O.; Pučić, M.; Igl, W.; Peeters, H.; Crepel, A.; Steyaert, J.; Novokmet, M.; Redžić, I.; et al. Human plasma glycome in attention-deficit hyperactivity disorder and autism spectrum disorders. Mol. Cell. Proteom. 2011, 10, 110–004200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagata, H.; Uchida, S.; Matsuo, K.; Harada, K.; Kobayashi, A.; Nakashima, M.; Higuchi, F.; Watanuki, T.; Matsubara, T.; Watanabe, Y. Altered plasma protein glycosylation in a mouse model of depression and in patients with major depression. J. Affect. Disord. 2018, 233, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, Y.; Liu, A. Increased levels of serum glycosylated hemoglobin are associated with depressive symptoms in a population with cancer (>/=49 Years): An antidepressant-stratified analysis. Clin. Interv. Aging 2021, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Harmer, B.; Lee, S.; Duong, T.V.H.; Saadabadi, A. Suicidal Ideation; StatPearls: Treasure Island, FL, USA, 2021.
- Song, J.; Sjölander, A.; Joas, E.; Bergen, S.; Runeson, B.; Larsson, H.; Landén, M.; Lichtenstein, P. suicidal behavior during lithium and valproate treatment: A within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am. J. Psychiatry 2017, 174, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pinto, A.; Mosquera, F.; Alonso, M.; López, P.; Ramírez, F.; Vieta, E.; Baldessarini, R.J. Suicidal risk in bipolar I disorder patients and adherence to long-term lithium treatment. Bipolar Disord. 2006, 8, 618–624. [Google Scholar] [CrossRef]
- Wasserman, D.; Rihmer, Z.; Rujescu, D.; Sarchiapone, M.; Sokolowski, M.; Titelman, D.; Zalsman, G.; Zemishlany, Z.; Carli, V. The European Psychiatric Association (EPA) guidance on suicide treatment and prevention. Eur. Psychiatry 2012, 27, 129–141. [Google Scholar] [CrossRef] [PubMed]






| References | Sample | Main Findings | Type of Study |
|---|---|---|---|
| [16] | 79 individuals with bipolar I 30 individuals with bipolar II 86 healthy controls | No association between bipolar disorder and the SERT gene. No association was found between suicidal behavior and the SERT gene. | Candidate gene, cases and controls |
| [17] | 67 individuals with depressive disorders 28 individuals with bipolar disorder 106 healthy controls | No association between the 5-HT2A polymorphism 1438G/A and the patient group or suicide attempts. | Candidate gene, cases and controls |
| [18] | 46 individuals with depressive disorders 34 individuals with bipolar disorder 92 healthy controls | No association between the serotonin transporter polymorphism in SLC6A4 gene and mood disorders or suicide attempts. | Candidate gene, cases and controls |
| [19] | 70 individuals with a history of suicide attempts and various psychiatric disorders 42 individuals with MDD 10 individuals with bipolar disorder 97 healthy controls | No association between the G2457A polymorphism in ABCG1 gene and affective disorders or suicidal behavior. | Candidate gene, cases and controls |
| [20] | 2025 affected relative pairs with depressive disorders and mood disorders | Significant association between regions at 2p, 5q, 6q, 11q and Xq and suicide attempt. Strongest evidence for the phenotype Depression Spectrum Disorder was found at D8S1145 marker at 8p22-p21. Significant association between recurrent, early-onset major depressive disorder (RE-MDD) and Xq at DXS1047 marker. For all depressive phenotypes significant correlation with D8S1145 and suicide attempt. | Genome-wide linkage |
