Renal Cell Carcinoma in Tuberous Sclerosis Complex
Abstract
:1. Tuberous Sclerosis Complex: Genetic and Clinical Features
1.1. TSC Overview
1.2. Renal Manifestations of TSC
2. TSC-RCC, Clinical and Pathologic Features
| Authors | Types of RCC Seen in TSC Patients | # Type of Tumor/Total | % |
|---|---|---|---|
| Yang et al. [18] | TSC-associated papillary RCC | 24/46 | 52 |
| Hybrid oncocytic chromophobe tumor | 15/46 | 32 | |
| Unclassifiable | 7/46 | 15 | |
| Guo et al. [17] | Chromophobe | 34/57 | 59 |
| Eosinophilic hybrid macrocystic | 6/57 | 10 | |
| RCC with smooth muscle stroma | 17/57 | 29 |
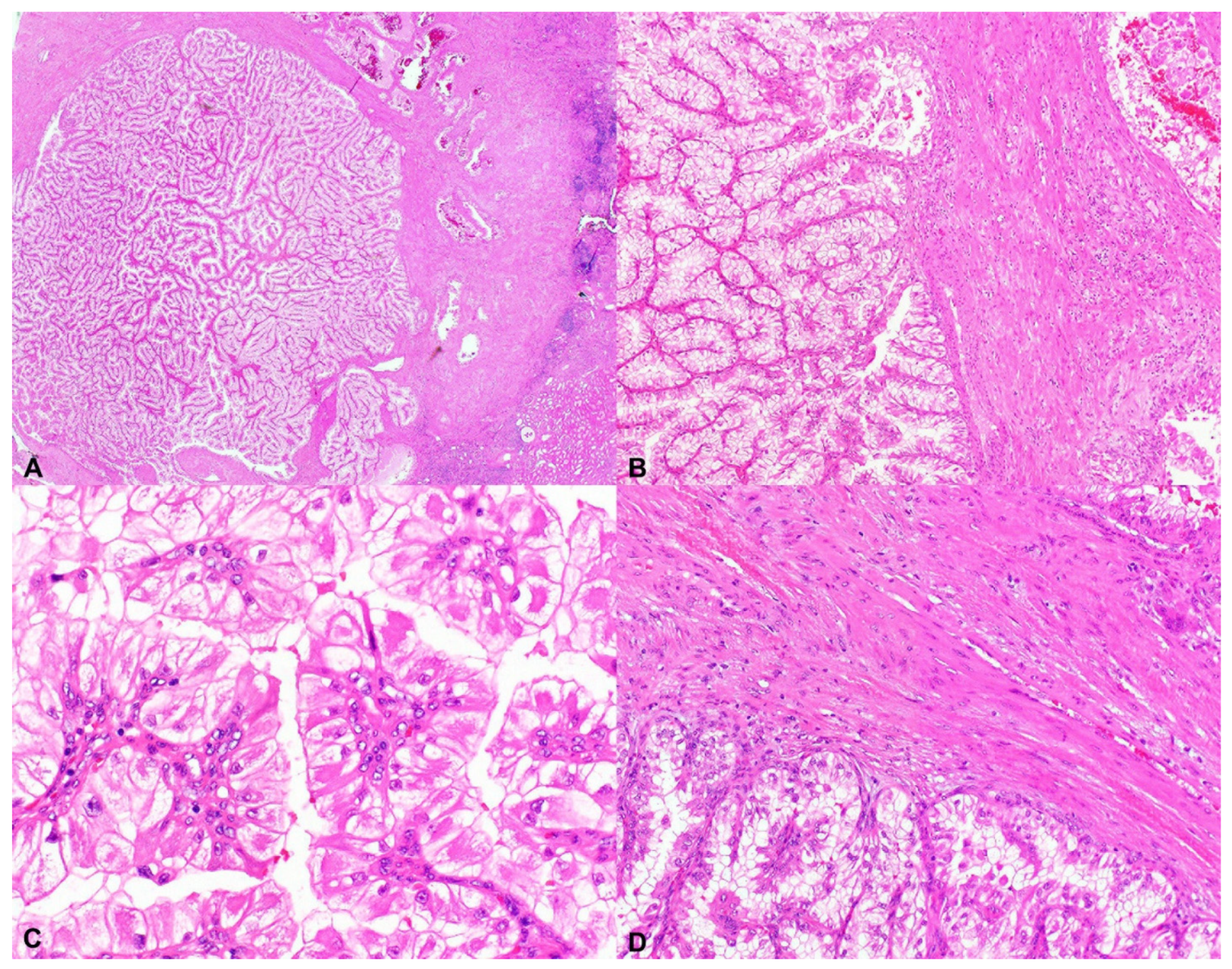
2.1. Tuberous Sclerosis Complex-Associated Papillary Renal Cell Carcinoma (TSC-Associated PRCC)
2.2. Hybrid Oncocytic/Chromophobe Tumor (HOCT); Chromophobe-Like RCC
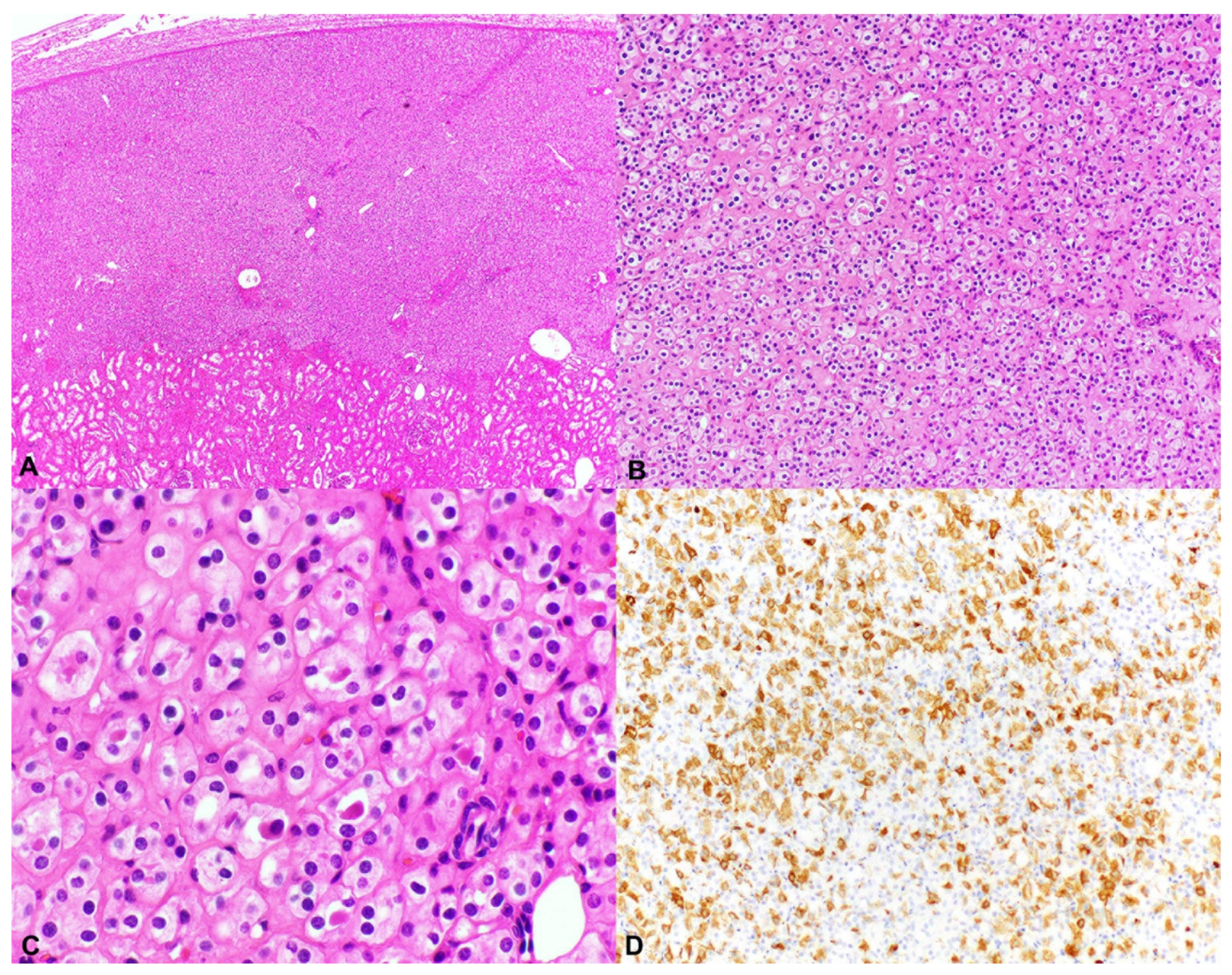
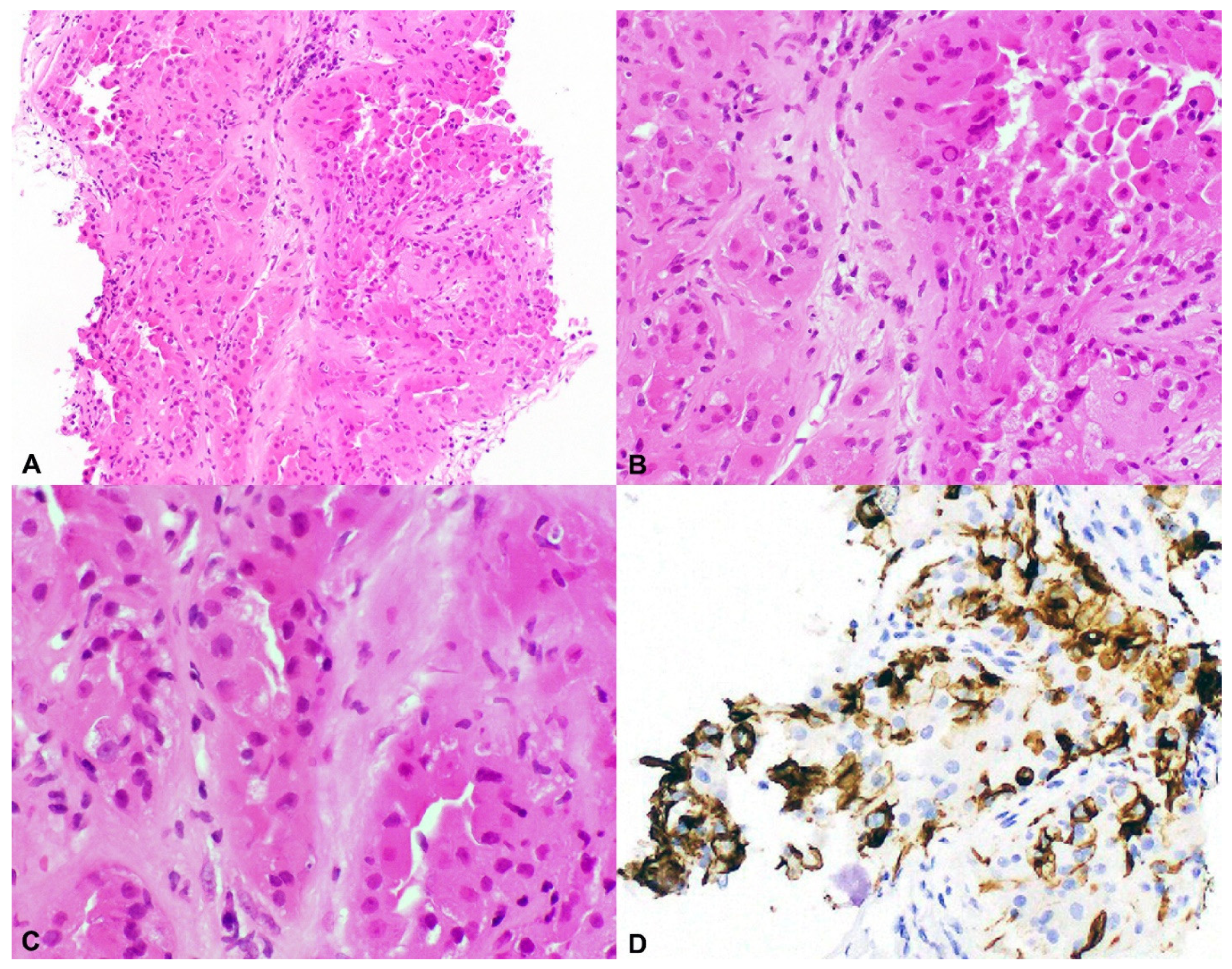
2.3. Unclassified Renal Cell Carcinoma (Unclassified RCC); Granular Eosinophilic Macrocystic RCC
2.4. Conclusions from Two Large TSC-RCC Series
3. Genetic Features of TSC-RCC
4. TSC1 and TSC2 Mutations in Sporadic RCC
4.1. TSC1 and TSC2 Mutations in Sporadic ccRCC and chRCC
4.2. TSC1 and TSC2 Mutations in Sporadic Unclassified Eosinophilic RCC
4.3. TSC1 and TSC2 Mutations in Sporadic RCC-LMS (RCC with Leiomyomatous Stroma)
4.4. TSC1 and TSC2 Mutations in Sporadic Eosinophilic Solid and Cystic Renal Cell Carcinoma (ESC-RCC)
4.5. TSC1 and TSC2 Mutations in Sporadic Hybrid Oncocytic/Chromophobe Tumor (HOCT) and Chromophobe-Like RCC
4.6. TSC1 and TSC2 Mutations in Sporadic Low-Grade Oncocytic Tumor (LOT) of the Kidney
4.7. TSC1 and TSC2 Mutations in Eosinophilic Vacuolated Tumor (EVT)
5. Pathogenesis of TSC-RCC: Links to TFEB/TFE3 and BHD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; A Zonnenberg, B.; Frost, M.; Belousova, E.D.; Sauter, M.; Nonomura, N.; Brakemeier, S.; de Vries, P.J.; et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013, 381, 817–824. [Google Scholar] [CrossRef]
- Franz, D.N.; Belousova, E.D.; Sparagana, S.; Bebin, E.M.; Frost, M.; Kuperman, R.; Witt, O.; Kohrman, M.H.; Flamini, J.R.; Wu, J.Y.; et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef]
- McCormack, F.X.; Inoue, Y.; Moss, J.; Singer, L.G.; Strange, C.; Nakata, K.; Barker, A.F.; Chapman, J.T.; Brantly, M.L.; Stocks, J.M.; et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011, 364, 1595–1606. [Google Scholar] [CrossRef]
