Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation and Protects Mice from Radiation Gastrointestinal Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Survival Assays
2.3. Apoptosis Assay
2.4. Colony Formation Assay
2.5. Immunoblotting
2.6. Immunofluorescence
2.7. Mice
2.8. Mouse Toxicity Test
2.9. Induction of Acute Radiation Syndromes
2.10. Statistical Analyses
3. Results
3.1. Isorhamnetin Inhibits Radiation Cell Death
3.2. Isorhamnetin Inhibits Radiation Cell Death Independent of p53-Mediated Apoptosis
3.3. Isorhamnetin Protects Mice from SBI-Induced GI Death
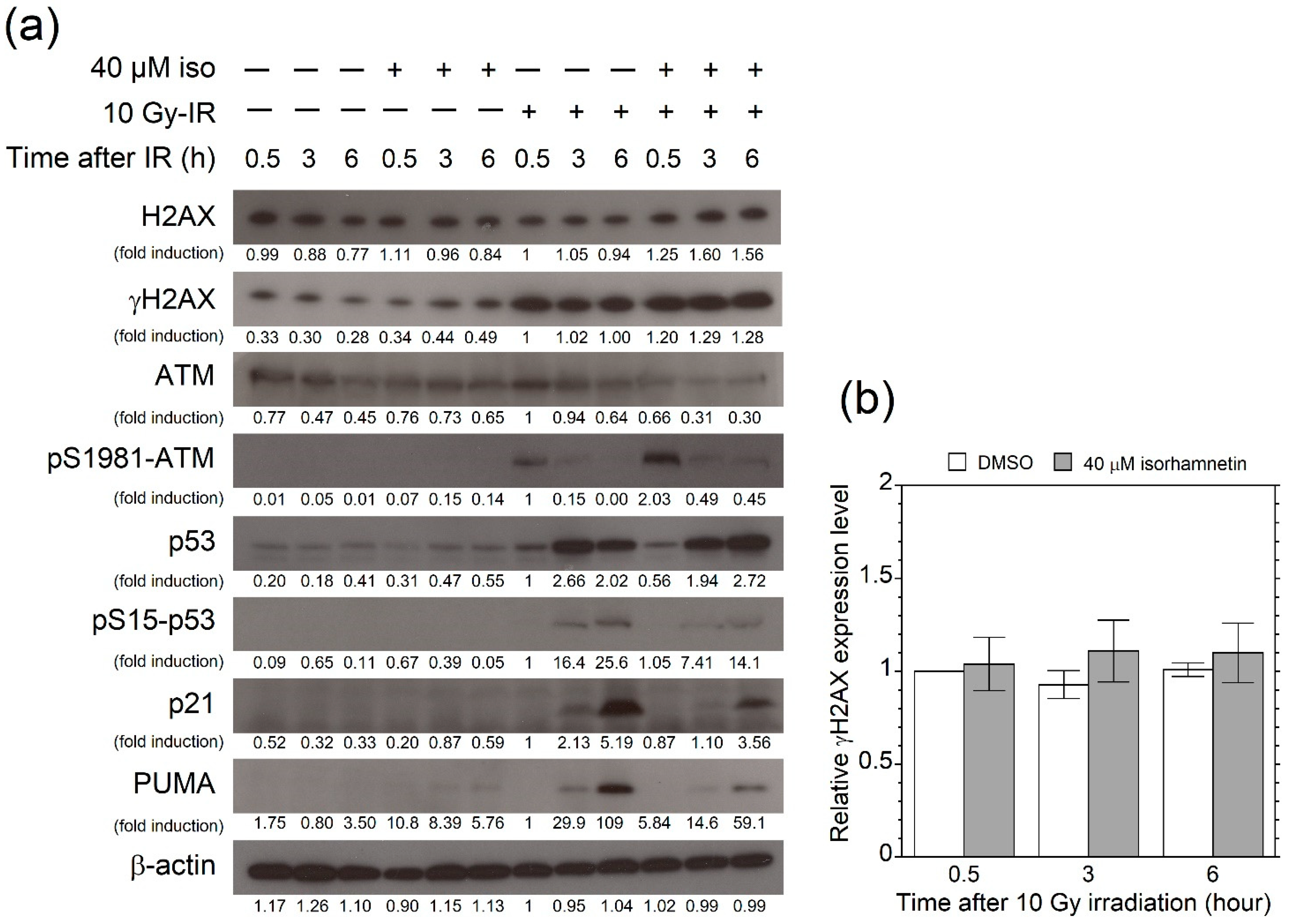
3.4. Isorhamnetin Promotes Phosphorylation of ATM after Irradiation
3.5. Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, S.W.; Schmitt, E.M.; Smith, S.W.; Osborne, B.A.; Jacks, T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 1993, 362, 847–849. [Google Scholar] [CrossRef]
- Kuerbitz, S.J.; Plunkett, B.S.; Walsh, W.V.; Kastan, M.B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc. Natl. Acad. Sci. USA 1992, 89, 7491–7495. [Google Scholar] [CrossRef]
- Nelson, W.G.; Kastan, M.B. DNA strand breaks: The DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol. Cell. Biol. 1994, 14, 1815–1823. [Google Scholar] [CrossRef]
- Komarova, E.A.; Kondratov, R.V.; Wang, K.; Christov, K.; Golovkina, T.V.; Goldblum, J.R.; Gudkov, A.V. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 2004, 23, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.G.; Santiago, P.M.; di Tomaso, E.; Sullivan, J.M.; Hou, W.-S.; Dayton, T.; Jeffords, L.B.; Sodha, P.; Mercer, K.L.; Cohen, R.; et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science 2010, 327, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Morita, A.; Wang, B.; Sakai, T.; Ramadhani, D.; Satoh, H.; Tanaka, K.; Sasatani, M.; Ochi, S.; Tominaga, M.; et al. Evaluation of sodium orthovanadate as a radioprotective agent under total-body irradiation and partial-body irradiation conditions in mice. Int. J. Radiat. Biol. 2021, 97, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Strom, E.; Sathe, S.; Komarov, P.G.; Chernova, O.B.; Pavlovska, I.; Shyshynova, I.; Bosykh, D.A.; Burdelya, L.G.; Macklis, R.M.; Skaliter, R.; et al. Small-molecule inhibitor of p53 binding to mitochondria protects mice from γ radiation. Nat. Chem. Biol. 2006, 2, 474–479. [Google Scholar] [CrossRef]
- Komarov, P.G.; Komarova, E.A.; Kondratov, R.V.; Christov-Tselkov, K.; Coon, J.S.; Chernov, M.V.; Gudkov, A.V. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999, 285, 1733–1737. [Google Scholar] [CrossRef]
- Morita, A.; Ariyasu, S.; Wang, B.; Asanuma, T.; Onoda, T.; Sawa, A.; Tanaka, K.; Takahashi, I.; Togami, S.; Nenoi, M.; et al. AS-2, a novel inhibitor of p53-dependent apoptosis, prevents apoptotic mitochondrial dysfunction in a transcription-independent manner and protects mice from a lethal dose of ionizing radiation. Biochem. Biophys. Res. Commun. 2014, 450, 1498–1504. [Google Scholar] [CrossRef]
