Identification and Validation of a QTL for Bacterial Leaf Streak Resistance in Rice (Oryza sativa L.) against Thai Xoc Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Condition
2.2. Inoculum Preparation and Phenotyping
2.3. Heritability of the Trait
2.4. Sample Bulking, DNA Isolation and Whole-Genome Resequencing
2.5. Data Analysis Via the QTL-Seq Pipeline and Candidate Gene Annotation
2.6. Development of a KASP Marker and Validation in Populations with Different Genetic Backgrounds
3. Results
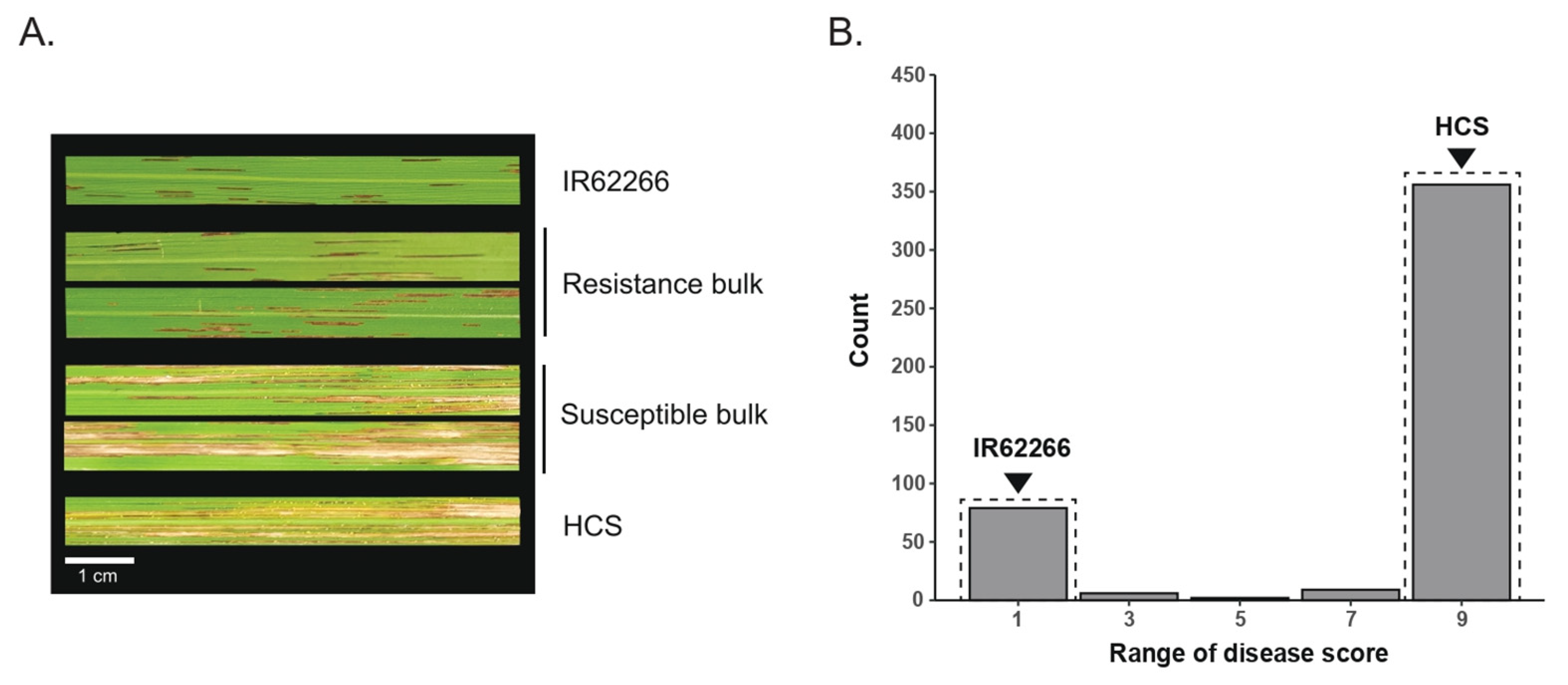
3.1. Phenotype of F2 Population and the Construction of Resistant and Susceptible Bulks
3.2. Whole-Genome Re-Sequencing of the Two Bulks and Parents
3.3. QTL-Seq Analysis
3.4. Development of a Kompetitive Allele-Specific PCR (KASP) Marker for BLS Resistance and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, L.-Y.; Liu, X.-L.; Xiao, Y.-H.; Wang, G.-L. Recent Advances in Cloning and Characterization of Disease Resistance Genes in Rice. J. Integr. Plant Biol. 2007, 49, 112–119. [Google Scholar] [CrossRef]
- Sparks, A.; Nelson, A.; Castilla, N. Where Rice Pests and Diseases Do the Most Damage. Rice Today 2012, 11, 27. [Google Scholar]
- NIÑO-LIU, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae Pathovars: Model Pathogens of a Model Crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance Genes and Their Interactions with Bacterial Blight/Leaf Streak Pathogens (Xanthomonas oryzae) in Rice (Oryza sativa L.)—An Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, J.; Triplett, L.; Leach, J.E.; Wang, G.-L. Novel Insights into Rice Innate Immunity against Bacterial and Fungal Pathogens. Annu. Rev. Phytopathol. 2014, 52, 213–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thind, B.S. Phytopathogenic Bacteria and Plant Diseases; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780429512506. [Google Scholar]
- Onaga, G.; Murori, R.; Habarugira, G.; Nyongesa, O.; Bigirimana, J.; Oliva, R.; Vera Cruz, C.; Onyango, G.; Andaku, J.; Ongom, J. First Report of Xanthomonas oryzae Pv.oryzicola Causing Bacterial Leaf Streak of Rice in Kenya. Plant Dis. 2018, 102, 1025. [Google Scholar] [CrossRef]
- Wonni, I.; Djedatin, G.; Ouédraogo, L.; Verdier, V. Evaluation of Rice Germplasm against Bacterial Leaf Streak Disease Reveals Sources of Resistance in African Varieties. J. Plant Pathol. Microbiol. 2015, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Wu, W.; Li, W.; Lu, H.; Worland, A.J. Mapping of QTLs Conferring Resistance to Bacterial Leaf Streak in Rice. Theor. Appl. Genet. 2000, 101, 286–291. [Google Scholar] [CrossRef]
- Chen, C.-H.; Zheng, W.; Huang, X.-M.; Zhang, D.-P.; Lin, X.-H. Major QTL Conferring Resistance to Rice Bacterial Leaf Streak. Agric. Sci. China 2006, 5, 216–220. [Google Scholar] [CrossRef]
