Genotype-Specific Lesion Growth Rates in Stargardt Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Approval
2.3. Imaging Procedures and Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Heath Jeffery, R.C.; Mukhtar, S.A.; McAllister, I.L.; Morgan, W.H.; Mackey, D.A.; Chen, F.K. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Runhart, E.H.; Dhooge, P.; Meester-Smoor, M.; Pas, J.; Pott, J.W.R.; van Leeuwen, R.; Kroes, H.Y.; Bergen, A.A.; Jong-Hesse, Y.; Thiadens, A.A.; et al. Stargardt disease: Monitoring incidence and diagnostic trends in the Netherlands using a nationwide disease registry. Acta Ophthalmol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Thompson, J.A.; Lo, J.; Lamey, T.M.; McLaren, T.L.; McAllister, I.L.; Mackey, D.A.; Constable, I.J.; De Roach, J.N.; Chen, F.K. Atrophy Expansion Rates in Stargardt Disease Using Ultra-Widefield Fundus Autofluorescence. Ophthalmol. Sci. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Chen, F.K. Stargardt disease: Multimodal imaging: A review. Clin. Exp. Ophthalmol. 2021, 49, 498–515. [Google Scholar] [CrossRef]
- Feuer, W.J.; Yehoshua, Z.; Gregori, G.; Penha, F.M.; Chew, E.Y.; Ferris, F.; Clemons, T.E.; Lindblad, A.S.; Rosenfeld, P.J. Square Root Transformation of Geographic Atrophy Area Measurements to Eliminate Dependence of Growth Rates on Baseline Lesion Measurements: A Reanalysis of Age-Related Eye Disease Study Report No. 26. JAMA Ophthalmol. 2013, 131, 110–111. [Google Scholar] [CrossRef]