- Bissler, J.J.; McCormack, F.; Young, L.R.; Elwing, J.M.; Chuck, G.; Leonard, J.M.; Schmithorst, V.J.; Laor, T.; Brody, A.S.; Bean, J.; et al. Sirolimus for Angiomyolipoma in Tuberous Sclerosis Complex or Lymphangioleiomyomatosis. N. Engl. J. Med. 2008, 358, 140–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zöllner, J.P.; Franz, D.N.; Hertzberg, C.; Nabbout, R.; Rosenow, F.; Sauter, M.; Schubert-Bast, S.; Wiemer-Kruel, A.; Strzelczyk, A. A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J. Rare Dis. 2020, 15, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trnka, P.; Kennedy, S.E. Renal tumors in tuberous sclerosis complex. Pediatr. Nephrol. 2020, 36, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Kingswood, J.C.; Belousova, E.; Benedik, M.P.; Carter, T.; Cottin, V.; Curatolo, P.; Dahlin, M.; Amato, L.D.; D’Augères, G.B.; De Vries, P.J.; et al. Renal angiomyolipoma in patients with tuberous sclerosis complex: Findings from the TuberOus SClerosis registry to increase disease Awareness. Nephrol. Dial. Transplant. 2018, 34, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, S.; Lux, A.; Calder, N.; Laugharne, M.; Osborne, J.; O’Callaghan, F. Causes of mortality in individuals with tuberous sclerosis complex. Dev. Med. Child Neurol. 2016, 59, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Janssens, P.; Van Hoeve, K.; De Waele, L.; De Rechter, S.; Claes, K.J.; Van De Perre, E.; Wissing, K.M.; Bammens, B.; Jansen, A.; Mekahli, D. Renal progression factors in young patients with tuberous sclerosis complex: a retrospective cohort study. Pediatr. Nephrol. 2018, 33, 2085–2093. [Google Scholar] [CrossRef]
- Lam, H.C.; Siroky, B.J.; Henske, E.P. Renal disease in tuberous sclerosis complex: Pathogenesis and therapy. Nat. Rev. Nephrol. 2018, 14, 704–716. [Google Scholar] [CrossRef]
- Henske, E.P. Tuberous sclerosis and the kidney: From mesenchyme to epithelium, and beyond. Pediatr. Nephrol. 2005, 20, 854–857. [Google Scholar] [CrossRef]
- Henske, E.P.; Jozwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primers 2016, 2, 16035. [Google Scholar] [CrossRef]
- Bjornsson, J.; Short, M.P.; Kwiatkowski, D.J.; Henske, E.P. Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am. J. Pathol. 1996, 149, 1201–1208. [Google Scholar]
- Bissler, J.J.; Kingswood, J.C. Renal manifestation of tuberous sclerosis complex. Am. J. Med. Genet. Part C Semin. Med. Genet. 2018, 178, 338–347. [Google Scholar] [CrossRef]
- Karbowniczek, M.; Henske, E.P. The role of tuberin in cellular differentiation: Are B-Raf and MAPK involved? Ann. N. Y. Acad. Sci. 2005, 1059, 168–173. [Google Scholar] [CrossRef] [PubMed]
