The Staphylococcus aureus CC398 Lineage: An Evolution Driven by the Acquisition of Prophages and Other Mobile Genetic Elements
Abstract
:1. Introduction
2. Clinical Importance of the CC398 Lineage
3. Prophage Content of the CC398 Sub-Populations, Phage-Encoded Factors
4. Roles of CC398-Borne Prophages in Bacterial Virulence
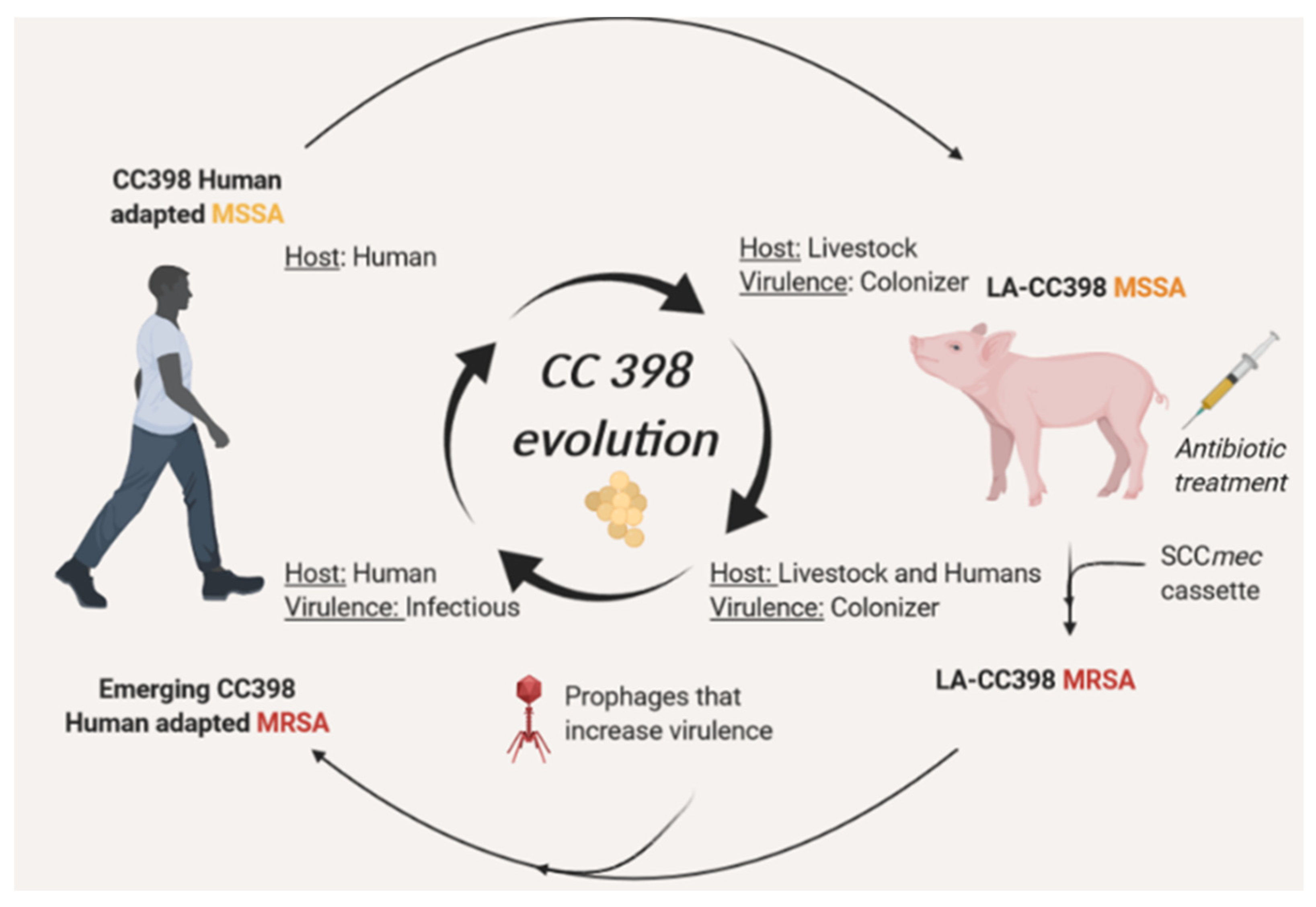
5. Continuous Evolution of CC398 towards Highly Virulent and Resistant Phenotypes through MGEs Acquisition
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- van Belkum, A.; Verkaik, N.J.; de Vogel, C.P.; Boelens, H.A.; Verveer, J.; Nouwen, J.L.; Verbrugh, H.A.; Wertheim, H.F. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 2009, 199, 1820–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, M.O.; Foster, S.J. Stress resistance in Staphylococcus aureus. Trends Microbiol. 1999, 7, 458–462. [Google Scholar] [CrossRef]
- van Loo, I.; Huijsdens, X.; Tiemersma, E.; de Neeling, A.; van de Sande-Bruinsma, N.; Beaujean, D.; Voss, A.; Kluytmans, J. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg. Infect. Dis. 2007, 13, 1834–1839. [Google Scholar] [CrossRef]
- Armand-Lefevre, L.; Ruimy, R.; Andremont, A. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg. Infect. Dis. 2005, 11, 711–714. [Google Scholar] [CrossRef]
- Ip, M.; Yung, R.W.; Ng, T.K.; Luk, W.K.; Tse, C.; Hung, P.; Enright, M.; Lyon, D.J. Contemporary methicillin-resistant Staphylococcus aureus clones in Hong Kong. J. Clin. Microbiol. 2005, 43, 5069–5073. [Google Scholar] [CrossRef] [Green Version]
- Huijsdens, X.W.; van Dijke, B.J.; Spalburg, E.; van Santen-Verheuvel, M.G.; Heck, M.E.; Pluister, G.N.; Voss, A.; Wannet, W.J.; de Neeling, A.J. Community-acquired MRSA and pig-farming. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Witte, W.; Strommenger, B.; Stanek, C.; Cuny, C. Methicillin-resistant Staphylococcus aureus ST398 in humans and animals, Central Europe. Emerg. Infect. Dis. 2007, 13, 255–258. [Google Scholar] [CrossRef]
- Cuny, C.; Kuemmerle, J.; Stanek, C.; Willey, B.; Strommenger, B.; Witte, W. Emergence of MRSA infections in horses in a veterinary hospital: Strain characterisation and comparison with MRSA from humans. Euro. Surveill. 2006, 11, 44–47. [Google Scholar] [CrossRef]
- van Belkum, A.; Melles, D.C.; Peeters, J.K.; van Leeuwen, W.B.; van Duijkeren, E.; Huijsdens, X.W.; Spalburg, E.; de Neeling, A.J.; Verbrugh, H.A.; Dutch Working Party on, S.; et al. Methicillin-resistant and -susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg. Infect. Dis. 2008, 14, 479–483. [Google Scholar] [CrossRef]
- Bhat, M.; Dumortier, C.; Taylor, B.S.; Miller, M.; Vasquez, G.; Yunen, J.; Brudney, K.; Sanchez, E.J.; Rodriguez-Taveras, C.; Rojas, R.; et al. Staphylococcus aureus ST398, New York City and Dominican Republic. Emerg. Infect. Dis. 2009, 15, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Stegger, M.; Lindsay, J.A.; Sorum, M.; Gould, K.A.; Skov, R. Genetic diversity in CC398 methicillin-resistant Staphylococcus aureus isolates of different geographical origin. Clin. Microbiol. Infect. 2010, 16, 1017–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin-Domelier, A.S.; Girard, M.; Bertrand, X.; Violette, J.; Francois, P.; Donnio, P.Y.; Talon, D.; Quentin, R.; Schrenzel, J.; van der Mee-Marquet, N. Methicillin-Susceptible ST398 Staphylococcus aureus Responsible for Bloodstream Infections: An Emerging Human-Adapted Subclone? Plos ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.N.; Velez, L.A.; Mediavilla, J.R.; Ocampo, A.M.; Vanegas, J.M.; Rodriguez, E.A.; Kreiswirth, B.N.; Correa, M.M. Livestock-associated methicillin-susceptible Staphylococcus aureus ST398 infection in woman, Colombia. Emerg. Infect. Dis. 2011, 17, 1970–1971. [Google Scholar] [CrossRef] [PubMed]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; et al. