Senescence: The Compromised Time of Death That Plants May Call on Themselves
Abstract
:1. Senescence and Aging in Plants
2. Age-Dependent/-Independent Senescence in Plants
3. Whole Plant Senescence
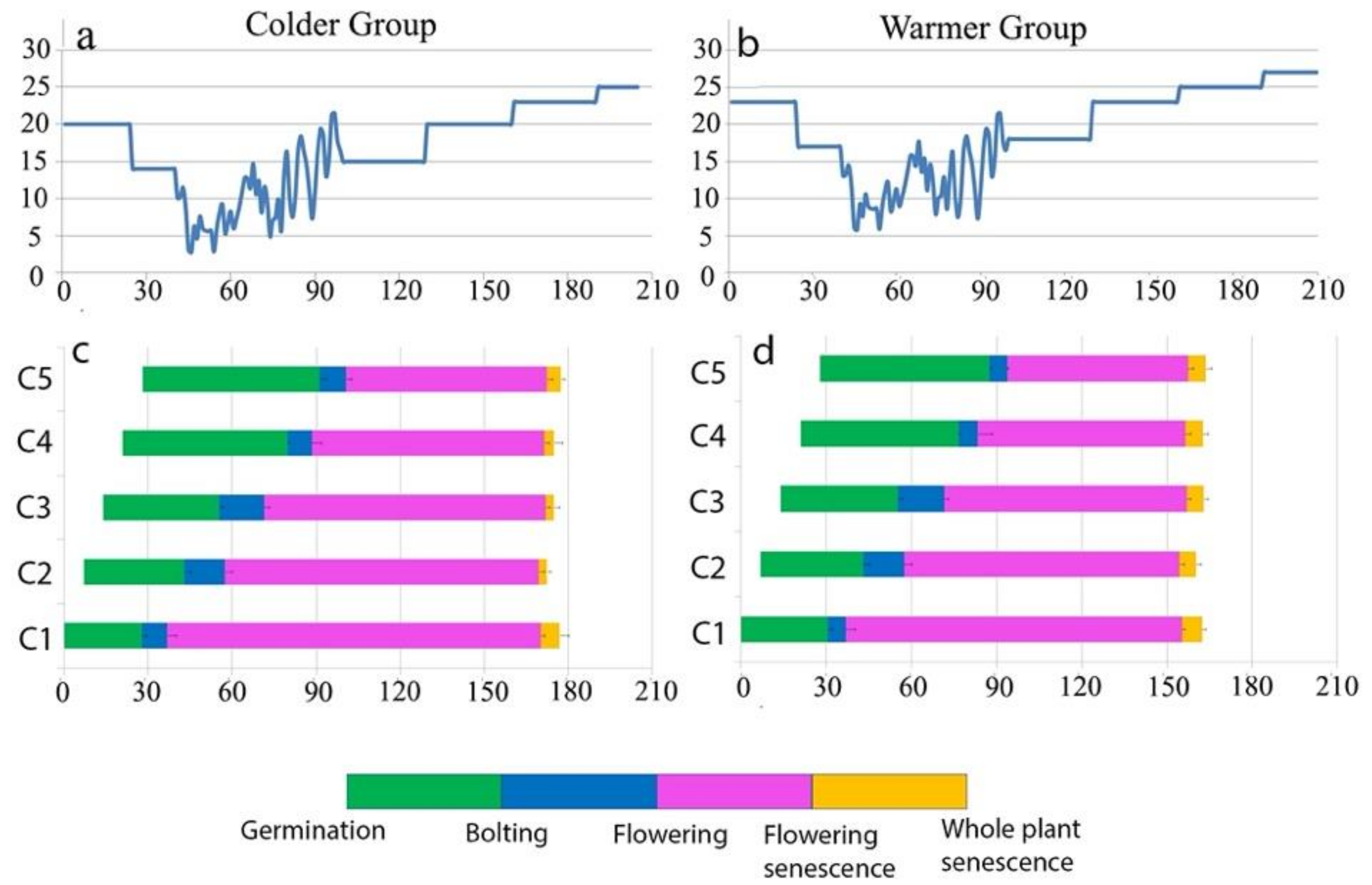
4. Plants Schedule Their Life Events Based on Environmental Signals
5. The Art of Senescence Synchrony and Harmonizing with Environmental Cues
6. Senescence Timing and Productivity in Plants
7. The Importance of Molecular Analyses of Senescence
8. “Premature Senescence”: A Forced, Unwanted Type of Senescence
9. Conclusions and Future Plans
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caswell, H.; Salguero-Gómez, R. Age, stage and senescence in plants. J. Ecol. 2013, 101, 585–595. [Google Scholar] [CrossRef]
- Harper, J.L. Plant demography and ecological theory. Oikos 1980, 35, 244–253. [Google Scholar] [CrossRef]
- Lanner, R.M.; Connor, K.F. Does bristlecone pine senesce? Exp. Gerontol. 2001, 36, 675–685. [Google Scholar] [CrossRef]
- García, M.B.; Dahlgren, J.P.; Ehrlén, J. No evidence of senescence in a 300-year-old mountain herb. J. Ecol. 2011, 99, 1424–1430. [Google Scholar] [CrossRef]
- Morales, M.; Oñate, M.; García, M.B.; Munné-Bosch, S. Photo-oxidative stress markers reveal absence of physiological deterioration 1 with ageing in Borderea pyrenaica, an extraordinarily long-lived herb. J. Ecol. 2013, 101, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Medawar, P.B. An Unsolved Problem of Biology; H.K. Lewis: London, UK, 1952. [Google Scholar]
- Williams, G.C. Pleiotropy, natural selection, and the evolution of senescence. Evolution 1957. [Google Scholar] [CrossRef]
- Hamilton, W.D. The moulding of senescence by natural selection. J. Theor. Biol. 1966, 12, 12–45. [Google Scholar] [CrossRef]
- Pujol, B.; Marrot, P.; Pannell, J.R. A quantitative genetic signature of senescence in a short-lived perennial plant. Curr. Biol. 2014, 24, 744–747. [Google Scholar] [CrossRef] [Green Version]
- Brommer, J.E. Senescence: Detecting an evolutionary fingerprint in plants. Curr. Biol. 2014, 24, 267–269. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.R.; Masclaux-Daubresse, C.; Lim, P.O. Plant senescence: How plants know when and how to die. J. Exp. Bot. 2018, 69, 715–718. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Page, T.; Harrison, E.; Breeze, E.; Lim, P.O.; Nam, H.G.; Lin, J.F.; Wu, S.H.; Swidzinski, J.; Ishizaki, K.; et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005, 42, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H. Senescence, ageing and death of the whole plant. New Phytol. 2013, 197, 696–711. [Google Scholar] [CrossRef]
- Schippers, J.H. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M.; Yamaguchi, M.; Kudoh, H. Synchronisation of Arabidopsis flowering time and whole-plant senescence in seasonal environments. Sci. Rep. 2018, 8, 10282. [Google Scholar] [CrossRef] [Green Version]