- The European Chromosome 16 Tuberous Sclerosis Consortium, Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993, 75, 1305–1315. [CrossRef]
- Guo, J.; Tretiakova, M.S.; Troxell, M.L.; Osunkoya, A.O.; Fadare, O.; Sangoi, A.R.; Shen, S.S.; Lopez-Beltran, A.; Mehra, R.; Heider, A.; et al. Tuberous sclerosis-associated renal cell carcinoma: a clinicopathologic study of 57 separate carcinomas in 18 patients. Am. J. Surg. Pathol. 2014, 38, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cornejo, K.M.; Sadow, P.; Cheng, L.; Wang, M.; Xiao, Y.; Jiang, Z.; Oliva, E.; Jozwiak, S.; Nussbaum, R.L.; et al. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Am. J. Surg. Pathol. 2014, 38, 895–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, S.R.; Hornick, J.L.; Eble, J.N.; Gupta, N.S.; Rogers, C.G.; True, L.; Grignon, D.J.; Cheng, L. Renal cell carcinoma with angioleiomyoma-like stroma and clear cell papillary renal cell carcinoma: Exploring SDHB protein immunohistochemistry and the relationship to tuberous sclerosis complex. Hum. Pathol. 2018, 75, 10–15. [Google Scholar] [CrossRef]
- Schreiner, A.; Daneshmand, S.; Bayne, A.; Countryman, G.; Corless, C.L.; Troxell, M.L. Distinctive Morphology of Renal Cell Carcinomas in Tuberous Sclerosis. Int. J. Surg. Pathol. 2009, 18, 409–418. [Google Scholar] [CrossRef]
- Lam, H.C.; Nijmeh, J.S.; Henske, E.P. New developments in the genetics and pathogenesis of tumours in tuberous sclerosis complex. J. Pathol. 2016, 241, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henske, E.P.; Scheithauer, B.W.; Short, M.P.; Wollmann, R.; Nahmias, J.; Hornigold, N.; Van Slegtenhorst, M.; Welsh, C.T.; Kwiatkowski, D.J. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am. J. Hum. Genet. 1996, 59, 400–406. [Google Scholar] [PubMed]
- Henske, E.P.; Wessner, L.L.; Golden, J.; Scheithauer, B.W.; Vortmeyer, A.O.; Zhuang, Z.; Klein-Szanto, A.J.; Kwiatkowski, D.J.; Yeung, R.S. Loss of tuberin in both subependymal giant cell astrocytomas and angiomyolipomas supports a two-hit model for the pathogenesis of tuberous sclerosis tumors. Am. J. Pathol. 1997, 151, 1639–1647. [Google Scholar] [PubMed]
- Tyburczy, M.E.; Jozwiak, S.; Malinowska, I.A.; Chekaluk, Y.; Pugh, T.; Wu, C.-L.; Nussbaum, R.L.; Seepo, S.; Dzik, T.; Kotulska, K.; et al. A shower of second hit events as the cause of multifocal renal cell carcinoma in tuberous sclerosis complex. Hum. Mol. Genet. 2014, 24, 1836–1842. [Google Scholar] [CrossRef] [Green Version]
