PAPPA2 Promote the Proliferation of Dermal Papilla Cells in Hu Sheep (Ovis aries) by Regulating IGFBP5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dermal Papilla Cells Culture
2.2. The Extraction of Total Cellular RNA and Synthesis of cDNA
2.3. Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
2.3.1. Primer Design
2.3.2. Operation Steps
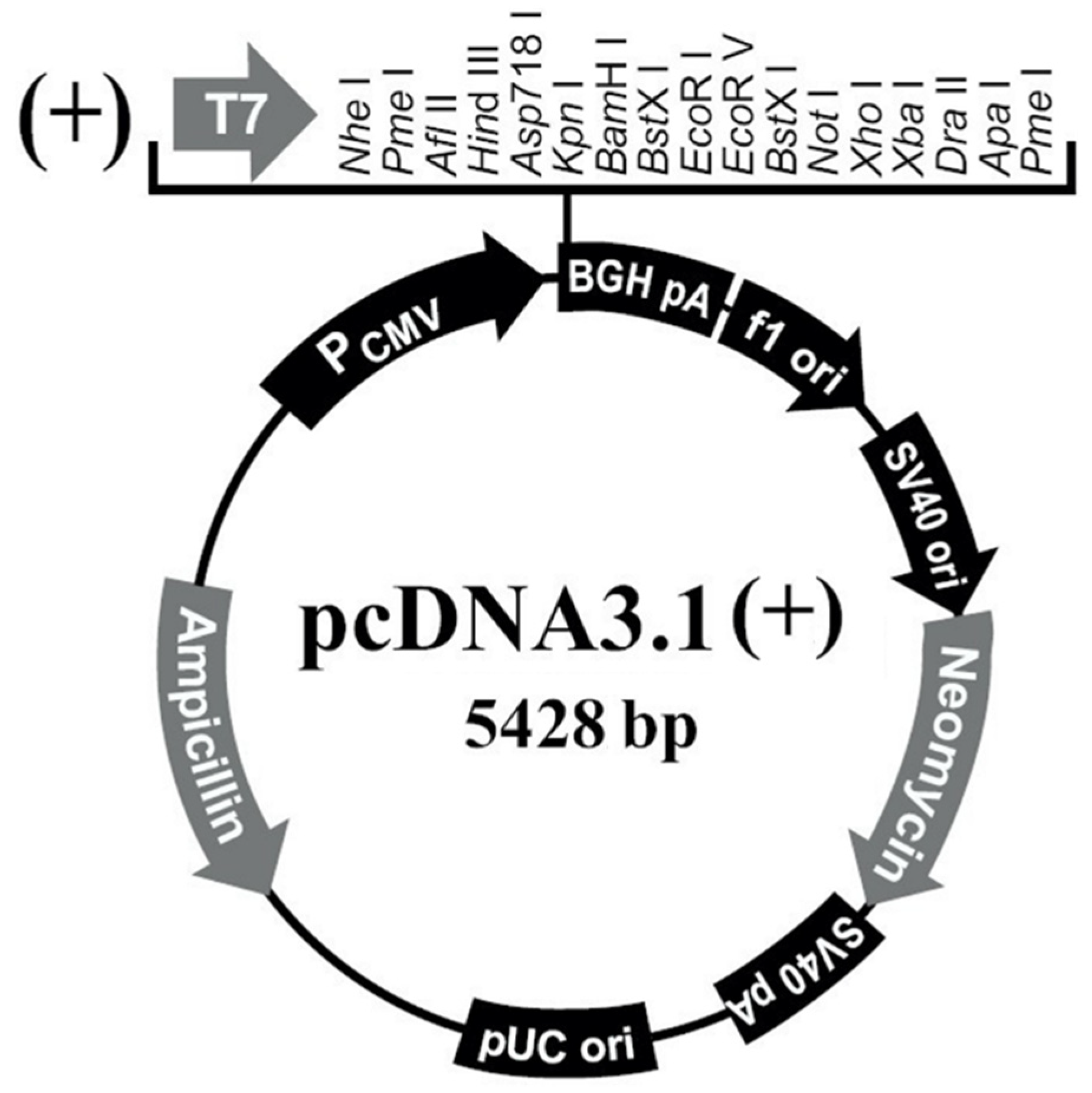
2.4. Construction of PAPPA2 Overexpression Vector
2.4.1. Primer Design
2.4.2. Full-Length High-Fidelity Amplification of the PAPPA2 CDS Region
2.4.3. Restriction of pcDNA3.1 Plasmid
2.4.4. Ligation of PAPPA2 Fragment with Linear Vector
2.4.5. Construction of IGFBP5 Overexpression Vector
2.5. Synthesis of PAPPA2 Small Interfering RNA (siRNA)
2.6. Cell Transfection
2.7. Cell Cycle Assay
2.8. EdU and High-Content Image Acquisition and Analysis
2.9. Data Analysis
3. Results
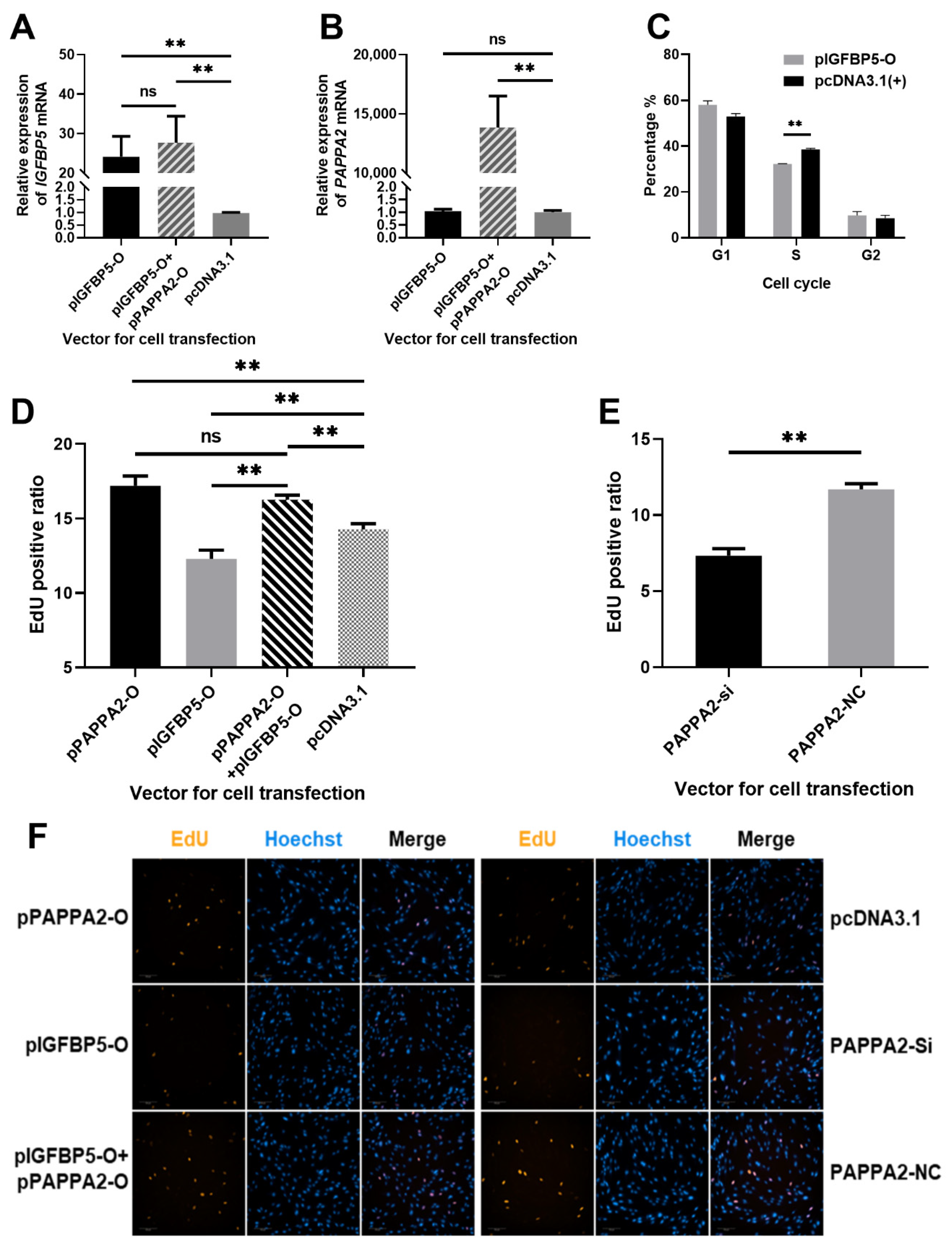
3.1. Construction of PAPPA2 and IGFBP5 Eukaryotic Overexpression Vectors
3.2. PAPPA2 Promotes the Proliferation of Hu Sheep DPCs
3.3. PAPPA2 Can Restore the Proliferation of DPCs from Inhibition by IGFBP5
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, X.; Chen, W.; Sun, W.; Hussain, Z.; Wang, S.; Wang, J. Analysis of lncRNAs Expression Profiles in Hair Follicle of Hu Sheep Lambskin. Animals 2020, 10, 1035. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Du, J.; Han, A.; Chang, Y.; Li, T.; Zhang, F.; Zhang, R. Morphological observation of pattern formation in lambskins of Hu sheep (China). Sci. Agric. Sin. 1982, 15, 73–82. [Google Scholar]
- Nissimov, J.N.; Das Chaudhuri, A.B. Hair curvature: A natural dialectic and review. Biol. Rev. Camb. Philos. Soc. 2014, 89, 723–766. [Google Scholar] [CrossRef]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Stenn, K.S.; Cotsarelis, G. Bioengineering the hair follicle: Fringe benefits of stem cell technology. Curr. Opin. Biotechnol. 2005, 16, 493–497. [Google Scholar] [CrossRef]