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. MBio. 2012, 3, e00305-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlemann, A.C.; Porcella, S.F.; Trivedi, S.; Sullivan, S.B.; Hafer, C.; Kennedy, A.D.; Barbian, K.D.; McCarthy, A.J.; Street, C.; Hirschberg, D.L.; et al. Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. MBio 2012, 3, e00027-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkade, E.; Bergmans, A.M.; Budding, A.E.; van Belkum, A.; Savelkoul, P.; Buiting, A.G.; Kluytmans, J. Recent emergence of Staphylococcus aureus clonal complex 398 in human blood cultures. PLoS ONE 2012, 7, e41855. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Z.; Liu, C.; Zhang, X.; Lin, X.; Chi, S.; Zhou, T.; Chen, Z.; Chen, X. Prevalence of Staphylococcus aureus carrying Panton-Valentine leukocidin genes among isolates from hospitalised patients in China. Clin. Microbiol. Infect. 2008, 14, 381–384. [Google Scholar] [CrossRef] [Green Version]
- Schijffelen, M.J.; Boel, C.H.; van Strijp, J.A.; Fluit, A.C. Whole genome analysis of a livestock-associated methicillin-resistant Staphylococcus aureus ST398 isolate from a case of human endocarditis. BMC Genomics 2010, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Hallin, M.; De Mendonca, R.; Denis, O.; Lefort, A.; El Garch, F.; Butaye, P.; Hermans, K.; Struelens, M.J. Diversity of accessory genome of human and livestock-associated ST398 methicillin resistant Staphylococcus aureus strains. Infect. Genet. Evol. 2011, 11, 290–299. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Witney, A.A.; Gould, K.A.; Moodley, A.; Guardabassi, L.; Voss, A.; Denis, O.; Broens, E.M.; Hinds, J.; Lindsay, J.A. The distribution of mobile genetic elements (MGEs) in MRSA CC398 is associated with both host and country. Genome. Biol. Evol. 2011, 3, 1164–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Mee-Marquet, N.; Corvaglia, A.R.; Valentin, A.S.; Hernandez, D.; Bertrand, X.; Girard, M.; Kluytmans, J.; Donnio, P.Y.; Quentin, R.; Francois, P. Analysis of prophages harbored by the human-adapted subpopulation of Staphylococcus aureus CC398. Infect. Genet. Evol. 2013, 18, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Mee-Marquet, N.L.; Corvaglia, A.; Haenni, M.; Bertrand, X.; Franck, J.B.; Kluytmans, J.; Girard, M.; Quentin, R.; Francois, P. Emergence of a novel subpopulation of CC398 Staphylococcus aureus infecting animals is a serious hazard for humans. Front. Microbiol. 2014, 5, 652. [Google Scholar] [CrossRef] [PubMed]
- Diene, S.M.; Corvaglia, A.R.; Francois, P.; van der Mee-Marquet, N.; Regional Infection Control Group of the Centre, R. Prophages and adaptation of Staphylococcus aureus ST398 to the human clinic. BMC Genomics 2017, 18, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senneville, E.; Briere, M.; Neut, C.; Messad, N.; Lina, G.; Richard, J.L.; Sotto, A.; Lavigne, J.P.; French Study Group on the Diabetic, F. First report of the predominance of clonal complex 398 Staphylococcus aureus strains in osteomyelitis complicating diabetic foot ulcers: A national French study. Clin. Microbiol. Infect. 