- Bah, I.; Fahiminiya, S.; Bégin, L.R.; Hamel, N.; D’Agostino, M.D.; Tanguay, S.; Foulkes, W.D. Atypical tuberous sclerosis complex presenting as familial renal cell carcinoma with leiomyomatous stroma. J. Pathol. Clin. Res. 2018, 4, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Kucejova, B.; Peña-Llopis, S.; Yamasaki, T.; Sivanand, S.; Tran, T.A.T.; Alexander, S.; Wolff, N.C.; Lotan, Y.; Xie, X.-J.; Kabbani, W.; et al. Interplay Between pVHL and mTORC1 Pathways in Clear-Cell Renal Cell Carcinoma. Mol. Cancer Res. 2011, 9, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Casuscelli, J.; Weinhold, N.; Gundem, G.; Wang, L.; Zabor, E.C.; Drill, E.; Wang, P.I.; Nanjangud, G.J.; Redzematovic, A.; Nargund, A.M.; et al. Genomic landscape and evolution of metastatic chromophobe renal cell carcinoma. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Durinck, S.; Stawiski, E.W.; Pavía-Jiménez, A.; Modrusan, Z.; Kapur, P.; Jaiswal, B.S.; Zhang, N.; Toffessi-Tcheuyap, V.; Nguyen, T.; Pahuja, K.B.; et al. Spectrum of diverse genomic alterations define non–clear cell renal carcinoma subtypes. Nat. Genet. 2014, 47, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef]
- Davis, C.; Ricketts, C.J.; Wang, M.; Yang, L.; Cherniack, A.D.; Shen, H.; Buhay, C.; Kang, H.; Kim, S.C.; Fahey, C.C.; et al. The Somatic Genomic Landscape of Chromophobe Renal Cell Carcinoma. Cancer Cell 2014, 26, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Scelo, G.; Riazalhosseini, Y.; Greger, L.; Greger, L.; Letourneau, L.; Gonzàlez-Porta, M.; Wozniak, M.B.; Bourgey, M.; Harnden, P.; Egevad, L.; et al. Variation in genomic landscape of clear cell renal cell carcinoma across Europe. Nat. Commun. 2014, 5, 5135. [Google Scholar] [CrossRef]
- Palsgrove, D.N.; Li, Y.; Pratilas, C.A.; Lin, M.T.; Pallavajjalla, A.; Gocke, C.; De Marzo, A.M.; Matoso, A.; Netto, G.J.; Epstein, J.I.; et al. Eosinophilic Solid and Cystic (ESC) Renal Cell Carcinomas Harbor TSC Mutations: Molecular Analysis Supports an Expanding Clinicopathologic Spectrum. Am. J. Surg. Pathol. 2018, 42, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-B.; Mirsadraei, L.; Jayakumaran, G.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Sirintrapun, S.J.; Tickoo, S.K.; Reuter, V.E. Somatic Mutations of TSC2 or MTOR Characterize a Morphologically Distinct Subset of Sporadic Renal Cell Carcinoma With Eosinophilic and Vacuolated Cytoplasm. Am. J. Surg. Pathol. 2019, 43, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.B.; Stohr, B.A.; Tu, Z.J.; Gao, Y.; Przybycin, C.G.; Nguyen, J.; Cox, R.M.; Rashid-Kolvear, F.; Weindel, M.D.; Farkas, D.H.; et al. “Renal Cell Carcinoma With Leiomyomatous Stroma” Harbor Somatic Mutations of TSC1, TSC2, MTOR, and/or ELOC (TCEB1): Clinicopathologic and Molecular Characterization of 18 Sporadic Tumors Supports a Distinct Entity. Am. J. Surg. Pathol. 2019, 44, 571–581. [Google Scholar] [CrossRef]