- Shen, L.L.; Sun, M.; Grossetta Nardini, H.K.; Del Priore, L.V. Natural History of Autosomal Recessive Stargardt Disease in Untreated Eyes: A Systematic Review and Meta-analysis of Study- and Individual-Level Data. Ophthalmology 2019, 126, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- De Roach, J.N.; McLaren, T.L.; Paterson, R.L.; O’Brien, E.C.; Hoffmann, L.; Mackey, D.A.; Hewitt, A.W.; Lamey, T.M. Establishment and evolution of the Australian Inherited Retinal Disease Register and DNA Bank. Clin. Exp. Ophthalmol. 2013, 41, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremers, F.P.; Lee, W.; Collin, R.W.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.B.; Molday, L.L.; Garces, F.A.; Molday, R.S. Functional analysis and classification of homozygous and hypomorphic ABCA4 variants associated with Stargardt macular degeneration. Hum. Mutat. 2020, 41, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Fakin, A.; Robson, A.; Chiang, J.; Fujinami, K.; Moore, A.T.; Michaelides, M.; Holder, G.E.; Webster, A.R. The Effect on Retinal Structure and Function of 15 Specific ABCA4 Mutations: A Detailed Examination of 82 Hemizygous Patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5963–5973. [Google Scholar] [CrossRef]
- Sun, H.; Smallwood, P.M.; Nathans, J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat. Genet. 2000, 26, 242–246. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Thompson, J.A.; Lamey, T.M.; McLaren, T.L.; McAllister, I.L.; Constable, I.J.; Chen, F.K. Classifying ABCA4 mutation severity using age-dependent ultra-widefield fundus autofluorescence-derived total lesion size. Retina 2021, 41, 2578–2588, Online Ahead of Print. [Google Scholar] [CrossRef]