- Bossa-Castro, A.M.; Tekete, C.; Raghavan, C.; Delorean, E.E.; Dereeper, A.; Dagno, K.; Koita, O.; Mosquera, G.; Leung, H.; Verdier, V.; et al. Allelic Variation for Broad-Spectrum Resistance and Susceptibility to Bacterial Pathogens Identified in a Rice MAGIC Population. Plant Biotechnol. J. 2018, 16, 1559–1568. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Chen, Z.; Cao, J.; Guan, H.; Lin, D.; Li, C.; Lan, T.; Duan, Y.; Mao, D.; Wu, W. Toward the Positional Cloning of QBlsr5a, a QTL Underlying Resistance to Bacterial Leaf Streak, Using Overlapping Sub-CSSLs in Rice. PLoS ONE 2014, 9, e95751. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.L.; Grondin, A.; Courtois, B.; Gantet, P. Next-Generation Sequencing Accelerates Crop Gene Discovery. Trends Plant Sci. 2019, 24, 263–274. [Google Scholar] [CrossRef]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-Seq: Rapid Mapping of Quantitative Trait Loci in Rice by Whole Genome Resequencing of DNA from Two Bulked Populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ogiso-Tanaka, E.; Tanaka, T.; Tanaka, K.; Nonoue, Y.; Sasaki, T.; Fushimi, E.; Koide, Y.; Okumoto, Y.; Yano, M.; Saito, H. Detection of Novel QTLs QDTH4.5 and QDTH6.3, Which Confer Late Heading under Short-Day Conditions, by SSR Marker-Based and QTL-Seq Analysis. Breed. Sci. 2017, 67, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Xia, X.; Zhang, Z.; Nong, B.; Zeng, Y.; Xiong, F.; Wu, Y.; Gao, J.; Deng, G.; Li, D. QTL Mapping by Whole Genome Re-Sequencing and Analysis of Candidate Genes for Nitrogen Use Efficiency in Rice. Front. Sci. 2017, 8, 1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadambari, G.; Vemireddy, L.R.; Srividhya, A.; Nagireddy, R.; Jena, S.S.; Gandikota, M.; Patil, S.; Veeraghattapu, R.; Deborah, D.A.K.; Reddy, G.E.; et al. QTL-Seq-Based Genetic Analysis Identifies a Major Genomic Region Governing Dwarfness in Rice (Oryza sativa L.). Plant Cell Rep. 2018, 37, 677–687. [Google Scholar] [CrossRef]
- Yaobin, Q.; Peng, C.; Yichen, C.; Yue, F.; Derun, H.; Tingxu, H.; Xianjun, S.; Jiezheng, Y. QTL-Seq Identified a Major QTL for Grain Length and Weight in Rice Using Near Isogenic F 2 Population. Rice Sci. 2018, 25, 121–131. [Google Scholar] [CrossRef]
- Arikit, S.; Wanchana, S.; Khanthong, S.; Saensuk, C.; Thianthavon, T.; Vanavichit, A.; Toojinda, T. QTL-Seq Identifies Cooked Grain Elongation QTLs near Soluble Starch Synthase and Starch Branching Enzymes in Rice (Oryza sativa L.). Sci. Rep. 2019, 9, 8328. [Google Scholar] [CrossRef] [Green Version]
- Nubankoh, P.; Wanchana, S.; Saensuk, C.; Ruanjaichon, V.; Cheabu, S.; Vanavichit, A.; Toojinda, T.; Malumpong, C.; Arikit, S. QTL-Seq Reveals Genomic Regions Associated with Spikelet Fertility in Response to a High Temperature in Rice (Oryza sativa L.). Plant Cell Rep. 2020, 39, 149–162. [Google Scholar] [CrossRef]