- Hardy, M.H. The secret life of the hair follicle. Trends Genet. 1992, 8, 55–61. [Google Scholar] [CrossRef]
- Rogers, G.E.; Reis, P.J.; Ward, K.A.; Marshall, R.C. The Biology of Wool and Hair; Springer: Berlin, Germany, 1989. [Google Scholar]
- Reddy, S.; Andl, T.; Bagasra, A.; Lu, M.M.; Epstein, D.J.; Morrisey, E.E.; Millar, S.E. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001, 107, 69–82. [Google Scholar] [CrossRef]
- Rendl, M.; Lewis, L.; Fuchs, E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005, 3, e331. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem. Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Iida, M.; Ihara, S.; Matsuzaki, T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev. Growth Differ. 2007, 49, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Du Cros, D.L.; LeBaron, R.G.; Couchman, J.R. Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J. Investig. Dermatol. 1995, 105, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Batch, J.A.; Mercuri, F.A.; Werther, G.A. Identification and localization of insulin-like growth factor-binding protein (IGFBP) messenger RNAs in human hair follicle dermal papilla. J. Investig. Dermatol. 1996, 106, 471–475. [Google Scholar] [CrossRef]
- Weger, N.; Schlake, T. Igf-I signalling controls the hair growth cycle and the differentiation of hair shafts. J. Investig. Dermatol. 2005, 125, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Sriwiriyanont, P.; Hachiya, A.; Pickens, W.L.; Moriwaki, S.; Kitahara, T.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Takema, Y.; Kobinger, G.P. Effects of IGF-binding protein 5 in dysregulating the shape of human hair. J. Investig. Dermatol. 2011, 131, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Overgaard, M.T.; Boldt, H.B.; Laursen, L.S.; Sottrup-Jensen, L.; Conover, C.A.; Oxvig, C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J. Biol. Chem. 2001, 276, 21849–21853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, M.; Hwa, V.; Dauber, A. Novel Modulators of the Growth Hormone—Insulin-Like Growth Factor Axis: Pregnancy-Associated Plasma Protein-A2 and Stanniocalcin-2. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Gkiatas, I.; Boptsi, A.; Tserga, D.; Gelalis, I.; Kosmas, D.; Pakos, E. Developmental dysplasia of the hip: A systematic literature review of the genes related with its occurrence. EFORT Open Rev. 2019, 4, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Baulcombe, D.C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 1999, 286, 950–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutvágner, G.; McLachlan, J.; Pasquinelli, A.E.; Bálint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, K.; Feng, Y.; Ren, C.; Li, W.; Xiao, J.; Fan, L.; Beejadhursing, R.; Xi, L.; Chen, S. The potential role of pregnancy-associated plasma protein-A2 in angiogenesis and development of preeclampsia. Hypertens. Res. 2019, 42, 970–980. [Google Scholar] [CrossRef]
- Dauber, A.; Muñoz-Calvo, M.T.; Barrios, V.; Domené, H.M.; Kloverpris, S.; Serra-Juhé, C.; Desikan, V.; Pozo, J.; Muzumdar, R.; Martos-Moreno, G.Á.; et al. Mutations in pregnancy-associated plasma protein A2 cause short stature due to low IGF-I availability. EMBO Mol. Med. 2016, 8, 363–374. [Google Scholar] [CrossRef]
- Muñoz-Calvo, M.T.; Barrios, V.; Pozo, J.; Chowen, J.A.; Martos-Moreno, G.Á.; Hawkins, F.; Dauber, A.; Domené, H.M.; Yakar, S.; Rosenfeld, R.G.; et al. Treatment with Recombinant Human Insulin-Like Growth Factor-1 Improves Growth in Patients With PAPP-A2 Deficiency. J. Clin. Endocrinol. Metab. 2016, 101, 3879–3883. [Google Scholar] [CrossRef] [Green Version]
- Andress, D.L.; Birnbaum, R.S. Human osteoblast-derived insulin-like growth factor (IGF) binding protein-5 stimulates osteoblast mitogenesis and potentiates IGF action. J. Biol. Chem. 1992, 267, 22467–22472. [Google Scholar] [CrossRef]