| [21] | 9265 individuals, probands with alcohol dependence and biological relatives | Significant association for the phenotype “ever tried suicide” and chromosome 2 near D2S1790. Some association between the quantitative suicidality index and chromosome 1 near D1S1602, and chromosome 3 near D1S1602. | Genome-wide linkage |
| [22] | 106 individuals with completed suicide and MDD or depression not otherwise specified 152 controls with MDD | The variants 5-HTTLPR and STin2 in 5-HTT were considered. A significant association was found between suicide completion and having at least one copy of the STin2 10 allele. Added a positive family history of suicide risk increases the risk of suicide 5.56 times after adjustment for other clinical risk factors. | Candidate gene, cases and controls |
| [23] | 1060 individuals with bipolar disorder from 154 multiplex families | Genome-wide significance between 6q25.2 at D6S2436 and suicidal behavior. Suggestive linkage was observed on 2q24.1 at D2S1353, 4p16.1 at D4S2366, 6q24.3 at D6S1848 and 10q25.3 at D10S1237. | Genome-wide linkage |
| [24] | 162 individuals, multiplex bipolar pedigrees | Suggestive linkage signal between 2p12 and suicide attempt; from D2S1394 on 2p13 to D2S2972 on 2q11, including TACR1 and TGOLN2. The second suggestive association was found at 6q26 at D6S1277. | Genome-wide linkage |
| [25] | 154 individuals with MDD 154 healthy, age and gender matched controls | No association between the Val66Met polymorphism of the BDNF and development of MDD. Significant association between the dose of the Met allele and the clinical features psychotic and suicidal behavior, which suggest association with severe MDD. | Candidate gene, cases and controls |
| [26] | 3117 individuals with bipolar disorder 1273 individuals with MDD | Suicide attempts in the bipolar sample were associated with following SNPs: rs1466846 (TBL1XR1), rs924134 (IRX2), rs6548036 (CAPN13), rs1457463 (ZNF409), rs11130703 (FLJ42117). Suicide attempts in the MDD sample were associated with following SNPs: rs2576377 (ABI3BP), rs2601098 (SLC4A4), rs1417259 (LRRC44), rs7655668 (SLC4A4), rs12462673 (HAS1), rs6737169 (ARL6IP2). None of these results were replicated. Modest support was found for candidate genes FKBP5 and NGFR (p75NTR). | Genome-wide association study |
| [27] | 2023 individuals with MDD | The quantitative SSU score showed suggested association for rs4751955 (GFRA1). For the discrete trait of serious suicidal attempts suggested association was found at rs203136 (KIAA1244). None of these results were replicated. Candidate gene analysis supported the association of a polymorphism in NTRK2 with suicidality. | Genome-wide association study |
| [28] | 2836 individuals with bipolar disorder | Associated SNP (rs300774) on 2p25 related to the ACP1 gene was marginally associated with suicide risk. | Genome-wide association study |
| [29] | 250 individuals with treatment resistant MDD | No association was found between genotyped SNPs in the COMT gene and suicide attempts and suicide risk. Significant association between suicide risk and non-responders to antidepressant treatment was found. | Candidate gene, genome-wide association study |