2014, 20, O274–O277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouiller, K.; Gbaguidi-Haore, H.; Hocquet, D.; Cholley, P.; Bertrand, X.; Chirouze, C. Clonal complex 398 methicillin-susceptible Staphylococcus aureus bloodstream infections are associated with high mortality. Clin. Microbiol. Infect. 2016, 22, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Sieber, R.N.; Skov, R.L.; Nielsen, J.; Schulz, J.; Price, L.B.; Aarestrup, F.M.; Larsen, A.R.; Stegger, M.; Larsen, J. Drivers and Dynamics of Methicillin-Resistant Livestock-Associated Staphylococcus aureus CC398 in Pigs and Humans in Denmark. MBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Laumay, F.; Corvaglia, A.R.; Diene, S.M.; Girard, M.; Oeschlin, F.; Van der Mee-Marquet, N.; Entenza, J.; Francois, P. Temperate prophages increase bacterial adhesin expression and virulence in an experimental model of endocarditis due to Staphylococcus aureus from the CC398 lineage. Front. Microbiol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Kashif, A.; McClure, J.A.; Lakhundi, S.; Pham, M.; Chen, S.; Conly, J.M.; Zhang, K. Staphylococcus aureus ST398 Virulence Is Associated With Factors Carried on Prophage varphiSa3. Front Microbiol. 2019, 10, 2219. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.C.; Pearson, N. The emergence of Staphylococcus aureus ST398. Vector Borne Zoonotic Dis. 2011, 11, 327–339. [Google Scholar] [CrossRef]
- Cuny, C.; Nathaus, R.; Layer, F.; Strommenger, B.; Altmann, D.; Witte, W. Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS ONE 2009, 4, e6800. [Google Scholar] [CrossRef]
- Cuny, C.; Friedrich, A.; Kozytska, S.; Layer, F.; Nubel, U.; Ohlsen, K.; Strommenger, B.; Walther, B.; Wieler, L.; Witte, W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med. Microbiol. 2010, 300, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, F.; Mazzolini, E.; Bacchin, C.; Bano, L.; Berto, G.; Rigoli, R.; Muffato, G.; Coato, P.; Tonon, E.; Drigo, I. First reporting of methicillin-resistant Staphylococcus aureus (MRSA) ST398 in an industrial rabbit holding and in farm-related people. Veter. Microbiol. 2014, 170, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Graveland, H.; Wagenaar, J.A.; Bergs, K.; Heesterbeek, H.; Heederik, D. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS ONE 2011, 6, e16830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirolo, M.; Visaggio, D.; Gioffre, A.; Artuso, I.; Gherardi, M.; Pavia, G.; Samele, P.; Ciambrone, L.; Di Natale, R.; Spatari, G.; et al. Unidirectional animal-to-human transmission of methicillin-resistant Staphylococcus aureus ST398 in pig farming; evidence from a surveillance study in southern Italy. Antimicrob. Resist. Infect. Control. 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluytmans, J.; Van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, S.; Grumann, D.; Balau, V.; Barwich, A.; Kolata, J.; Goehler, A.; Weiss, S.; Holtfreter, B.; Bauerfeind, S.S.; Doring, P.; et al. Molecular Epidemiology of Staphylococcus aureus in the General Population in Northeast Germany: Results of the Study of Health in Pomerania (SHIP-TREND-0). J. Clin. Microbiol. 2016, 54, 2774–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquemond, I.; Muggeo, A.; Lamblin, G.; Tristan, A.; Gillet, Y.; Bolze, P.A.; Bes, M.; Gustave, C.A.; Rasigade, J.P.; Golfier, F.; et al. Complex ecological interactions of Staphylococcus aureus in tampons during menstruation. Sci. Rep. 2018, 8, 9942. [Google Scholar] [CrossRef] [Green Version]