- Miryeganeh, M. Synchronization of senescence and desynchronization of flowering in Arabidopsis thaliana. AoB Plants 2020, 12, plaa018. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Balanza, V.; Martınez-Fernandez, I.; Sato, S.; Yanofsky, M.F.; Kaufmann, K.; Angenent, G.C.; Bemer, M.; Ferrandiz, C. Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat. Commun. 2018, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Gan, S.S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2012, 35, 644–655. [Google Scholar] [CrossRef]
- Khan, M.; Rozhon, W.; Poppenberger, B. The role of hormones in the aging of plants—A mini-review. Gerontology 2014, 60, 49–55. [Google Scholar] [CrossRef]
- Pico, F.X.; Retana, J. Age-specific, density-dependent and environment-based mortality of a short-lived perennial herb. Plant Biol. 2008, 10, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.A.; Ridley, C.E.; Dudycha, J.L. Longitudinal analysis of Plantago: Age-by-environment interactions reveal aging. Ecology 2009, 90, 1427–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shefferson, R.P.; Roach, D.A. Longitudinal analysis in Plantago: Strength of selection and reverse age analysis reveal age-indeterminate senescence. J. Ecol. 2013, 101, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.H.; Bennett, T. Forbidden fruit: Dominance relationships and the control of shoot architecture. Annu. Plant Rev. 2018, 1, 1–38. [Google Scholar]
- González-Suárez, P.; Walker, C.H.; Bennett, T. Bloom and bust: Understanding the nature and regulation of the end of flowering. Curr. Opin. Plant Biol. 2020, 57, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Fournier-Level, A.; Korte, A.; Cooper, M.D.; Nordborg, M.; Schmitt, J.; Wilczek, A.M. A map of local adaptation in Arabidopsis thaliana. Science 2011, 334, 86–89. [Google Scholar] [CrossRef]
- Ono, K.; Nishi, Y.; Watanabe, A.; Terashima, I. Possible mechanismsof adaptive leaf senescence. Curr. Opin. Plant Biol. 2001, 3, 234–243. [Google Scholar]
- Parrott, D.L.; Downs, E.P.; Fischer, A.M. Control of barley (Hordeum vulgare L.) development and senescence by the interaction between a chromosome six grain protein content locus, day length, and vernalization. J. Exp. Bot. 2012, 63, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.O.; Davies, P.J. Photoperiodic and genetic control of carbon partitioning in peas and its relationship to apical senescence. Plant Physiol. 1988, 86, 978–982. [Google Scholar] [CrossRef] [Green Version]
- Noodén, L.D.; Guiamét, J.J.; John, I. Whole plant senescence. In Plant Cell Death Processes, 1st ed.; Noodén, L.D., Ed.; Academic Press: San Diego, CA, USA, 2004; pp. 227–244. [Google Scholar]
- Leopold, A.C. Senescence in plant development. Science 1961, 134, 1727–1732. [Google Scholar] [CrossRef]
- Leopold, A.C. Aging, senescence and turnover in plants. BioScience 1975, 25, 659. [Google Scholar] [CrossRef]
- Guiboileau, A.; Sormani, R.; Meyer, C.; Masclaux-Daubresse, C. Senescence and death of plant organs: Nutrient recycling and developmental regulation. Comptes Rendus Biologies 2010, 333, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, F. Die Lebensdauer und Vegetationsweise der Pflanzen, ihre Ursahe und ihre Entwicklung. Bot Jahrb Syst Pflan- zengesch Pflanzengeogr. 1881, 2, 51–135. [Google Scholar]