- Garces, F.; Jiang, K.; Molday, L.L.; Stöhr, H.; Weber, B.H.; Lyons, C.J.; Maberley, D.; Molday, R.S. Correlating the Expression and Functional Activity of ABCA4 Disease Variants With the Phenotype of Patients With Stargardt Disease. Investig. Opthalmology Vis. Sci. 2018, 59, 2305–2315. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Tsybovsky, Y.; Kolesnikov, A.V.; Rozanowska, M.; Swider, M.; Schwartz, S.B.; Stone, E.M.; Palczewska, G.; Maeda, A.; Kefalov, V.; et al. Protein misfolding and the pathogenesis of ABCA4-associated retinal degenerations. Hum. Mol. Genet. 2015, 24, 3220–3237. [Google Scholar] [CrossRef]
- Kong, X.; Strauss, R.W.; Munoz, B.; West, S.; Cheetham, J.; Scholl, H.P.N. Re: Shen et al.: Natural history of autosomal recessive Stargardt disease in untreated eyes: A systematic review and meta-analysis of study and individual level data (Ophthalmology. 2019;126:1288–1296). Ophthalmology 2020, 127, e28–e29. [Google Scholar] [CrossRef]
- Kugelman, J.; Alonso-Caneiro, D.; Chen, Y.; Arunachalam, S.; Huang, D.; Vallis, N.; Collins, M.J.; Chen, F.K. Retinal Boundary Segmentation in Stargardt Disease Optical Coherence Tomography Images Using Automated Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.K.; Patel, P.J.; Webster, A.R.; Coffey, P.J.; Tufail, A.; Da Cruz, L. Nidek MP1 is able to detect subtle decline in function in inherited and age-related atrophic macular disease with stable visual acuity. Retina 2011, 31, 371–379. [Google Scholar] [CrossRef]
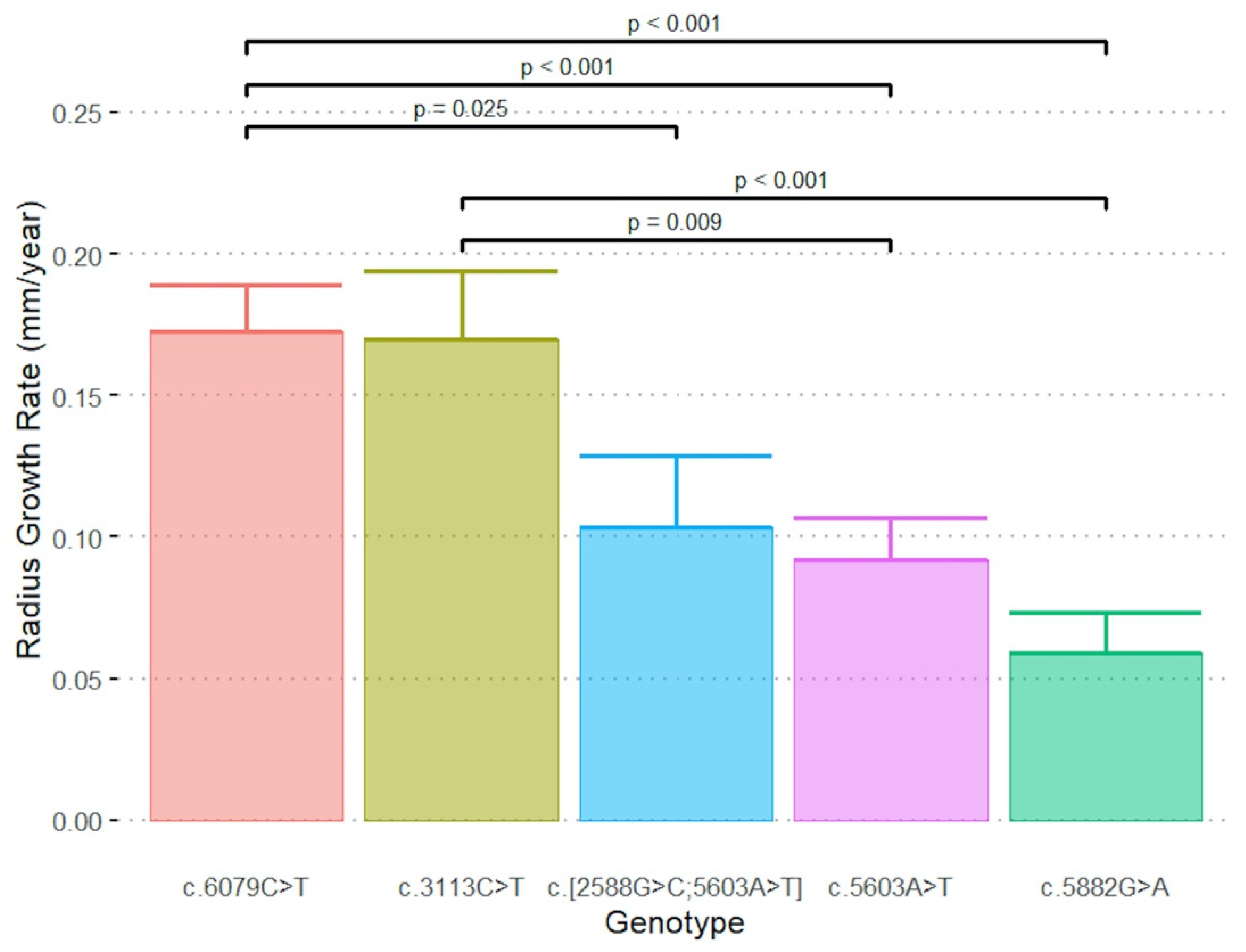