- Andress, D.L. Heparin modulates the binding of insulin-like growth factor (IGF) binding protein-5 to a membrane protein in osteoblastic cells. J. Biol. Chem. 1995, 270, 28289–28296. [Google Scholar] [CrossRef]
- Andress, D.L. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates phosphorylation of the IGFBP-5 receptor. Am. J. Physiol. 1998, 274, E744–E750. [Google Scholar] [CrossRef]
- Hwa, V.; Oh, Y.; Rosenfeld, R.G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999, 20, 761–787. [Google Scholar] [CrossRef]
- Argente, J.; Chowen, J.A.; Pérez-Jurado, L.A.; Frystyk, J.; Oxvig, C. One level up: Abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology. EMBO Mol. Med. 2017, 9, 1338–1345. [Google Scholar] [CrossRef]
- Banaszak-Ziemska, M.; Niedziela, M. PAPP-A2 a new key regulator of growth. Endokrynol. Pol. 2017, 68, 682–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Bruick, R.K.; Yu, Y. Secreted IGFBP5 mediates mTORC1-dependent feedback inhibition of IGF-1 signalling. Nat. Cell Biol. 2016, 18, 319–327. [Google Scholar] [CrossRef]
- Chi, W.; Wu, E.; Morgan, B.A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013, 140, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, R.; Sun, W.; Yin, J.; Su, R.; Ding, J.; Zhang, Y.; Chen, L.; Wu, W.; Zhou, H. Histological studies on the hair follicles of lambs with different patterns in Hu sheep. Chin. J. Anim. Sci. 2013, 49, 23–25. [Google Scholar]



| Genes | Accession No. | Sequences (5′→3′) (F: Forward R: Reverse) | Product Length (bp) |
|---|---|---|---|
| PAPPA2 | XM_004013823.4 | F: CAGGGGCTCCATTCAACAAC | 124 |
| R: CTCTGGCTCCACTGCTGATAC | |||
| IGFBP5 | NM_001129733.1 | F: CTGTGACCGCAAAGGGTTCT | 135 |
| R: CACTGAAAGTCCCCGTCCAC | |||
| GAPDH | NM_001190390.1 | F: TCTCAAGGGCATTCTAGGCTAC | 151 |
| R: GCCGAATTCATTGTCGTACCAG |
| Name | Volume or Mass |
|---|---|
| pcDNA3.1(+) | 2 µg |
| 10× QuickCut Green Buffer | 4 µL |
| QuickCut™ Hind III | 2 µL |
| QuickCut™ Xho I | 2 µL |
| RNase Free ddH2O | Up to 40 µL |
| Name | Binding Sites | Sequences (5′→3′) |