- Bommisetty, R.; Chakravartty, N.; Bodanapu, R.; Naik, J.B.; Panda, S.K.; Lekkala, S.P.; Lalam, K.; Thomas, G.; Mallikarjuna, S.J.; Eswar, G.R.; et al. Discovery of Genomic Regions and Candidate Genes for Grain Weight Employing next Generation Sequencing Based QTL-Seq Approach in Rice (Oryza sativa L.). Mol. Biol. Rep. 2020, 47, 8615–8627. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; Liu, Z.; Guo, Y.; Qiu, L.-J. Next-Generation Sequencing from Bulked-Segregant Analysis Accelerates the Simultaneous Identification of Two Qualitative Genes in Soybean. Front. Plant Sci. 2017, 8, 919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, W.; Guo, N.; Zhang, Y.; Bu, Y.; Zhao, J.; Xing, H. Combining QTL-Seq and Linkage Mapping to Fine Map a Wild Soybean Allele Characteristic of Greater Plant Height. BMC Genom. 2018, 19, 226. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Khan, A.W.; Jaganathan, D.; Thudi, M.; Roorkiwal, M.; Takagi, H.; Garg, V.; Kumar, V.; Chitikineni, A.; Gaur, P.M.; et al. QTL-Seq for Rapid Identification of Candidate Genes for 100-Seed Weight and Root/Total Plant Dry Weight Ratio under Rainfed Conditions in Chickpea. Plant Biotechnol. J. 2016, 14, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Deokar, A.; Sagi, M.; Daba, K.; Tar’an, B. QTL Sequencing Strategy to Map Genomic Regions Associated with Resistance to Ascochyta Blight in Chickpea. Plant Biotechnol. J. 2019, 17, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Illa-Berenguer, E.; Van Houten, J.; Huang, Z.; van der Knaap, E. Rapid and Reliable Identification of Tomato Fruit Weight and Locule Number Loci by QTL-Seq. Theor. Appl. Genet. 2015, 128, 1329–1342. [Google Scholar] [CrossRef]
- Luo, H.; Pandey, M.K.; Khan, A.W.; Guo, J.; Wu, B.; Cai, Y.; Huang, L.; Zhou, X.; Chen, Y.; Chen, W.; et al. Discovery of Genomic Regions and Candidate Genes Controlling Shelling Percentage Using QTL-Seq Approach in Cultivated Peanut (Arachis hypogaea L.). Plant Biotechnol. J. 2019, 17, 1248–1260. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ma, J.; Li, M.; Deng, L.; Li, G.; Xia, H.; Zhao, S.; Hou, L.; Li, P.; Ma, C.; et al. Whole-Genome Resequencing-Based QTL-Seq Identified AhTc1 Gene Encoding a R2R3-MYB Transcription Factor Controlling Peanut Purple Testa Colour. Plant Biotechnol. J. 2020, 18, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Janila, P.; Vishwakarma, M.K.; Khan, A.W.; Manohar, S.S.; Gangurde, S.S.; Variath, M.T.; Shasidhar, Y.; Pandey, M.K.; Varshney, R.K. Whole-Genome Resequencing-Based QTL-Seq Identified Candidate Genes and Molecular Markers for Fresh Seed Dormancy in Groundnut. Plant Biotechnol. J. 2020, 18, 992–1003. [Google Scholar] [CrossRef]
- Sattayachiti, W.; Wanchana, S.; Arikit, S.; Nubankoh, P.; Patarapuwadol, S.; Vanavichit, A.; Darwell, C.T.; Toojinda, T. Genome-Wide Association Analysis Identifies Resistance Loci for Bacterial Leaf Streak Resistance in Rice (Oryza sativa L.). Plants 2020, 9, 1673. [Google Scholar] [CrossRef]