| [12] | 4047 individuals with MDD, recurrent MDD, and bipolar disorder | Suggestive significance for suicide attempt and Rs935194. Meta-analysis found SNPs with suggestive significance: rs17173608 (RARRES2), rs17387100 (PROM1), rs3781878 (NCAM1), rs17010519 (HK2), rs13049531 (RCAN1), rs9394433 (RNF8). Polygenic scores for MDD significantly predicted suicidal ideation, this was also found for suicide attempt in a validation dataset. | Genome-wide association study, polygenic score analysis, meta-analysis. |
| [30] | 959 individuals with bipolar disorder | Associated genes with suicide severity were found at chromosome 8q12 (LINC000968/PENK), and at chromosome 10p11.2 (CCDC7/C10orf6/ITGB1) Suggestive genes associated with suicide attempt were found at 8q12-q21 (IL7) and at 18q22 (TMX3). | Genome-wide association study, meta-analysis. |
| [31] | 475 individuals, suicide attempters and suicides 1133 controls, with MDD or healthy | No association between suicidal behavior and CNV was found at genome-wide significant level. Highlighted results were CNVs at 6p22.2 including a H1 gene cluster and at 12q12 (LRRK2). | Cases and controls, PCR. |
| [13] | 577 individuals, suicide attempters and suicides 1233 individuals, non-attempter psychiatric and healthy controls | Comparing suicidal behavior (SB) to no SB, no SNPs reached genome wide significance, five SNPs had significant levels; rs11852984 (intergenic), rs6480463 (ADAMTS14), rs4575 (PSME2/RNF31), rs336284 (TBX20) and rs3019286 (STK3). Pathway analysis identified: “Cellular assembly and organization”, “nervous system development and function”, “cell death and survival”, “immunological disease”, “infectious disease” and “inflammatory response”. | Genome-wide association study, pathway analysis |
| [32] | 660 individuals with severe suicide attempt 88 individuals with SCZ-related diagnoses 85 individuals with MDD 489 healthy individuals | The top polygenes associated with neurodevelopment and suicide attempt were: CDH4, CDH12, CDH11, CDH13, CDH20, NRXN1, NRXN3, FGF12, NELL1, EPHB2, EPHA6, GLI2, MIXL1, MAML2, MS12, NTRK3, NPAS3, ODZ4, MYCBP2. Support evidence of a polygenic neurodevelopmental etiology in SB, also in absence of major psychiatric diagnoses. | Genome-wide association study, polygenic risk scores |
| [11] | GWAS: 473 individuals, cases 9778 individuals, controls Including psychiatric disorders and suicide attempters Clinical case-control: 51 individuals, suicide attempters 112 controls | Meta-analysis found significant association between suicide attempt and a locus on chromosome 6, near MRAP2 and CEP162, this consisted of 12 SNPs, peak SNP rs12524136-T, this was replicated in a meta-analysis of all studies and ancestral subgroups. Suggestive association was found for suicide attempt and bipolar disorder regarding the polygenic risk scores. | Genome-wide association study, meta-analysis, cases and controls |
| [33] | 1780 individuals with schizophrenia 1768 healthy matched controls | A 10 times higher mortality rate as well as high risk of multiple suicide attempts was replicated for persons with schizophrenia compared to the controls. No genetic overlap was found between PRS and mortality, or between PRS and multiple suicide attempts. Family history of mental disorders was found to be associated with higher mortality and multiple suicide attempts. | Cases and controls, polygenic risk scores |