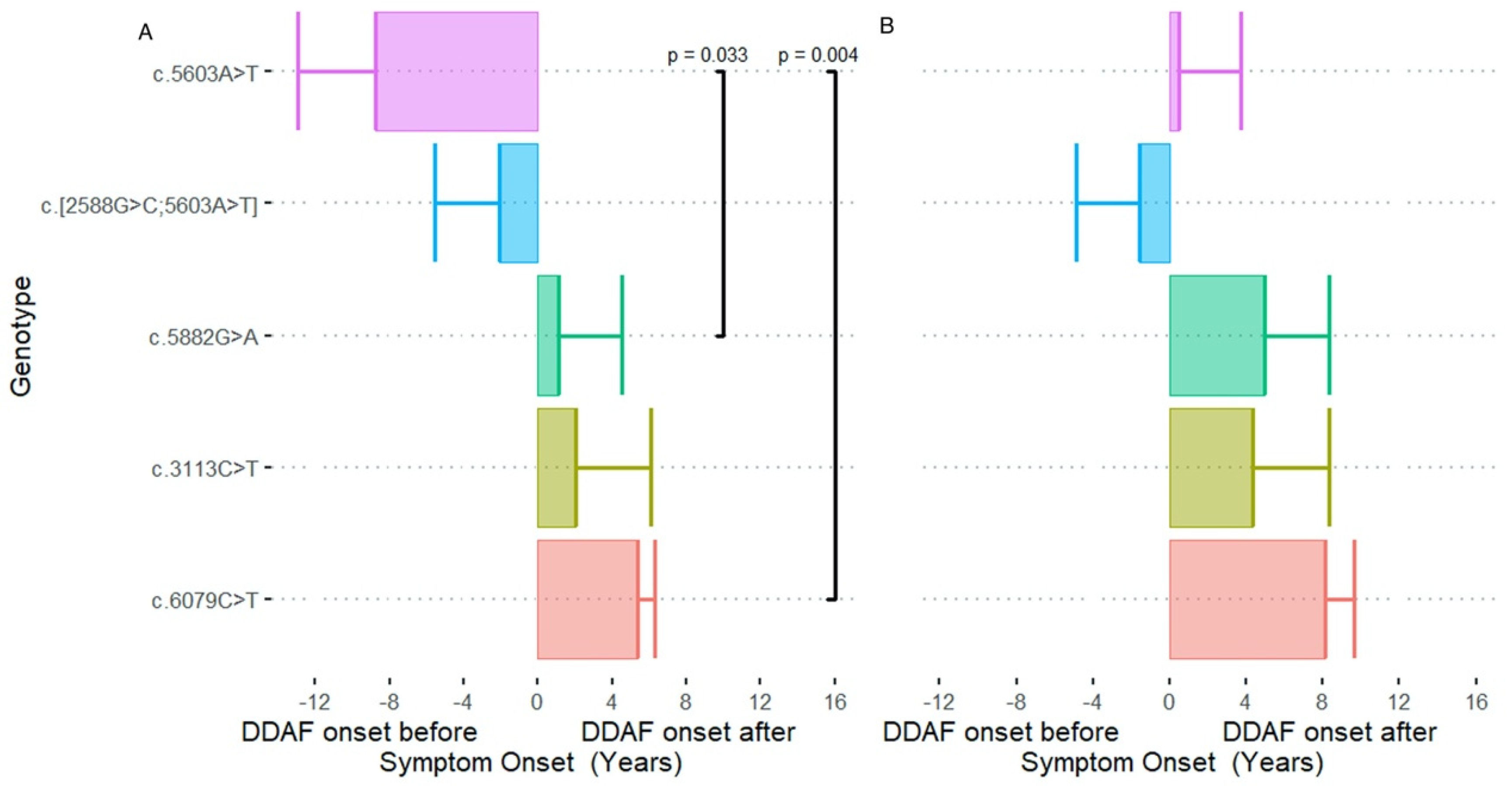
| Genotype | N | Radius Growth Rate (mm / Year) | ΔT1 Mean ± SE (95% CI) (Years) | ΔT2 Mean ± SE (Years) |
|---|---|---|---|---|
| Mean ± SE (95% CI) | Mean ± SE (95% CI) | Mean ± SE (95% CI) | ||
| c.[2588G>C;5603A>T] | 4 | 0.10 ± 0.02 (0.05, 0.15) | −2.1 ± 3.5 † (−46.7, 42.5) | −1.6 ± 3.8 † (−49.4, 46.2) |
| c.3113C>T | 4 | 0.17 ± 0.02 (0.12, 0.22) | 2.0 ± 4.1 (−11.0, 15.0) | 4.3 ± 4.0 (−8.3, 16.9) |
| c.5603A>T | 11 | 0.09 ± 0.01 (0.06, 0.12) | −8.7 ± 4.2 ‡ (−18.2, 0.6) | 0.5 ± 3.2 ‡ (−6.9, 7.8) |
| c.5882G>A | 11 | 0.06 ± 0.01 (0.03, 0.09) | 1.1 ± 3.5 ‡ (−6.8, 8.9) | 4.9 ± 3.4 ‡ (−2.8, 12.6) |
| c.6079C>T | 9 | 0.17 ± 0.02 (0.14, 0.21) | 5.4 ± 0.9 (3.3, 7.4) | 8.1 ± 1.5 (4.7, 11.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heath Jeffery, R.C.; Thompson, J.A.; Lo, J.; Lamey, T.M.; McLaren, T.L.; McAllister, I.L.; Constable, I.J.; De Roach, J.N.; Chen, F.K. Genotype-Specific Lesion Growth Rates in Stargardt Disease. Genes 2021, 12, 1981. https://doi.org/10.3390/genes12121981
Heath Jeffery RC, Thompson JA, Lo J, Lamey TM, McLaren TL, McAllister IL, Constable IJ, De Roach JN, Chen FK. Genotype-Specific Lesion Growth Rates in Stargardt Disease. Genes. 2021; 12(12):1981. https://doi.org/10.3390/genes12121981
Chicago/Turabian StyleHeath Jeffery, Rachael C., Jennifer A. Thompson, Johnny Lo, Tina M. Lamey, Terri L. McLaren, Ian L. McAllister, Ian J. Constable, John N. De Roach, and Fred K. Chen. 2021. "Genotype-Specific Lesion Growth Rates in Stargardt Disease" Genes 12, no. 12: 1981. https://doi.org/10.3390/genes12121981