- Warner, J.N. A Method for Estimating Heritability 1. Agron. J. 1952, 44, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, Y.; Young, L.; Yaegashi, H.; Natsume, S.; Shea, D.J.; Takagi, H.; Booker, H.; Innan, H.; Terauchi, R.; Abe, A. High-Performance Pipeline for MutMap and QTL-Seq. BioRxiv 2020. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Korinsak, S.; Sirithunya, K.; Toojinda, T. Identifying a Source of a Bacterial Blight Resistance Gene Xa5 in Rice Variety ‘IR62266′and Development of a Functional Marker ‘PAxa5′, the Easy Agarose Based Detection. Genom. Genet. 2014, 7, 164–172. [Google Scholar]
- Wongkhamchan, A.; Chankaew, S.; Monkham, T.; Saksirirat, W.; Sanitchon, J. Broad Resistance of RD6 Introgression Lines with Xa5 Gene from IR62266 Rice Variety to Bacterial Leaf Blight Disease for Rice Production in Northeastern Thailand. Agric. Nat. Resour. 2018, 52, 241–245. [Google Scholar] [CrossRef]
- Khwanngam, P.; Watcharachaiyakup, J.; Kositratana, W.; Patarapuwadol, S. Genetic Diversity Assessment of Xanthomonas Oryzae Pv. Oryzicola in Thailand Using Repetitive Sequence-Based PCR (Rep-PCR) Technique. Agric. Sci. J. 2016, 47, 29–45. [Google Scholar]
- Xia, Y.; Lin, W.; Chen, O. Factor Influencing Resistance-Identification in Rice Varieties to Bacterial Leaf Streak. J. Fujian Agric. Col. 1991, 20, 272–275. [Google Scholar]
- Xia, Y.; Lin, W.; Chen, O. Resistance-Identification and Resistant-Source Screening for Rice Varieties against Bacterial Leaf Streak. J. Fujian Agric. Col. 1992, 21, 32–36. [Google Scholar]
- Dingzhong, T.; Weiming, L.; Weiren, W. Inheritance of the Resistance to Rice Bacterial Leaf Streak. J. Fujian Agric. Univ. 1998, 27, 133–137. [Google Scholar]
- Sheng, Z.J.; Zhen, L.Y.; Jun, F.X. Detection of QTL Conferring Resistance to Bacterial Leaf Streak in Rice Chromosome 2 (O. sativa L. spp. Indica). Sci. Agric. Sin. 2005, 38, 1923–1925. [Google Scholar]
- He, W.; Huang, D.; Li, R.; Qiu, Y.; Song, J.; Yang, H.; Zheng, J.; Huang, Y.; Li, X.; Liu, C.; et al. Identification of a Resistance Gene Bls1 to Bacterial Leaf Streak in Wild Rice Oryza rufipogon Griff. J. Integr. Agric. 2012, 11, 962–969. [Google Scholar] [CrossRef]
- Triplett, L.R.; Cohen, S.P.; Heffelfinger, C.; Schmidt, C.L.; Huerta, A.I.; Tekete, C.; Verdier, V.; Bogdanove, A.J.; Leach, J.E. A Resistance Locus in the American Heirloom Rice Variety Carolina Gold Select Is Triggered by TAL Effectors with Diverse Predicted Targets and Is Effective against African Strains of Xanthomonas oryzae Pv. Oryzicola. Plant J. 2016, 87, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Zegeye, W.A.; Zhang, Y.; Cao, L.; Cheng, S. Whole Genome Resequencing from Bulked Populations as a Rapid QTL and Gene Identification Method in Rice. Int. J. Mol. Sci. 2018, 19, 4000. [Google Scholar] [CrossRef] [Green Version]