| [9] | 6569 individuals with psychiatric disorders, all suicide attempters 17232 individuals with psychiatric disorders, all non-attempters | Three significant loci were found: for MDD a SNP rs45593736 (an intron of the ARL5B), for BD an insertion-deletion polymorphism chr4_23273116_D (an intronic variant in the noncoding RNA LOC105374524). Polygenic risk scores for MDD were significantly associated with suicide attempt in MDD (R2 = 0.25%), BD (R2 = 0.24%) and schizophrenia (R2 = 0.40%). | Genome-wide association study, polygenic risk scores |
| [34] | 6320 individuals with psychiatric disorders and SUD. | One genome-wide significant SNP s1677091 (LDHB). Other associations were rs683813 (ARNTL2), rs72740082 (FAH) and s11876255. Significant genetic overlap between MDD and suicide attempt severity was estimated up to 0.7% using PRS. | Genome-wide association study, polygenic risk scores |
| [10] | 2433 individuals, all attempters, including psychiatric disorders 334766 controls 61676 individuals from electronic health records | For suicide attempt significant heritability from common variation was estimated to 4%, and significant genetic correlation was found for depressive symptoms, neuroticism, MDD, schizophrenia and insomnia. For one sample two genomic regions with genome-wide significance were identified on chromosomes 5 and 19, the most significant SNPs being rs12972617 and rs12972618. | Genome-wide association study, polygenic risk scores, machine learning |
| [14] | 6024 individuals, all attempters, including psychiatric disorders 44240 controls, non-attempters, including psychiatric disorders | Suggestive associations between SNPs, rs6880062 and rs6880461, and suicide attempt. Adjusted for mental disorders three significant associations were found on chromosome 20; rs4809706, rs4810824 and rs6019297. Heritability was found to be 4.6%, adjusted for mental disorders heritability was 1.9%. | Genome-wide association study |
| Not_Worth | Fantasies about Hurting Suicide | Tried Suicide | |
|---|---|---|---|
| T1: Case/control less similar | p = 0.209928 | p = 0.209928 | p = 0.553834 |
| T2: Case/control more similar | p = 0.790082 | p = 0.790082 | p = 0.446176 |
| T3: Case/case less similar than control/control | p = 0.207738 | p = 0.207738 | p = 0.553984 |
| T4: Case/case more similar than control/control | p = 0.792272 | p = 0.792272 | p = 0.446026 |
| T5: Case/case less similar | p = 0.200218 | p = 0.200218 | p = 0.563414 |
| T6: Case/case more similar | p = 0.799792 | p = 0.799792 | p = 0.436596 |
| T7: Control/control less similar | p = 0.791442 | p = 0.791442 | p = 0.446156 |
| T8: Control/control more similar | p = 0.208568 | p = 0.208568 | p = 0.553854 |
| T9: Case/case less similar than case/control | p = 0.788322 | p = 0.788322 | p = 0.446346 |
| T10: Case/case more similar than case/control | p = 0.211688 | p = 0.211688 | p = 0.553664 |
| T11: Control/control less similar than case/control | p = 0.790802 | p = 0.790802 | p = 0.446166 |
| T12: Control/control more similar than case/control | p = 0.209208 | p = 0.209208 | p = 0.553844 |