- Chiaruzzi, M.; Barbry, A.; Muggeo, A.; Tristan, A.; Jacquemond, I.; Badiou, C.; Cluzeau, L.; Bourdeau, S.; Durand, T.; Engelmann, A.; et al. Vaginal Tampon Colonization by Staphylococcus aureus in Healthy Women. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Smith, T.C.; Wardyn, S.E. Human Infections with Staphylococcus aureus CC398. Curr. Environ. Health Rep. 2015, 2, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, Y.; Jiang, X.; Chen, M.; Wang, H. Rapid change of methicillin-resistant Staphylococcus aureus clones in a Chinese tertiary care hospital over a 15-year period. Antimicrob. Agents Chemother. 2010, 54, 1842–1847. [Google Scholar] [CrossRef] [Green Version]
- Wagenaar, J.A.; Yue, H.; Pritchard, J.; Broekhuizen-Stins, M.; Huijsdens, X.; Mevius, D.J.; Bosch, T.; Van Duijkeren, E. Unexpected sequence types in livestock associated methicillin-resistant Staphylococcus aureus (MRSA): MRSA ST9 and a single locus variant of ST9 in pig farming in China. Veter. Microbiol. 2009, 139, 405–409. [Google Scholar] [CrossRef] [PubMed]
- van Cleef, B.A.; Monnet, D.L.; Voss, A.; Krziwanek, K.; Allerberger, F.; Struelens, M.; Zemlickova, H.; Skov, R.L.; Vuopio-Varkila, J.; Cuny, C.; et al. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg. Infect. Dis. 2011, 17, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Bouiller, K.; Bertrand, X.; Hocquet, D.; Chirouze, C. Human Infection of Methicillin-Susceptible Staphylococcus aureus CC398: A Review. Microorganisms 2020, 8, 1737. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xu, S.; Zhao, H.; Hu, F.; Xu, X.; Jin, S.; Yang, H.; Gong, F.; Liu, Q. Leukotoxin and pyrogenic toxin Superantigen gene backgrounds in bloodstream and wound Staphylococcus aureus isolates from eastern region of China. BMC Infect. Dis. 2018, 18, 395. [Google Scholar] [CrossRef] [Green Version]
- Bouiller, K.; Hocquet, D.; Sauget, M.; Bertrand, X.; Chirouze, C. Epidemiology and risk factors of Staphylococcus aureus CC398 bone and joint infections. BMC Infect. Dis. 2020, 20, 384. [Google Scholar] [CrossRef] [PubMed]
- van der Mee-Marquet, N.; Domelier, A.S.; Girard, N.; Quentin, R.; Bloodstream Infection Study Group of the Relais d’Hygiene du, C. Epidemiology and typing of Staphylococcus aureus strains isolated from bloodstream infections. J. Clin. Microbiol. 2004, 42, 5650–5657. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Chen, H.; Zhao, C.; Zhang, F.; Li, H.; Wang, Q.; Wang, X.; Wang, H. Population structure and characterisation of Staphylococcus aureus from bacteraemia at multiple hospitals in China: Association between antimicrobial resistance, toxin genes and genotypes. Int. J. Antimicrob. Agents. 2013, 42, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, S.; Yang, C.; Chen, H.; Yin, Y.; Li, H.; Zhao, C.; Wang, H. The Changing Pattern of Population Structure of Staphylococcus aureus from Bacteremia in China from 2013 to 2016: ST239-030-MRSA Replaced by ST59-t437. Front. Microbiol. 2018, 9, 332. [Google Scholar] [CrossRef]
- van der Mee-Marquet, N.; Diene, S.M.; Barbera, L.; Courtier-Martinez, L.; Lafont, L.; Ouachee, A.; Valentin, A.S.; Santos, S.D.; Quentin, R.; Francois, P. Analysis of the prophages carried by human infecting isolates provides new insight into the evolution of Group B Streptococcus species. Clin. Microbiol. Infect. 2018, 24, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Renard, A.; Barbera, L.; Courtier-Martinez, L.; Dos Santos, S.; Valentin, A.S.; Mereghetti, L.; Quentin, R.; van der Mee-Marquet, N.L. phiD12-Like Livestock-Associated Prophages Are Associated With Novel Subpopulations of Streptococcus agalactiae Infecting Neonates. Front. Cell Infect. Microbiol. 