- Hensel, L.L.; Nelson, M.A.; Richmond, T.A.; Bleecker, A.B. The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol. 1994, 106, 863–876. [Google Scholar] [CrossRef] [Green Version]
- Ware, A.; Walker, C.; Simura, J.; González-Suárez, P.; Ljung, K.; Bishopp, A.; Wilson, Z.; Bennett, T. Auxin export from proximal fruits drives arrest in competent inflorescences. Nat. Plants 2020, 6, 699–707. [Google Scholar] [CrossRef]
- Wuest, S.E.; Philipp, M.A.; Guthorl, D.; Schmid, B.; Grossniklaus, U. Seed production affects maternal growth and senescence in Arabidopsis. Plant Physiol. 2016, 171, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Murneek, A.E. Effects of correlation between vegetative and reproductive functions in the tomato (Lycopersicon esculentum Mill.). Plant Physiol. 1926, 1, 3–56. [Google Scholar] [CrossRef] [Green Version]
- Gan, S. Mitotic and postmitotic senescence in plants. Sci. Aging Knowl. Environ. 2003, 38, 7. [Google Scholar] [CrossRef] [Green Version]
- Hensel, L.L.; Grbić, V.; Baumgarten, D.A.; Bleecker, A.B. Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. Plant Cell. 1993, 5, 553–564. [Google Scholar]
- Molisch, H. Die Lebensdauer der Pflanze; Verlag von Gustav Fischer: Jena, Germany, 1929. [Google Scholar]
- Leopold ACNiedergang-Kamien, E.; Janick, J. Experimental modification of plant senescence. Plant Physiol. 1959, 34, 570–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.K.; Hill, S.A. Altered resource allocation during seed development in Arabidopsis caused by the abi3 mutation. Plant Cell Environ. 1999, 22, 117–123. [Google Scholar] [CrossRef]
- Noodén, L.D.; Penney, J.P. Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J Exp Bot. 2001, 52, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Lindoo, S.J.; Noodén, L.D. Studies on the behavior of the senescence signal in anoka soybeans. Plant Physiol. 1977, 59, 1136–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.B. An evolutionary perspective on the “death hormone” hypothesis in plants. Physiol. Plant. 1997, 99, 511–516. [Google Scholar] [CrossRef]
- Fischer, R.A. The importance of grain or kernel number in wheat: A reply to Sinclair and Jamieson. Field Crops Res. 2008, 105, 15–21. [Google Scholar] [CrossRef]
- Diaz, C.; Purdy, S.; Christ, A.; Morot-Gaudry, J.F.; Wingler, A.; MasclauxDaubresse, C. Characterization of markers to determine the extent and variability of leaf senescence in Arabidopsis. A metabolic profiling approach. Plant Physiol. 2005, 138, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Luquez, V.M.; Sasal, Y.; Medrano, M.; Martín, M.I.; Mujica, M.; Guiamét, J.J. Quantitative trait loci analysis of leaf and plant longevity in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 1363–1372. [Google Scholar] [CrossRef] [Green Version]
- Woolhouse, H.W. Hormonal control of senescence allied to reproduction in plants. In Beltsville Symposia in Agricultural Research-Strategies of Plant Reproduction; Allanheld, Osmun & Co.: Totowa, NJ, USA, 1983; pp. 201–236. [Google Scholar]
- Gan, S.; Amasino, R.M. Making sense of senescence. Plant Physiol. 1997, 113, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Martre, P.; Jamieson, P.D.; Semenov, M.A.; Zyskowski, R.F.; Porter, J.R.; Triboi, E. Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. Eur. J. Agron. 2006, 25, 138–154. [Google Scholar] [CrossRef]