| Variable | Not Worth Class (Yes, No) | Hurt Class (Yes, No) | Suicide Attempters Class (Yes, No) | |
|---|---|---|---|---|
| Age | ||||
| mean: 41.69 +/− 12.26 | Yes: 41.1 +/− 11.49 | Yes: 39.85 +/− 11.14 | Yes: 35.28 +/− 11.24 | |
| No: 42.24 +/− 12.9 | No: 42.45 +/− 12.62 | No: 41.98 +/− 12.22 | ||
| t = 1.5936, df = 1153.9, p-value = 0.111 | t = 3.4719, df = 706.97, p-value = 0.0005 | t = 4.1073, df = 54.366, p-value = 0.0001 | ||
| Gender | ||||
| Males = 670 (58.01%) | Females = 485 (41.99%) | X-squared = 0.19584, df = 1, p-value = 0.6581 | X-squared = 0.74851, df = 1, p-value = 0.3869 | X-squared = 0.37699, df = 1, p-value = 0.5392 |
| Race | ||||
| Asian or Pacific Islander n = 25 (2.13%) | No Primary Race n = 6 (0.51%) | X-squared = 12.689, df = 6, p-value = 0.04826 | X-squared = 11.531, df = 6, p-value = 0.07328 | X-squared = 1.9257, df = 6, p-value = 0.9264 |
| Black or African American n = 53 (4.51%) | Other, Specify n = 8 (0.68%) | |||
| Native American, Eskimo or Aleut n = 5 (0.43%) | N/A n = 17 (1.45%) | |||
| White or Caucasian n = 1060 (90.29%) | ||||
| Marital status | ||||
| Divorced n = 234 (19.93%) | Separated/No longer living as married n = 56 (4.77%) | X-squared = 16.528, df = 6, p-value = 0.01118 | X-squared = 17.963, df = 6, p-value = 0.006327 | X-squared = 11.739, df = 6, p-value = 0.06806 |
| Living as Married n = 28 (2.39%) | Widowed n = 15 (1.28%) | |||
| Married n = 435 (37.05%) | Unknown n = 17 (1.45%) | |||
| Never Married (never lived as) n = 389 (33.13%) | ||||
| Living alone | ||||
| Yes n = 297 (25.3%) | Unknown n = 16 (1.36%) | X-squared = 11.023, df = 2, p-value = 0.00404 | X-squared = 2.4716, df = 2, p-value = 0.2906 | X-squared = 1.6656, df = 2, p-value = 0.4348 |
| No n = 861 (73.34%) | ||||
| Less than seventh grade n = 0 (0%) | College Diploma (Bachelors Degree) n = 342 (29.13%) | X-squared = 13.408, df = 7, p-value = 0.06278 | X-squared = 6.6033, df = 7, p-value = 0.4713 | X-squared = 14.007, df = 7, p-value = 0.05105 |
| Seventh grade–ninth grade n = 7 (0.6%) | Technical School or Associates Degree n = 131 (11.16%) | |||
| Partial High School n = 17 (1.45%) | Graduate or Professional Degree n = 219 (18.65%) | |||
| High School Diploma or GED n = 156 (13.29%) | Unknown n = 17 (1.45%) | |||
| Some college (at least one year) n = 285 (24.28%) | ||||
| Job | ||||
| Clerical and sales workers n = 237 (20.19%) | Professional n = 349 (29.73%) | X-squared = 12.654, df = 6, p-value = 0.04888 | X-squared = 13.007, df = 6, p-value = 0.04292 | X-squared = 11.523, df = 6, p-value = 0.07349 |
| Craftsmen and kindred workers n = 135 (11.5%) | Other n = 170 (14.48%) | |||
| Laborers, operatives and kindred workers n = 91 (7.75%) | Unknown n = 40 (3.41%) | |||
| Managers and administrators n = 152 (12.95%) | ||||
| Employment | ||||
| Disabled n = 230 (19.59%) | Part-time for pay n = 164 (13.97%) | X-squared = 31.957, df = 8, p-value = 9.48 × 10−5 | X-squared = 14.495, df = 8, p-value = 0.06974 | X-squared = 13.29, df = 8, p-value = 0.1022 |
| Full-time n = 358 (30.49%) | Retired n = 46 (3.92%) | |||
| Homemaker n = 56 (4.77%) | Unemployed n = 259 (22.06%) | |||
| Leave of Absence n = 22 (1.87%) | Unknown n = 22 (1.87%) | |||
| Other n = 17 (1.45%) | ||||