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohmer, C.; Wolz, C. The Role of hlb-Converting Bacteriophages in Staphylococcus aureus Host Adaption. Microb. Physiol. 2021, 31, 109–122. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.J.; van Wamel, W.; Vandendriessche, S.; Larsen, J.; Denis, O.; Garcia-Graells, C.; Uhlemann, A.C.; Lowy, F.D.; Skov, R.; Lindsay, J.A. Staphylococcus aureus CC398 clade associated with human-to-human transmission. Appl. Environ. Microbiol. 2012, 78, 8845–8848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuny, C.; Abdelbary, M.; Layer, F.; Werner, G.; Witte, W. Prevalence of the immune evasion gene cluster in Staphylococcus aureus CC398. Veter. Microbiol. 2015, 177, 219–223. [Google Scholar] [CrossRef]
- Sieber, R.N.; Urth, T.R.; Petersen, A.; Moller, C.H.; Price, L.B.; Skov, R.L.; Larsen, A.R.; Stegger, M.; Larsen, J. Phage-Mediated Immune Evasion and Transmission of Livestock-Associated Methicillin-Resistant Staphylococcus aureus in Humans. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Sung, J.M.; Lloyd, D.H.; Lindsay, J.A. Staphylococcus aureus host specificity: Comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology 2008, 154, 1949–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wamel, W.J.; Rooijakkers, S.H.; Ruyken, M.; van Kessel, K.P.; van Strijp, J.A. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Chabelskaya, S.; Gaillot, O.; Felden, B. A Staphylococcus aureus small RNA is required for bacterial virulence and regulates the expression of an immune-evasion molecule. PLoS Pathogens 2010, 6, e1000927. [Google Scholar] [CrossRef]
- Atwood, D.N.; Beenken, K.E.; Loughran, A.J.; Meeker, D.G.; Lantz, T.L.; Graham, J.W.; Spencer, H.J.; Smeltzer, M.S. XerC Contributes to Diverse Forms of Staphylococcus aureus Infection via agr-Dependent and agr-Independent Pathways. Infect. Immun. 2016, 84, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Michel, A.; Agerer, F.; Hauck, C.R.; Herrmann, M.; Ullrich, J.; Hacker, J.; Ohlsen, K. Global regulatory impact of ClpP protease of Staphylococcus aureus on regulons involved in virulence, oxidative stress response, autolysis, and DNA repair. J. Bacteriol. 2006, 188, 5783–5796. [Google Scholar] [CrossRef] [Green Version]
- Lemire, S.; Figueroa-Bossi, N.; Bossi, L. Bacteriophage crosstalk: Coordination of prophage induction by trans-acting antirepressors. PLoS Genet. 2011, 7, e1002149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fieldhouse, R.J.; Turgeon, Z.; White, D.; Merrill, A.R. Cholera- and anthrax-like toxins are among several new ADP-ribosyltransferases. PLoS Comput. Biol. 2010, 6, e1001029. [Google Scholar] [CrossRef] [PubMed]
- Gibert, L.; Didi, J.; Marlinghaus, L.; Lesouhaitier, O.; Legris, S.; Szabados, F.; Pons, J.L.; Pestel-Caron, M. The major autolysin of Staphylococcus lugdunensis, AtlL, is involved in cell separation, stress-induced autolysis and contributes to bacterial pathogenesis. FEMS Microbiol. Lett. 2014, 352, 78–86. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, N.; Dong, J.; Gao, Y.; Zhang, X.; Shao, N.; Yang, G. SsrA (tmRNA) acts as an antisense RNA to regulate Staphylococcus aureus pigment synthesis by base pairing with crtMN mRNA. FEBS Lett. 2010, 584, 4325–4329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purves, J.; Cockayne, A.; Moody, P.C.; Morrissey, J.A. Comparison of the regulation, metabolic functions, and roles in virulence of the glyceraldehyde-3-phosphate dehydrogenase homologues gapA and gapB in Staphylococcus aureus. Infect. Immun. 