- Tjota, M.Y.; Wanjari, P.; Segal, J.; Antic, T. TSC/MTOR-mutated eosinophilic renal tumors are a distinct entity that is CK7+/CK20-/vimentin-: a validation study. Hum. Pathol. 2020, 115, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Tjota, M.; Chen, H.; Parilla, M.; Wanjari, P.; Segal, J.; Antic, T. Eosinophilic Renal Cell Tumors With a TSC and MTOR Gene Mutations Are Morphologically and Immunohistochemically Heterogenous: Clinicopathologic and Molecular Study. Am. J. Surg. Pathol. 2020, 44, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R.; Cardili, L.; Whiteley, L.J.; Bs, J.S.; Kis, O.; Ba, L.J.W. Sclerosing TSC1 mutated renal cell carcinoma: An unusual pattern mimicking MITF family translocation renal cell carcinoma. Genes Chromosom. Cancer 2020, 59, 591–594. [Google Scholar] [CrossRef]
- Mihaela, F.; Zoran, G.; Kiril, T.; Jeffrey, S.; Ming, Z.; Reza, A.; Williamson, S.R.; Cristina, M.-G.; Gill, A.J.; Maria, T.; et al. Eosinophilic vacuolated tumor (EVT) of kidney demonstrates sporadic TSC/MTOR mutations: Next-generation sequencing multi-institutional study of 19 cases. Mod. Pathol. 2021, 1–8. [Google Scholar] [CrossRef]
- Kapur, P.; Gao, M.; Zhong, H.; Chintalapati, S.; Mitui, M.; Barnes, S.D.; Zhou, Q.; Miyata, J.; Carrillo, D.; Malladi, V.S.; et al. Germline and sporadic mTOR pathway mutations in low-grade oncocytic tumor of the kidney. Mod. Pathol. 2021, 1–11. [Google Scholar] [CrossRef]
- A Yeh, Y.; Constantinescu, M.; Chaudoir, C.; Tanner, A.; Serkin, F.; Yu, X.; Fazili, T.; A Lurie, A. Renal cell carcinoma with leiomyomatous stroma: a review of an emerging entity distinct from clear cell conventional renal cell carcinoma. Am. J. Clin. Exp. Urol. 2019, 7, 321–326. [Google Scholar] [PubMed]
- Trpkov, K.; Abou-Ouf, H.; Hes, O.; Lopez, J.I.; Nesi, G.; Comperat, E.; Sibony, M.; Osunkoya, A.O.; Zhou, M.; Gokden, N.; et al. Eosinophilic Solid and Cystic Renal Cell Carcinoma (ESC RCC): Further Morphologic and Molecular Characterization of ESC RCC as a Distinct Entity. Am. J. Surg. Pathol. 