| Earnings | ||||
| less than $10,000 n = 565 (48.13%) | $75,000–$99,999 n = 31 (2.64%) | X-squared = 18.28, df = 10, p-value = 0.05043 | X-squared = 5.8668, df = 10, p-value = 0.8263 | X-squared = 6.8626, df = 10, p-value = 0.7384 |
| $10,000–$19,999 n = 141 (12.01%) | $100,000–$149,999 n = 17 (1.45%) | |||
| $20,000–$29,999 n = 110 (9.37%) | $150,000 or more n = 18 (1.53%) | |||
| $30,000–$39,999 n = 96 (8.18%) | Refused n = 3 (0.26%) | |||
| $40,000–$49,999 n = 63 (5.37%) | Unknown n = 40 (3.41%) | |||
| $50,000–$74,999 n = 90 (7.67%) | ||||
| Home income | ||||
| less than $10,000 n = 166 (14.14%) | $75,000–$99,999 n = 107 (9.11%) | X-squared = 33.938, df = 11, p-value = 0.0003702 | X-squared = 15.01, df = 11, p-value = 0.1821 | X-squared = 18.084, df = 11, p-value = 0.07966 |
| $10,000–$19,999 n = 149 (12.69%) | $100,000–$149,999 n = 117 (9.97%) | |||
| $20,000–$29,999 n = 125 (10.65%) | $150,000–$199,999 n = 40 (3.41%) | |||
| $30,000–$39,999 n = 95 (8.09%) | $200,000 or more n = 37 (3.15%) | |||
| $40,000–$49,999 n = 86 (7.33%) | Refused n = 11 (0.94%) | |||
| $50,000–$74,999 n = 150 (12.78%) | Unknown n = 91 (7.75%) | |||
| Personal income | ||||
| less than $10,000 n = 454 (38.67%) | $75,000–$99,999 n = 36 (3.07%) | X-squared = 17.176, df = 10, p-value = 0.07057 | X-squared = 8.5879, df = 10, p-value = 0.5716 | X-squared = 10.109, df = 10, p-value = 0.431 |
| $10,000–$19,999 n = 199 (16.95%) | $100,000–$149,999 n = 32 (2.73%) | |||
| $20,000–$29,999 n = 122 (10.39%) | $150,000 or more n = 25 (2.13%) | |||
| $30,000–$39,999 n = 106 (9.03%) | Refused n = 4 (0.34%) | |||
| $40,000–$49,999 n = 69 (5.88%) | Unknown n = 43 (3.66%) | |||
| $50,000–$74,999 n = 84 (7.16%) | ||||
| Medical insurance | ||||
| Yes n = 955 (81.35%) | Unknown n = 23 (1.96%) | X-squared = 7.4714, df = 2, p-value = 0.02386 | X-squared = 0.35551, df = 2, p-value = 0.8371 | X-squared = 0.32662, df = 2, p-value = 0.8493 |
| No n = 196 (16.7%) | ||||
| Limited mental care | ||||
| Yes n = 469 (39.95%) | N/A n = 448 (38.16%) | X-squared = 23.657, df = 3, p-value = 2.945 × 10−5 | X-squared = 3.0221, df = 3, p-value = 0.3882 | X-squared = 3.6797, df = 3, p-value = 0.2982 |
| No n = 167 (14.22%) | Unknown n = 90 (7.67%) | |||
| inpatient day per year (Number of days admitted in the hospital as inpatients) | ||||
| Yes n = 351 (29.9%) | N/A n = 705 (60.05%) | X-squared = 16.111, df = 3, p-value = 0.001076 | X-squared = 3.9117, df = 3, p-value = 0.2712 | X-squared = 2.7955, df = 3, p-value = 0.4242 |
| No n = 72 (6.13%) | Unknown n = 46 (3.92%) | |||
| outpatient day per year (Number of days admitted in the hospital as outpatients) | ||||
| Yes n = 398 (33.9%) | N/A n = 705 (60.05%) | X-squared = 13.383, df = 3, p-value = 0.003878 | X-squared = 2.3971, df = 3, p-value = 0.4942 | X-squared = 2.7176, df = 3, p-value = 0.4372 |
| No n = 31 (2.64%) | Unknown n = 40 (3.41%) | |||
| Life not worth living | ||||
| Yes n = 567 | No n = 607 | / | / | / |
| Fantasies on a violent suicide | ||||
| Yes n = 347 | No n = 827 | / | / | / |
| Attempted suicide | ||||
| Yes n = 51 | No n = 1123 | / | / | / |
| Overview (on GRCh38.13) | |||
|---|---|---|---|
| Mutation | Gene | ||
| Name: | rs2767403 | 9:94811838 C/G | C9orf3 (AOPEP) |
| HGVS Nomenclature: | ENST00000277198.6:c.1364+10836C > G | 9:94726669-95148264 | |