2010, 78, 5223–5232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.Z.; Fujiwara, T.; Komatsuzawa, H.; Sugai, M.; Sakon, J. Cell wall-targeting domain of glycylglycine endopeptidase distinguishes among peptidoglycan cross-bridges. J. Biol. Chem. 2006, 281, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Kurokawa, K.; Nakamura, K.; Lee, B.L.; Sekimizu, K.; Kubagawa, H.; Hiramatsu, K.; Yagita, H.; Okumura, K.; Takai, T.; et al. Inhibitory receptor paired Ig-like receptor B is exploited by Staphylococcus aureus for virulence. J. Immunol. 2012, 189, 5903–5911. [Google Scholar] [CrossRef] [Green Version]
- Viboud, G.I.; Bliska, J.B. Yersinia outer proteins: Role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 2005, 59, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Rooijakkers, S.H.; Ruyken, M.; van Roon, J.; van Kessel, K.P.; van Strijp, J.A.; van Wamel, W.J. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol. 2006, 8, 1282–1293. [Google Scholar] [CrossRef]
- Coleman, D.C.; Sullivan, D.J.; Russell, R.J.; Arbuthnott, J.P.; Carey, B.F.; Pomeroy, H.M. Staphylococcus aureus bacteriophages mediating the simultaneous lysogenic conversion of beta-lysin, staphylokinase and enterotoxin A: Molecular mechanism of triple conversion. J. Gen. Microbiol. 1989, 135, 1679–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, J.D.; Cafferkey, M.T.; Coleman, D.C. Serotype F double- and triple-converting phage insertionally inactivate the Staphylococcus aureus beta-toxin determinant by a common molecular mechanism. FEMS Microbiol. Lett. 1993, 106, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Goerke, C.; Wirtz, C.; Fluckiger, U.; Wolz, C. Extensive phage dynamics in Staphylococcus aureus contributes to adaptation to the human host during infection. Mol. Microbiol. 2006, 61, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Messad, N.; Prajsnar, T.K.; Lina, G.; O'Callaghan, D.; Foster, S.J.; Renshaw, S.A.; Skaar, E.P.; Bes, M.; Dunyach-Remy, C.; Vandenesch, F.; et al. Existence of a Colonizing Staphylococcus aureus Strain Isolated in Diabetic Foot Ulcers. Diabetes 2015, 64, 2991–2995. [Google Scholar] [CrossRef] [Green Version]
- Jung, P.; Abdelbary, M.M.; Kraushaar, B.; Fetsch, A.; Geisel, J.; Herrmann, M.; Witte, W.; Cuny, C.; Bischoff, M. Impact of bacteriophage Saint3 carriage on the immune evasion capacity and hemolytic potential of Staphylococcus aureus CC398. Veter. Microbiol. 2017, 200, 46–51. [Google Scholar] [CrossRef]
- Sinha, B.; Francois, P.P.; Nusse, O.; Foti, M.; Hartford, O.M.; Vaudaux, P.; Foster, T.J.; Lew, D.P.; Herrmann, M.; Krause, K.H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin a5b1. Cell. Microbiol. 1999, 1, 101–117. [Google Scholar] [CrossRef]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Said-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Kreiswirth, B.N.; et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Gerlach, D.; Guo, Y.; De Castro, C.; Kim, S.H.; Schlatterer, K.; Xu, F.F.; Pereira, C.; Seeberger, P.H.; Ali, S.; Codee, J.; et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 2018, 563, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.J.; Hook, M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998, 6, 484–488. [Google Scholar] [CrossRef]
- Patti, J.M.; Allen, B.L.; McGavin, M.J.; Hook, M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 1994, 48, 585–617. [Google Scholar] [CrossRef] [PubMed]