2017, 41, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Hes, O.; Bonert, M.; Lopez, J.I.; Bonsib, S.M.; Nesi, G.; Comperat, E.; Sibony, M.; Berney, D.M.; Martinek, P.; et al. Eosinophilic, Solid, and Cystic Renal Cell Carcinoma: Clinicopathologic Study of 16 Unique, Sporadic Neoplasms Occurring in Women. Am. J. Surg. Pathol. 2016, 40, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Siadat, F.; Trpkov, K. ESC, ALK, HOT and LOT: Three Letter Acronyms of Emerging Renal Entities Knocking on the Door of the WHO Classification. Cancers 2020, 12, 168. [Google Scholar] [CrossRef] [Green Version]
- Parilla, M.; Kadri, S.; Patil, S.A.; Ritterhouse, L.; Segal, J.; Henriksen, K.J.; Antic, T. Are Sporadic Eosinophilic Solid and Cystic Renal Cell Carcinomas Characterized by Somatic Tuberous Sclerosis Gene Mutations? Am. J. Surg. Pathol. 2018, 42, 911–917. [Google Scholar] [CrossRef]
- Mehra, R.; Vats, P.; Cao, X.; Su, F.; Lee, N.D.; Lonigro, R.; Premkumar, K.; Trpkov, K.; McKenney, J.K.; Dhanasekaran, S.M.; et al. Somatic Bi-allelic Loss of TSC Genes in Eosinophilic Solid and Cystic Renal Cell Carcinoma. Eur. Urol. 2018, 74, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Munari, E.; Settanni, G.; Caliò, A.; Segala, D.; Lonardi, S.; Sandrini, S.; Vacca, P.; Tumino, N.; Marconi, M.; Brunelli, M.; et al. TSC loss is a clonal event in eosinophilic solid and cystic renal cell carcinoma: a multiregional tumor sampling study. Mod. Pathol. 2021, 1–10. [Google Scholar] [CrossRef]
- McKenney, J.K.; Przybycin, C.G.; Trpkov, K.; Magi-Galluzzi, C. Eosinophilic solid and cystic renal cell carcinomas have metastatic potential. Histopathology 2017, 72, 1066–1067. [Google Scholar] [CrossRef]
- Tretiakova, M.S. Eosinophilic solid and cystic renal cell carcinoma mimicking epithelioid angiomyolipoma: Series of 4 primary tumors and 2 metastases. Hum. Pathol. 2018, 80, 65–75. [Google Scholar] [CrossRef]
- Li, Y.; Reuter, V.E.; Matoso, A.; Netto, G.J.; Epstein, J.I.; Argani, P. Re-evaluation of 33 ‘unclassified’ eosinophilic renal cell carcinomas in young patients. Histopathology 2018, 72, 588–600. [Google Scholar] [CrossRef]
- He, H.; Trpkov, K.; Martinek, P.; Isikci, O.T.; Maggi-Galuzzi, C.; Alaghehbandan, R.; Gill, A.J.; Tretiakova, M.; Lopez, J.I.; Williamson, S.R.; et al. “High-grade oncocytic renal tumor”: Morphologic, immuno-histochemical, and molecular genetic study of 14 cases. Virchows Arch. 2018, 473, 725–738. [Google Scholar] [CrossRef]