| Analysis Results | |||
| Signal | Interpretation | ||
| New Donor splice site | Activation of a cryptic Donor site. Potential alteration of splicing | ||
| Details | |||
| Name | Position | Sequences | Variation |
| HSF Donor site (matrix GT) | chr9:94811835 | TCTCTCTGA > TCTGTCTGA | 38.58 > 65.72 (70.35%) |
| Analysis in silico was performed with Genomnis Human Splicing Finder software (www.genomnis.com/, accessed on 21 April 2021) | |||
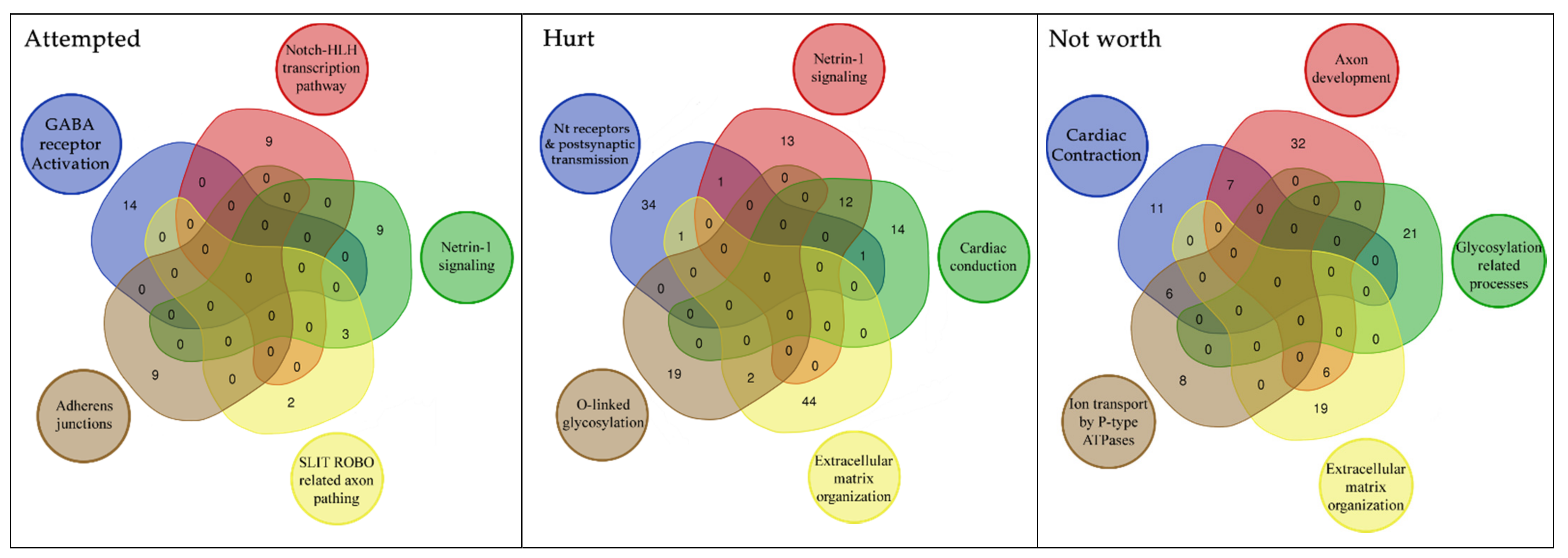
| Attempted | Not Worth | ||||||
|---|---|---|---|---|---|---|---|
| ID | Description | p.adjust | q Value | ID | Description | p.adjust | q Value |
| R-HSA-112316 | Neuronal System | 5.07 × 10−6 | 4.83 × 10−6 | R-HSA-5576891 | Cardiac conduction | 1.04 × 10−5 | 9.97 × 10−6 |
| R-HSA-112314 | Neurotransmitter receptors and postsynaptic signal transmission | 3.78 × 10−4 | 3.60 × 10−4 | R-HSA-373752 | Netrin-1 signaling | 2.09 × 10−5 | 2.01 × 10−5 |
| R-HSA-112315 | Transmission across Chemical Synapses | 6.87 × 10−4 | 6.55 × 10−4 | R-HSA-428542 | Regulation of commissural axon pathfinding by SLIT and ROBO | 3.17 × 10−5 | 3.04 × 10−5 |
| R-HSA-977443 | GABA receptor activation | 1.46 × 10−2 | 1.39 × 10−2 | R-HSA-112316 | Neuronal System | 4.16 × 10−5 | 3.99 × 10−5 |
| R-HSA-350054 | NOTCH-HLH transcription pathway | 1.84 × 10−2 | 1.76 × 10−2 | R-HSA-445095 | Interaction between L1 and Ankyrins | 8.71 × 10−5 | 8.35 × 10−5 |
| R-HSA-373752 | Netrin-1 signaling | 2.50 × 10−2 | 2.38 × 10−2 | R-HSA-397014 | Muscle contraction | 9.81 × 10−5 | 9.41 × 10−5 |
| R-HSA-446728 | Cell junction organization | 2.70 × 10−2 | 2.58 × 10−2 | R-HSA-373760 | L1CAM interactions | 1.50 × 10−4 | 1.43 × 10−4 |
| R-HSA-1500931 | Cell-Cell communication | 3.27 × 10−2 | 3.12 × 10−2 | R-HSA-5083635 | Defective B3GALTL causes Peters-plus syndrome (PpS) | 5.91 × 10−4 | 5.67 × 10−4 |
| R-HSA-428542 | Regulation of commissural axon pathfinding by SLIT and ROBO | 3.75 × 10−2 | 3.57 × 10−2 | R-HSA-5173214 | O-glycosylation of TSR domain-containing proteins | 7.40 × 10−4 | 7.09 × 10−4 |
| R-HSA-418990 | Adherens junctions interactions | 3.75 × 10−2 | 3.57 × 10−2 | R-HSA-1650814 | Collagen biosynthesis and modifying enzymes | 2.40 × 10−3 | 2.30 × 10−3 |
| R-HSA-421270 | Cell-cell junction organization | 4.29 × 10−2 | 4.10 × 10−2 | R-HSA-1474244 | Extracellular matrix organization | 2.81 × 10−3 | 2.69 × 10−3 |
| Hurt | R-HSA-8948216 | Collagen chain trimerization | 3.18 × 10−3 | 3.05 × 10−3 | |||
| ID | Description | p.adjust | q Value | R-HSA-375165 | NCAM signaling for neurite out-growth | 3.18 × 10−3 | 3.05 × 10−3 |
| R-HSA-112316 | Neuronal System | 2.23 × 10−7 | 2.11 × 10−7 | R-HSA-983712 | Ion channel transport | 3.82 × 10−3 | 3.66 × 10−3 |
| R-HSA-5576891 | Cardiac conduction | 2.23 × 10−7 | 2.11 × 10−7 | R-HSA-3000178 | ECM proteoglycans | 8.98 × 10−3 | 8.61 × 10−3 |
| R-HSA-397014 | Muscle contraction | 2.49 × 10−6 | 2.36 × 10−6 | R-HSA-2022928 | HS-GAG biosynthesis | 9.01 × 10−3 | 8.64 × 10−3 |
| R-HSA-112315 | Transmission across Chemical Synapses | 4.62 × 10−4 | 4.37 × 10−4 | R-HSA-5578775 | Ion homeostasis | 9.01 × 10−3 | 8.64 × 10−3 |
| R-HSA-373760 | L1CAM interactions | 4.62 × 10−4 | 4.37 × 10−4 | R-HSA-936837 | Ion transport by P-type ATPases | 1.04 × 10−2 | 1.00 × 10−2 |