- Clevenger, J.; Chu, Y.; Chavarro, C.; Botton, S.; Culbreath, A.; Isleib, T.G.; Holbrook, C.C.; Ozias-Akins, P. Mapping Late Leaf Spot Resistance in Peanut (Arachis hypogaea) Using QTL-Seq Reveals Markers for Marker-Assisted Selection. Front. Plant Sci. 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, A.S.; McCouch, S.R. The Rice Bacterial Blight Resistance Gene Xa5 Encodes a Novel Form of Disease Resistance. Mol. Plant Microbe Interact. 2004, 17, 1348–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.; Ke, Y.; Huang, R.; Ma, L.; Yang, Z.; Chu, Z.; Xiao, J.; Li, X.; Wang, S. A Host Basal Transcription Factor Is a Key Component for Infection of Rice by TALE-Carrying Bacteria. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Z.; Zhang, B.; Guan, H.; Zheng, Y.; Lan, T.; Zhang, J.; Qin, M.; Wu, W. Transcriptome Analysis of Xa5-Mediated Resistance to Bacterial Leaf Streak in Rice (Oryza sativa L.). Sci. Rep. 2020, 10, 19439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, F.; Zhang, A.; Kong, D.; Liu, G.; Luo, L.; Yu, X. Development and Validation of Functional Markers (Tetra-Primer ARMS and KASP) for the Bacterial Blight Resistance Gene Xa5 in Rice. Austral. Plant Pathol. 2021, 50, 323–327. [Google Scholar] [CrossRef]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial Leaf Blight Resistance in Rice: A Review of Conventional Breeding to Molecular Approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef]
- Chen, X.M. Epidemiology and Control of Stripe Rust [Puccinia striiformis f. sp. Tritici] on Wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Sengupta, D.; Das, S.N.; Pandey, M.K.; Bohra, A.; Sharma, N.K.; Sinha, P.; Sk, H.; Ghazi, I.A.; et al. Deployment of Genetic and Genomic Tools Toward Gaining a Better Understanding of Rice-Xanthomonasoryzae Pv. Oryzae Interactions for Development of Durable Bacterial Blight Resistant Rice. Front. Plant Sci. 2020, 11, 1152. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F.; Osorio-Rodriguez, D. Predicting Thermal Adaptation by Looking into Populations’ Genomic Past. Front. Genet. 2020, 11, 564515. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Fu, J.; Wang, H.; Wang, J.; Huang, C.; Prasanna, B.M.; Olsen, M.S.; Wang, G.; Zhang, A. Enhancing Genetic Gain through Genomic Selection: From Livestock to Plants. Plant Commun. 2020, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Merrick, L.F.; Burke, A.B.; Chen, X.; Carter, A.H. Breeding with Major and Minor Genes: Genomic Selection for Quantitative Disease Resistance. Front. Plant Sci. 2021, 12, 713667. [Google Scholar] [CrossRef] [PubMed]
- Leng, P.; Lübberstedt, T.; Xu, M. Genomics-Assisted Breeding—A Revolutionary Strategy for Crop Improvement. J. Integr. Agric. 2017, 16, 2674–2685. [Google Scholar] [CrossRef]
- Guevara-Escudero, M.; Osorio, A.N.; Cortés, A.J. Integrative Pre-Breeding for Biotic Resistance in Forest Trees. Plants 2021, 10, 2022. [Google Scholar] [CrossRef]



| Sample | No. of Clean Read (Million) | Clean Base (Gb) | Read Alignment (%) | Genome Coverage (%) | Average Depth |
|---|---|---|---|---|---|
| R-bulk | 46.08 | 6.89 | 97.14 | 91 | 18.13 |
| S-bulk | 56.16 | 8.39 | 97.21 | 93 | 22.10 |
| HCS | 58.48 | 8.74 | 97.25 | 94 | 23.00 |
| IR62266 | 59.87 | 8.95 | 97.78 | 92 | 23.57 |