- Trpkov, K.; Bonert, M.; Gao, Y.; Kapoor, A.; He, H.; Yilmaz, A.; Gill, A.J.; Williamson, S.R.; Comperat, E.; Tretiakova, M.; et al. High-grade oncocytic tumour (HOT) of kidney in a patient with tuberous sclerosis complex. Histopathology 2019, 75, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Kapur, P.; Gao, M.; Zhong, H.; Rakheja, D.; Cai, Q.; Pedrosa, I.; Margulis, V.; Xu, L.; Kinch, L.; Brugarolas, J. Eosinophilic Vacuolated Tumor of the Kidney: A Review of Evolving Concepts in This Novel Subtype With Additional Insights From a Case With MTOR Mutation and Concomitant Chromosome 1 Loss. Adv. Anat. Pathol. 2021, 28, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, K.; Williamson, S.R.; Gao, Y.; Martinek, P.; Cheng, L.; Sangoi, A.R.; Yilmaz, A.; Wang, C.; San Miguel Fraile, P.; Perez Montiel, D.M.; et al. Low-grade oncocytic tumour of kidney (CD117-negative, cytokeratin 7-positive): a distinct entity? Histopathology 2019, 75, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerma, L.A.; Schade, G.R.; Tretiakova, M.S. Co-existence of ESC-RCC, EVT, and LOT as synchronous and metachronous tumors in six patients with multifocal neoplasia but without clinical features of tuberous sclerosis complex. Hum. Pathol. 2021, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Puertollano, R.; Ferguson, S.M.; Brugarolas, J.; Ballabio, A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. EMBO J. 2018, 37, e98804. [Google Scholar] [CrossRef] [PubMed]
- Roczniak-Ferguson, A.; Petit, C.S.; Fröhlich, F.; Qian, S.; Ky, J.; Angarola, B.; Walther, T.; Ferguson, S.M. The Transcription Factor TFEB Links mTORC1 Signaling to Transcriptional Control of Lysosome Homeostasis. Sci. Signal. 2012, 5, ra42-ra42. [Google Scholar] [CrossRef] [Green Version]
- Alesi, N.; Akl, E.W.; Khabibullin, D.; Liu, H.-J.; Nidhiry, A.S.; Garner, E.R.; Filippakis, H.; Lam, H.C.; Shi, W.; Viswanathan, S.R.; et al. TSC2 regulates lysosome biogenesis via a non-canonical RAGC and TFEB-dependent mechanism. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Di Malta, C.; Ballabio, A. MiT/TFE Family of Transcription Factors, Lysosomes, and Cancer. Annu. Rev. Cancer Biol. 2019, 3, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Di Malta, C.; Esposito, A.; de Araujo, M.E.G.; Pece, S.; Bertalot, G.; Matarese, M.; Benedetti, V.; Zampelli, A.; Stasyk, T.; et al. A substrate-specific mTORC1 pathway underlies Birt-Hogg-Dube syndrome. Nature 2020, 585, 597–602. [Google Scholar] [CrossRef] [PubMed]
| Authors, Journal, Year | Histology | # | TSC1 Mutations # (%) | TSC2 Mutations # (%) | mTOR Mutations # (%) |
|---|---|---|---|---|---|
| Davis et al., Cancer Cell, 2014 [32] | ccRCC | 448 | 4 (0.9%) | 6 (1.2%) | 32 (7%) |