- Morita, A.; Yamamoto, S.; Wang, B.; Tanaka, K.; Suzuki, N.; Aoki, S.; Ito, A.; Nanao, T.; Ohya, S.; Yoshino, M.; et al. Sodium orthovanadate inhibits p53-mediated apoptosis. Cancer Res. 2010, 70, 257–265. [Google Scholar] [CrossRef]
- Morita, A.; Takahashi, I.; Sasatani, M.; Aoki, S.; Wang, B.; Ariyasu, S.; Tanaka, K.; Yamaguchi, T.; Sawa, A.; Nishi, Y.; et al. A chemical modulator of p53 transactivation that acts as a radioprotective agonist. Mol. Cancer Ther. 2018, 17, 432–442. [Google Scholar] [CrossRef]
- Chen, J.; Chen, A.Y.; Huang, H.; Ye, X.; Rollyson, W.D.; Perry, H.E.; Brown, K.C.; Rojanasakul, Y.; Rankin, G.O.; Dasgupta, P.; et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Begum, N.; Prasad, N.R.; Thayalan, K. Apigenin protects γ-radiation induced oxidative stress, hematological changes and animal survival in whole body irradiated Swiss albino mice. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 45–52. [Google Scholar]
- Begum, N.; Prasad, N.R.; Kanimozhi, G.; Agilan, B. Apigenin prevents γ radiation-induced gastrointestinal damages by modulating inflammatory and apoptotic signalling mediators. Nat. Prod. Res. 2021. [Google Scholar] [CrossRef]
- Gu, Y.H.; Yamashita, T.; Inoue, T.; Kang, K.M. Inhibition of radioactive depletion of hemocytes and antitumor effects of flavonoid. Arch. Clin. Med. Case. Rep. 2021, 5, 118–128. [Google Scholar] [CrossRef]
- Cheng, J.; Haas, M. Frequent mutations in the p53 tumor suppressor gene in human leukemia T-cell lines. Mol. Cell. Biol. 1990, 10, 5502–5509. [Google Scholar] [CrossRef]
- Nakano, H.; Kohara, M.; Shinohara, K. Evaluation of the relative contribution of p53-mediated pathway in X-ray-induced apoptosis in human leukemic MOLT-4 cells by transfection with a mutant p53 gene at different expression levels. Cell Tissue Res. 2001, 306, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Ariyasu, S.; Ohya, S.; Takahashi, I.; Wang, B.; Tanaka, K.; Uchida, T.; Okazaki, H.; Hanaya, K.; Enomoto, A.; et al. Evaluation of zinc (II) chelators for inhibiting p53-mediated apoptosis. Oncotarget 2013, 4, 2439–2450. [Google Scholar] [CrossRef]
- Morita, A.; Zhu, J.; Suzuki, N.; Enomoto, A.; Matsumoto, Y.; Tomita, M.; Suzuki, T.; Ohtomo, K.; Hosoi, Y. Sodium orthovanadate suppresses DNA damage-induced caspase activation and apoptosis by inactivating p53. Cell Death Differ. 2006, 13, 499–511. [Google Scholar] [CrossRef]
- Lopez-Terrada, D.; Cheung, S.W.; Finegold, M.J.; Knowles, B.B. Hep G2 is a hepatoblastoma-derived cell line. Hum. Pathol. 2009, 40, 1512–1515. [Google Scholar] [CrossRef]
- Terry, N.H.; Travis, E.L. The influence of bone marrow depletion on intestinal radiation damage. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 569–573. [Google Scholar] [CrossRef]
- Radford, I.R.; Murphy, T.K.; Radley, J.M.; Ellis, S.L. Radiation response of mouse lymphoid and myeloid cell lines. Part II. Apoptotic death is shown by all lines examined. Int. J. Radiat. Biol. 1994, 65, 217–227. [Google Scholar] [CrossRef]