- Levey, S.; Wingler, A. Natural variation in the regulation of leaf senescence and relation to other traits in Arabidopsis. Plant Cell Environ. 2005, 28, 223–231. [Google Scholar] [CrossRef]
- Schemske, D.W.; Willson, M.R.; Melarnpy, M.N.; Miller, L.J.; Verner, L.; Schemske, K.M.; Best, L.B. Flowering ecology of some spring woodland herbs. Ecology 1978, 59, 351–366. [Google Scholar] [CrossRef]
- Schmitt, J. Individual flowering phenology, plant size, and reproductive success in Linanthus androsaceus, a California annual. Oecologia 1983, 59, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Waser, N.M. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology 1978, 59, 934–944. [Google Scholar] [CrossRef]
- Gross, R.S.; Werner, P.A. Relationships among flowering phenology, insect visitors, and seed set of individuals: Experimental studies on four co-occurring species of goldenrod (Solidago: Compositae). Ecol. Monogr. 1983, 53, 95–117. [Google Scholar] [CrossRef]
- Zimmerman, M.; Gross, R.S. The relationship between flowering phenology and seed set in a herbaceous perennial plant, Polemonium foliosissimum Gray. Am. MidI. Nat. 1984, 11, 185–191. [Google Scholar] [CrossRef]
- Wingler, A.; Purdy, S.J.; Edwards, S.A.; Chardon, F.; Masclaux-Daubresse, C. QTL analysis for sugar-regulated leaf senescence supports flowering-dependent and -independent senescence pathways. New Phytol. 2010, 185, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Diaz, C.; Saliba-Colombani, V.; Loudet, O.; Belluomo, P.; Moreau, L.; Daniel-Vedele, F.; Morot-Gaudry, J.F.; Masclaux-Daubresse, C. Leaf yellowing and anthocyanin accumulation are two genetically independent strategies in response to nitrogen limitation in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Masclaux-Daubresse, C.; Purdy, S.; Lemaitre, T.; Pourtau, N.; Taconnat, L.; Renou, J.P.; Wingler, A. Genetic variation suggests interaction between cold acclimation and metabolic regulation of leaf senescence. Plant Physiol. 2007, 143, 434–446. [Google Scholar] [CrossRef] [Green Version]
- Kudoh, H. Photoperiod-temperature phase lag: A universal environmental context of seasonal developmental plasticity. DGD 2019, 61, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Donohue, K. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 2002, 83, 1006–1016. [Google Scholar] [CrossRef]
- Cohen, D. Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J. Theor. Biol. 1967, 16, 1–14. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Blackman, B.K. Changing responses to changing seasons: Natural variation in the plasticity of flowering time. Plant Physiol. 2017, 173, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Barrett, R.D.H.; Schluter, D. Adaptation from standing genetic variation. Trends Ecol. Evol. 2009, 23, 38–44. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Letts. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Kimball, S.; Angert, A.L.; Huxman, T.E.; Venable, D.L. Differences in the timing of germination and reproduction relate to growth physiology and population dynamics of Sonoran Desert winter annuals. Am. J. Bot. 2011, 98, 1773–1781. [Google Scholar] [CrossRef]