|---|---|---|
| siRNA-1 | 2149 | sense: CCUCAACCCAGCCUAUUAUTT |
| antisense: AUAAUAGGCUGGGUUGAGGTT | ||
| siRNA-2 | 3965 | sense: CCGCUGGUUAUCAACAUAATT |
| antisense: UUAUGUUGAUAACCAGCGGTT | ||
| siRNA-3 | 4693 | sense: GGGAAGCACAGAGAAUAAATT |
| antisense: UUUAUUCUCUGUGCUUCCCTT | ||
| NC | - | sense: UUCUCCGAACGUGUCACGUTT |
| antisense: ACGUGACACGUUCGGAGAATT |
| Name | Position (bp) | Reference | Mutant | Mutation Type |
|---|---|---|---|---|
| SNP1 | 1116 | C | T | Missense mutation |
| SNP2 | 1171 | C | G | Same Sense mutation |
| SNP3 | 1272 | G | T | Same Sense mutation |
| SNP4 | 3489 | G | A | Same Sense mutation |
| SNP5 | 3692 | G | C | Missense mutation |
| SNP6 | 4704 | A | G | Same Sense mutation |
| SNP7 | 5089 | G | A | Missense mutation |
| Name | Position (N to C) | Reference 1 | Reference 2 | Hu Sheep |
|---|---|---|---|---|
| AAM1 | 391 | H | D | D |
| AAM2 | 1231 | R | P | P |
| AAM3 | 1442 | M | L | M |
| AAM4 | 1457 | R | G | R |
| AAM5 | 1640 | Q | R | Q |
| AAM6 | 1697 | V | I | I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wang, S.; Jin, Q.; Lv, X.; Sun, W. PAPPA2 Promote the Proliferation of Dermal Papilla Cells in Hu Sheep (Ovis aries) by Regulating IGFBP5. Genes 2021, 12, 1490. https://doi.org/10.3390/genes12101490
Wu T, Wang S, Jin Q, Lv X, Sun W. PAPPA2 Promote the Proliferation of Dermal Papilla Cells in Hu Sheep (Ovis aries) by Regulating IGFBP5. Genes. 2021; 12(10):1490. https://doi.org/10.3390/genes12101490
Chicago/Turabian StyleWu, Tianyi, Shanhe Wang, Qiunan Jin, Xiaoyang Lv, and Wei Sun. 2021. "PAPPA2 Promote the Proliferation of Dermal Papilla Cells in Hu Sheep (Ovis aries) by Regulating IGFBP5" Genes 12, no. 10: 1490. https://doi.org/10.3390/genes12101490
APA StyleWu, T., Wang, S., Jin, Q., Lv, X., & Sun, W. (2021). PAPPA2 Promote the Proliferation of Dermal Papilla Cells in Hu Sheep (Ovis aries) by Regulating IGFBP5. Genes, 12(10), 1490. https://doi.org/10.3390/genes12101490