| Chromosome | Length (bp) | Total Number of SNPs (Depth ≥ 5) | Selected SNPs (Depth ≥ 15) |
|---|---|---|---|
| 1 | 43,270,923 | 26,917 | 6289 |
| 2 | 35,937,250 | 28,870 | 7161 |
| 3 | 36,413,819 | 26,643 | 6837 |
| 4 | 35,502,694 | 23,729 | 5391 |
| 5 | 29,958,434 | 26,619 | 6563 |
| 6 | 31,248,787 | 20,645 | 4985 |
| 7 | 29,697,621 | 24,758 | 5423 |
| 8 | 28,443,022 | 20,409 | 4534 |
| 9 | 23,012,720 | 12,899 | 2564 |
| 10 | 23,207,287 | 14,167 | 3000 |
| 11 | 29,021,106 | 21,047 | 4394 |
| 12 | 27,531,856 | 18,173 | 4050 |
| Total | 373,245,519 | 264,876 | 61,191 |
| Chr. | Position | p99 | p95 | SNP Index R-Bulk | SNP Index S-Bulk | ∆(SNP Index) | Candidate Gene |
|---|---|---|---|---|---|---|---|
| 5 | 0.44 | 0.46 | 0.36 | 0.00 | 0.90 | 0.90 | xa5 (Os05g0107700) |
| KASP Marker | Primer Name | Sequence (5′-3′) |
|---|---|---|
| xa5-KASP | FAM_primer | GAGCTCGCCATTCAAGTTCTTGA |
| HEX_primer | GAGCTCGCCATTCAAGTTCTTGT | |
| Common R-primer | GATAGTAATAGGTGGAAGGAGATATGGTA |
| Cross | Generation | Total Sample | Tested Sample | PVE (%) | p-Value | Xoc Isolate |
|---|---|---|---|---|---|---|
| IR62266 × HCS | F2 | 450 | 96 | 66.62 | <0.001 | 2NY2-2 |
| MNTK75 × HCS | F2 | 461 | 96 | 70.84 | <0.001 | 2NY2-2 |
| DV85 × HCS | F2 | 441 | 96 | 69.05 | <0.001 | 2NY2-2 |
| IR62266 × RD47 | F2 | 453 | 96 | 66.42 | <0.001 | 2NY2-2 |
| MNTK75 × RD47 | F2 | 358 | 96 | 59.04 | <0.001 | 2NY2-2 |
| DV85 × RD47 | F2 | 441 | 96 | 65.14 | <0.001 | 2NY2-2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thianthavon, T.; Aesomnuk, W.; Pitaloka, M.K.; Sattayachiti, W.; Sonsom, Y.; Nubankoh, P.; Malichan, S.; Riangwong, K.; Ruanjaichon, V.; Toojinda, T.; et al. Identification and Validation of a QTL for Bacterial Leaf Streak Resistance in Rice (Oryza sativa L.) against Thai Xoc Strains. Genes 2021, 12, 1587. https://doi.org/10.3390/genes12101587
Thianthavon T, Aesomnuk W, Pitaloka MK, Sattayachiti W, Sonsom Y, Nubankoh P, Malichan S, Riangwong K, Ruanjaichon V, Toojinda T, et al. Identification and Validation of a QTL for Bacterial Leaf Streak Resistance in Rice (Oryza sativa L.) against Thai Xoc Strains. Genes. 2021; 12(10):1587. https://doi.org/10.3390/genes12101587
Chicago/Turabian StyleThianthavon, Tripop, Wanchana Aesomnuk, Mutiara K. Pitaloka, Wannapa Sattayachiti, Yupin Sonsom, Phakchana Nubankoh, Srihunsa Malichan, Kanamon Riangwong, Vinitchan Ruanjaichon, Theerayut Toojinda, and et al. 2021. "Identification and Validation of a QTL for Bacterial Leaf Streak Resistance in Rice (Oryza sativa L.) against Thai Xoc Strains" Genes 12, no. 10: 1587. https://doi.org/10.3390/genes12101587
APA StyleThianthavon, T., Aesomnuk, W., Pitaloka, M. K., Sattayachiti, W., Sonsom, Y., Nubankoh, P., Malichan, S., Riangwong, K., Ruanjaichon, V., Toojinda, T., Wanchana, S., & Arikit, S. (2021). Identification and Validation of a QTL for Bacterial Leaf Streak Resistance in Rice (Oryza sativa L.) against Thai Xoc Strains. Genes, 12(10), 1587. https://doi.org/10.3390/genes12101587