| Sato et al., Nat Gen, 2013 [27] | ccRCC | 106 | 2 (2%) | 2 (2%) | 6 (6%) |
| Kucejova et al., Mol Cancer Res, 2011 [26] | ccRCC | 77 | 4 (5%) | 0 (0%) | not done |
| Scelo et al., Nat Comm, 2014 [33] | ccRCC | 94 | not done | not done | 8 (8.5%) |
| Authors, Journal, Year | Histology | # | TSC1 Mutations # (%) | TSC2 Mutations # (%) | mTOR Mutations # (%) |
|---|---|---|---|---|---|
| Davis et al., Cancer Cell, 2014 [32] | ChRCC | 66 | 3 (5%) | 3 (5%) | 2 (3%) |
| Durinck et al., Nat Gen, 2015 [30] | ChRCC | 49 | 1 (2%) | 1 (2%) | 2 (4%) |
| Casuscelli et al., JCI Insight, 2017 [29] | ChRCC | 79 | 2 (2.5%) | 4 (5%) | 4(5%) |
| Bah et al., J Pathol Clin Res, 2018 [25] | RCCLS | 5 | 0 | 5 (100%) | not done |
| Palsgrove et al., Am J Surg Pathol, 2018 [34] | ESC-RCC | 15 | 6 (40%) | 8 (53%) | not done |
| Unclassified ESC-like | 3 | 1 (33%) | 1 (33%) | not done | |
| Oncocytoid RCC | 1 | 0% | 1 (100%) | not done | |
| Chen et al., Am J Surg Pathol, 2019 [35] | ESC-RCC | 7 | 0 | 4 (42%) | 2 (28%) |
| Shah et al., Am J Surg Pathol, 2020 [36] | RCCLS | 18 | 4 (22%) | 4 (22%) | 6 (33%) |
| Tjota et al., Hum Pathol, 2020 [37] | ChRCC, eosinophilic variant | 6 | 1 (16%) | 2 (33%) | 3 (50%) |
| ChRCC | 2 | 0 | 1(50%) | 1 (50%) | |
| Tjota et al., Am J Surg Pathol, 2020 [38] | ChRCC, eosinophilic variant | 4 | 1 (25%) | 2 (50%) | 1(25%) |
| Unclassified RCC | 8 | 1 (12%) | 5 (62%) | 1(12%) | |
| CHRCC | 3 | 2 (66%) | 1 (33%) | 0 | |
| Renal oncocytoma | 2 | 2 (100%) | 0 | 0 | |
| Williamson et al., Genes Chromosome Cancer, 2020 [39] | ESC-RCC or HOCT | 1 | 1 (100%) | 0 | 0 |
| Mihaela et al., Mod Pathology, 2021 [40] | Eosinophilic vacuolated tumor (EVT) | 19 | 4 (21%) | 7 (36%) | 0 |
| Kapur et al., Mod Pathology, 2021 [41] | Low-grade oncocytic tumor (LOT) | 21 | 1 (4%) | 0 | 7 (33%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henske, E.P.; Cornejo, K.M.; Wu, C.-L. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Genes 2021, 12, 1585. https://doi.org/10.3390/genes12101585
Henske EP, Cornejo KM, Wu C-L. Renal Cell Carcinoma in Tuberous Sclerosis Complex. Genes. 2021; 12(10):1585. https://doi.org/10.3390/genes12101585
Chicago/Turabian StyleHenske, Elizabeth P., Kristine M. Cornejo, and Chin-Lee Wu. 2021. "Renal Cell Carcinoma in Tuberous Sclerosis Complex" Genes 12, no. 10: 1585. https://doi.org/10.3390/genes12101585
APA StyleHenske, E. P., Cornejo, K. M., & Wu, C.-L. (2021). Renal Cell Carcinoma in Tuberous Sclerosis Complex. Genes, 12(10), 1585. https://doi.org/10.3390/genes12101585