- Garcia-Cao, I.; Garcia-Cao, M.; Martin-Caballero, J.; Criado, L.M.; Klatt, P.; Flores, J.M.; Weill, J.C.; Blasco, M.A.; Serrano, M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002, 21, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Pant, V.; Xiong, S.; Wasylishen, A.R.; Larsson, C.A.; Aryal, N.K.; Chau, G.; Tailor, R.C.; Lozano, G. Transient enhancement of p53 activity protects from radiation-induced gastrointestinal toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 17429–17437. [Google Scholar] [CrossRef]
- Wang, B.; Matsuoka, S.; Carpenter, P.B.; Elledge, S.J. 53BP1, a mediator of the DNA damage checkpoint. Science 2002, 298, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Bunting, S.F.; Callen, E.; Wong, N.; Chen, H.T.; Polato, F.; Gunn, A.; Bothmer, A.; Feldhahn, N.; Fernandez-Capetillo, O.; Cao, L.; et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 2010, 141, 243–254. [Google Scholar] [CrossRef]
- Ward, I.M.; Minn, K.; Jorda, K.G.; Chen, J. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J. Biol. Chem. 2003, 278, 19579–19582. [Google Scholar] [CrossRef]
- Morales, J.C.; Xia, Z.; Lu, T.; Aldrich, M.B.; Wang, B.; Rosales, C.; Kellems, R.E.; Hittelman, W.N.; Elledge, S.J.; Carpenter, P.B. Role for the BRCA1 C-terminal repeats (BRCT) protein 53BP1 in maintaining genomic stability. J. Biol. Chem. 2003, 278, 14971–14977. [Google Scholar] [CrossRef]
- Ward, I.M.; Minn, K.; van Deursen, J.; Chen, J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol. Cell. Biol. 2003, 23, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Jones, N.; Pryde, F.; Adachi, Y.; Sansom, O.J. 53BP1 deficiency in intestinal enterocytes does not alter the immediate response to ionizing radiation, but leads to increased nuclear area consistent with polyploidy. Oncogene 2007, 26, 6349–6355. [Google Scholar] [CrossRef] [PubMed][Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, Y.; Morita, A.; Tatsuta, S.; Kanamaru, M.; Sakaue, M.; Ueda, K.; Shono, M.; Fujita, R.; Wang, B.; Hosoi, Y.; et al. Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation and Protects Mice from Radiation Gastrointestinal Syndrome. Genes 2021, 12, 1514. https://doi.org/10.3390/genes12101514
Nishiyama Y, Morita A, Tatsuta S, Kanamaru M, Sakaue M, Ueda K, Shono M, Fujita R, Wang B, Hosoi Y, et al. Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation and Protects Mice from Radiation Gastrointestinal Syndrome. Genes. 2021; 12(10):1514. https://doi.org/10.3390/genes12101514
Chicago/Turabian StyleNishiyama, Yuichi, Akinori Morita, Shogo Tatsuta, Misaki Kanamaru, Masahiro Sakaue, Kenta Ueda, Manami Shono, Rie Fujita, Bing Wang, Yoshio Hosoi, and et al. 2021. "Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation and Protects Mice from Radiation Gastrointestinal Syndrome" Genes 12, no. 10: 1514. https://doi.org/10.3390/genes12101514
APA StyleNishiyama, Y., Morita, A., Tatsuta, S., Kanamaru, M., Sakaue, M., Ueda, K., Shono, M., Fujita, R., Wang, B., Hosoi, Y., Aoki, S., & Sugai, T. (2021). Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation and Protects Mice from Radiation Gastrointestinal Syndrome. Genes, 12(10), 1514. https://doi.org/10.3390/genes12101514