- Entenza, J.M.; Foster, T.J.; Ni, E.D.; Vaudaux, P.; Francioli, P.; Moreillon, P. Contribution of clumping factor B to pathogenesis of experimental endocarditis due to Staphylococcus aureus. Infect. Immun. 2000, 68, 5443–5446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Que, Y.A.; Haefliger, J.A.; Piroth, L.; Francois, P.; Widmer, E.; Entenza, J.M.; Sinha, B.; Herrmann, M.; Francioli, P.; Vaudaux, P.; et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 2005, 201, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Elasri, M.O.; Thomas, J.R.; Skinner, R.A.; Blevins, J.S.; Beenken, K.E.; Nelson, C.L.; Smelter, M.S. Staphylococcus aureus collagen adhesin contributes to the pathogenesis of osteomyelitis. Bone 2002, 30, 275–280. [Google Scholar] [CrossRef]
- Patti, J.M.; Bremell, T.; Krajewska-Pietrasik, D.; Abdelnour, A.; Tarkowski, A.; Ryden, C.; Hook, M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect. Immun. 1994, 62, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Bonesso, M.F.; Yeh, A.J.; Villaruz, A.E.; Joo, H.S.; McCausland, J.; Fortaleza, C.M.; Cavalcante, R.S.; Sobrinho, M.T.; Ronchi, C.F.; Cheung, G.Y.; et al. Key Role of alpha-Toxin in Fatal Pneumonia Caused by Staphylococcus aureus Sequence Type 398. Am. J. Respir. Crit. Care Med. 2016, 193, 217–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirolo, M.; Sieber, R.N.; Moodley, A.; Visaggio, D.; Artuso, I.; Gioffre, A.; Casalinuovo, F.; Spatari, G.; Guardabassi, L.; Stegger, M.; et al. Local and Transboundary Transmissions of Methicillin-Resistant Staphylococcus aureus Sequence Type 398 through Pig Trading. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Hansen, J.E.; Ronco, T.; Stegger, M.; Sieber, R.N.; Fertner, M.E.; Martin, H.L.; Farre, M.; Toft, N.; Larsen, A.R.; Pedersen, K. LA-MRSA CC398 in Dairy Cattle and Veal Calf Farms Indicates Spillover From Pig Production. Front. Microbiol. 2019, 10, 2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J.E.; Stegger, M.; Pedersen, K.; Sieber, R.N.; Larsen, J.; Larsen, G.; Lilje, B.; Chriel, M.; Andersen, P.S.; Larsen, A.R. Spread of LA-MRSA CC398 in Danish mink (Neovison vison) and mink farm workers. Veter. Microbiol. 2020, 245, 108705. [Google Scholar] [CrossRef]
- Liang, B.; Mai, J.; Liu, Y.; Huang, Y.; Zhong, H.; Xie, Y.; Deng, Q.; Huang, L.; Yao, S.; He, Y.; et al. Prevalence and Characterization of Staphylococcus aureus Isolated From Women and Children in Guangzhou, China. Front. Microbiol. 2018, 9, 2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardyn, S.E.; Stegger, M.; Price, L.B.; Smith, T.C. Whole-Genome Analysis of Recurrent Staphylococcus aureus t571/ST398 Infection in Farmer, Iowa, USA. Emerg. Infect. Dis. 2018, 24, 153–154. [Google Scholar] [CrossRef] [Green Version]
- Carrel, M.; Goto, M.; Schweizer, M.L.; David, M.Z.; Livorsi, D.; Perencevich, E.N. Diffusion of clindamycin-resistant and erythromycin-resistant methicillin-susceptible Staphylococcus aureus (MSSA), potential ST398, in United States Veterans Health Administration Hospitals, 2003-2014. Antimicrob. Resist. Infect. Control. 2017, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Kruger, H.; Fessler, A.T.; Liu, J.; Zeng, Z.; Wang, Y.; Wu, C.; Schwarz, S. A novel SCCmec type V variant in porcine MRSA ST398 from China. J. Antimicrob. Chemother. 2020, 75, 484–486. [Google Scholar] [CrossRef] [PubMed]