- Anderson, J.T.; Inouye, D.W.; McKinney, A.M.; Colautti, R.I.; Mitchell-Olds, T. Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc. R Soc. Lond. 2012, 279, 3843–3852. [Google Scholar] [CrossRef] [Green Version]
- Iler, A.M.; Høye, T.T.; Inouye, D.W.; Schmidt, N.M. Long-term trends mask variation in the direction and magnitude of short-term phenological shifts. Am. J. Bot. 2013, 100, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Gremer, J.R.; Wilcox, C.J.; Chiono, A.; Suglia, E.; Schmitt, J. Germination timing and chilling exposure create contingency in life history and influence fitness in the native wildflower Streptanthus tortuosus. J. Ecol. 2020, 108, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Fitter, A.H.; Fitter, R.S. Rapid changes in flowering time in British plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Springthorpe, V.; Penfield, S. Flowering time and seed dormancy control use external coincidence to generate life history strategy. eLIFE 2015, 4, e05557. [Google Scholar] [CrossRef] [PubMed]
- Toomajian, C.; Hu, T.T.; Aranzana, M.J.; Lister, C.; Tang, C.; Zheng, H.; Nordborg, M. A nonparametric test reveals selection for rapid flowering in the Arabidopsis genome. PLoS Biol. 2006, 4, e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, D.B.; Willis, J.H. A widespread chromosomal inversion polymorphism contributes to a major life-history transition, local adaptation, and reproductive isolation. PLoS Biol. 2010, 8, e1000500. [Google Scholar] [CrossRef] [Green Version]
- Baduel, P.; Arnold, B.; Weisman, C.M.; Hunter, B.; Bomblies, K. Habitat-associated life history and stress-tolerance variation in Arabidopsis arenos. Plant Physiol. 2016, 171, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Fenner, M. The effects of parental environment on seed germinability. Seed Sci. Res. 1991, 1, 75–81. [Google Scholar] [CrossRef]
- Schmuths, H.; Bachmann, K.; Weber, W.E.; Horres, R.; Hoffmann, M.H. Effects of preconditioning and temperature during germination of 73 natural accessions of Arabidopsis thaliana. Ann. Bot. 2006, 97, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Chiang, G.C.; Barua, D.; Kramer, E.M.; Amasino, R.M.; Donohue, K. Major flowering time gene, flowering locus C, regulates seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2009, 106, 11661–11666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilczek, A.M.; Roe, J.L.; Knapp, M.C.; Cooper, M.D.; Lopez-Gallego, C.; Martin, L.J.; Schmitt, J. Effects of genetic perturbation on seasonal life history plasticity. Science 2009, 323, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Evidence for existence of 3 primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, D.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. Niche construction through germination cueing: Life-history responses to timing of germination in Arabidopsis thaliana. Evolution 2005, 59, 771–785. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evolution 2005, 59, 758–770. [Google Scholar] [CrossRef]