| R-HSA-445095 | Interaction between L1 and Ankyrins | 8.83 × 10−4 | 8.37 × 10−4 | R-HSA-5173105 | O-linked glycosylation | 1.49 × 10−2 | 1.43 × 10−2 |
| R-HSA-112314 | Neurotransmitter receptors and postsynaptic signal transmission | 1.60 × 10−3 | 1.52 × 10−3 | R-HSA-5576892 | Phase 0—rapid depolarization | 1.71 × 10−2 | 1.64 × 10−2 |
| R-HSA-5576892 | Phase 0—rapid depolarization | 2.02 × 10−3 | 1.92 × 10−3 | R-HSA-3906995 | Diseases associated with O-glycosylation of proteins | 1.89 × 10−2 | 1.81 × 10−2 |
| R-HSA-1474244 | Extracellular matrix organization | 4.49 × 10−3 | 4.26 × 10−3 | R-HSA-112315 | Transmission across Chemical Synapses | 1.90 × 10−2 | 1.82 × 10−2 |
| R-HSA-5578775 | Ion homeostasis | 5.64 × 10−3 | 5.34 × 10−3 | R-HSA-419037 | NCAM1 interactions | 2.28 × 10−2 | 2.18 × 10−2 |
| R-HSA-373752 | Netrin-1 signaling | 5.64 × 10−3 | 5.34 × 10−3 | R-HSA-1474290 | Collagen formation | 4.63 × 10−2 | 4.44 × 10−2 |
| R-HSA-5173105 | O-linked glycosylation | 4.12 × 10−2 | 3.90 × 10−2 | ||||
| ID | Description | p.adjust | q Value |
|---|---|---|---|
| R-HSA-112316 | Neuronal System | 3.94 × 10−4 | 3.67 × 10−4 |
| R-HSA-9013508 | NOTCH3 Intracellular Domain Regulates Transcription | 5.05 × 10−3 | 4.71 × 10−3 |
| R-HSA-210744 | Regulation of gene expression in late stage (branching morphogenesis) pancreatic bud precursor cells | 5.05 × 10−3 | 4.71 × 10−3 |
| R-HSA-350054 | NOTCH-HLH transcription pathway | 5.82 × 10−3 | 5.42 × 10−3 |
| R-HSA-5173105 | O-linked glycosylation | 5.95 × 10−3 | 5.54 × 10−3 |
| R-HSA-8941856 | RUNX3 regulates NOTCH signaling | 2.69 × 10−2 | 2.50 × 10−2 |
| R-HSA-186712 | Regulation of β-cell development | 3.11 × 10−2 | 2.89 × 10−2 |
| R-HSA-373760 | L1CAM interactions | 3.29 × 10−2 | 3.06 × 10−2 |
| R-HSA-112315 | Transmission across Chemical Synapses | 3.36 × 10−2 | 3.13 × 10−2 |
| R-HSA-445095 | Interaction between L1 and Ankyrins | 4.48 × 10−2 | 4.17 × 10−2 |
| R-HSA-2122947 | NOTCH1 Intracellular Domain Regulates Transcription | 4.48 × 10−2 | 4.17 × 10−2 |
| R-HSA-163685 | Integration of energy metolism | 4.48 × 10−2 | 4.17 × 10−2 |
| R-HSA-9012852 | Signaling by NOTCH3 | 4.48 × 10−2 | 4.17 × 10−2 |
| R-HSA-5576892 | Phase 0—rapid depolarisation | 4.68 × 10−2 | 4.35 × 10−2 |
| R-HSA-9013695 | NOTCH4 Intracellular Domain Regulates Transcription | 4.68 × 10−2 | 4.35 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lybech, L.K.M.; Calabró, M.; Briuglia, S.; Drago, A.; Crisafulli, C. Suicide Related Phenotypes in a Bipolar Sample: Genetic Underpinnings. Genes 2021, 12, 1482. https://doi.org/10.3390/genes12101482
Lybech LKM, Calabró M, Briuglia S, Drago A, Crisafulli C. Suicide Related Phenotypes in a Bipolar Sample: Genetic Underpinnings. Genes. 2021; 12(10):1482. https://doi.org/10.3390/genes12101482
Chicago/Turabian StyleLybech, Line K. M., Marco Calabró, Silvana Briuglia, Antonio Drago, and Concetta Crisafulli. 2021. "Suicide Related Phenotypes in a Bipolar Sample: Genetic Underpinnings" Genes 12, no. 10: 1482. https://doi.org/10.3390/genes12101482
APA StyleLybech, L. K. M., Calabró, M., Briuglia, S., Drago, A., & Crisafulli, C. (2021). Suicide Related Phenotypes in a Bipolar Sample: Genetic Underpinnings. Genes, 12(10), 1482. https://doi.org/10.3390/genes12101482