- van Alen, S.; Ballhausen, B.; Kaspar, U.; Kock, R.; Becker, K. Prevalence and Genomic Structure of Bacteriophage phi3 in Human-Derived Livestock-Associated Methicillin-Resistant Staphylococcus aureus Isolates from 2000 to 2015. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fessler, A.; Kadlec, K.; Wang, Y.; Zhang, W.J.; Wu, C.; Shen, J.; Schwarz, S. Small Antimicrobial Resistance Plasmids in Livestock-Associated Methicillin-Resistant Staphylococcus aureus CC398. Front. Microbiol. 2018, 9, 2063. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ripa, L.; Belles, A.; Garcia, M.; Torres, C. Detection of a cfr-positive MRSA CC398 strain in a pig farmer in Spain. Enferm Infecc. Microbiol. Clin. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Andersen, P.S.; Stegger, M.; Sieber, R.N.; Ingmer, H.; Staubrand, N.; Dalsgaard, A.; Leisner, J.J. Antimicrobial Resistance and Virulence Gene Profiles of Methicillin-Resistant and -Susceptible Staphylococcus aureus From Food Products in Denmark. Front. Microbiol. 2019, 10, 2681. [Google Scholar] [CrossRef] [PubMed]
- Mama, O.M.; Morales, L.; Ruiz-Ripa, L.; Zarazaga, M.; Torres, C. High prevalence of multidrug resistant S. aureus-CC398 and frequent detection of enterotoxin genes among non-CC398 S. aureus from pig-derived food in Spain. Int. J. Food Microbiol. 2020, 320, 108510. [Google Scholar] [CrossRef] [PubMed]
| Prophages | Characteristics | Putative roles | References |
|---|---|---|---|
| φ3 and Sa3int variants | β-converting, IEC, putative factors involved in bacterial virulence, biofilm formation, fitness, stress adaptation and genome plasticity | Immune escape | [21,22,56,57,74] |
| Host colonization, invasion | [22,24,58,59,60,61,62,63,64,65,66,67,68] | ||
| Long-term host colonization | [57,69,72,73] | ||
| Virulence in Caenorhabditis elegans | [29] | ||
| MR11-like variants | Putative factors involved in bacterial virulence, biofilm formation, fitness, stress adaptation and genome plasticity | Host colonization, invasion | [22,24,58,59,60,61,62,63,64,65,66,67,68] |
| Immune escape, when associated to φ3 | [22,69,70,71] | ||
| φtarP-Sa1int and φtarP-Sa9int | Alternative glycosyltransferase TarP | WTAs modification, immune escape | [78] |
| StauST398-2Pro and StauST398-3Pro | Adhesion to fibronectin/fibrinogen—modulation of adhesion genes expression, cell invasion, virulence in rats (infectious endocarditis) | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laumay, F.; Benchetrit, H.; Corvaglia, A.-R.; van der Mee-Marquet, N.; François, P. The Staphylococcus aureus CC398 Lineage: An Evolution Driven by the Acquisition of Prophages and Other Mobile Genetic Elements. Genes 2021, 12, 1752. https://doi.org/10.3390/genes12111752
Laumay F, Benchetrit H, Corvaglia A-R, van der Mee-Marquet N, François P. The Staphylococcus aureus CC398 Lineage: An Evolution Driven by the Acquisition of Prophages and Other Mobile Genetic Elements. Genes. 2021; 12(11):1752. https://doi.org/10.3390/genes12111752
Chicago/Turabian StyleLaumay, Floriane, Hugo Benchetrit, Anna-Rita Corvaglia, Nathalie van der Mee-Marquet, and Patrice François. 2021. "The Staphylococcus aureus CC398 Lineage: An Evolution Driven by the Acquisition of Prophages and Other Mobile Genetic Elements" Genes 12, no. 11: 1752. https://doi.org/10.3390/genes12111752
APA StyleLaumay, F., Benchetrit, H., Corvaglia, A. -R., van der Mee-Marquet, N., & François, P. (2021). The Staphylococcus aureus CC398 Lineage: An Evolution Driven by the Acquisition of Prophages and Other Mobile Genetic Elements. Genes, 12(11), 1752. https://doi.org/10.3390/genes12111752