- Wilczek, A.M.; Burghardt, L.T.; Cobb, A.R.; Cooper, M.D.; Welch, S.M.; Schmitt, J. Genetic and physiological bases for phenological responses to current and predicted climates. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2010, 365, 3129–3147. [Google Scholar] [CrossRef] [Green Version]
- Burghardt, L.T.; Metcalf, C.J.E.; Wilczek, A.M.; Schmitt, J.; Donohue, K. Modeling the influence of genetic and environmental variation on the expression of plant life cycles across landscapes. Am. Nat. 2015, 185, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Donohue, K.; de Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Akiyama, R.; Ågren, J. Conflicting selection on the timing of germination in a natural population of Arabidopsis thaliana. J. Evol. Biol. 2014, 27, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Postma, F.M.; Agren, J. Early life stages contribute strongly to local adaptation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 7590–7595. [Google Scholar] [CrossRef] [Green Version]
- Vidigal, D.S.; Marques, A.C.S.S.; Willems, L.A.J.; Buijs, G.; Mendez-Vigo, B.; Hilhorst, H.M.; Bentsink, L.; Pico, F.X.; Alonso-Blanco, C. Altitudinal and climatic associations of seed dormancy and flowering traits evidence adaptation of annual life cycle timing in Arabidopsis thaliana. Plant Cell Environ. 2016, 39, 1737–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, A.S.; Cabin, R.J. Can dormancy affect the evolution of post-germination traits? The case of Lesquerella fendleri. Ecology 1995, 76, 344–356. [Google Scholar] [CrossRef]
- Tyler, B.; Borrill, M.; Chorlton, K. Studies in Festuca. Observations on germination and seedling cold tolerance in diploid Festuca pratensis and tetraploid F. pratensis var. apennina in relation to their altitudinal distribution. J. Appl. Ecol. 1978, 15, 219–226. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution 2005, 59, 740–757. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Mullen, J.L.; Heiliger, A.; McKay, J.K. QTL analysis of root morphology, flowering time, and yield reveals trade-offs in response to drought in Brassica napus. J. Exp. Bot. 2015, 66, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Slade, N.A.; Horton, J.S.; Mooney, H.A. Yearly variation in the phenology of California annuals. Am. Midi. Nat. 1975, 94, 209–214. [Google Scholar] [CrossRef]
- Skulachev, V.P. The programmed death phenomena, aging, and the Samurai law of biology. Exp. Gerontol. 2001, 36, 995–1024. [Google Scholar] [CrossRef]
- Thomas, H. Ageing in the plant and animal kingdoms—The role of cell death. Rev. Clin. Gerontol. 1994, 4, 5–20. [Google Scholar] [CrossRef]
- Noodén, L.D. Whole plant senescence. In Senescence and Aging in Plants; Noodén, L.D., Leopold, A.C., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 392–439. [Google Scholar]
- Noodén, L.D. The phenomena of senescence and aging. In Senescence and Alterung in Plants; Noodén, L.D., Leopold, A.C., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 1–50. [Google Scholar]
- Davies, P.J.; Gan, S. Towards an integrated view of monocarpic plant senescence. Russ. J. Plant Physiol. 2012, 59, 467–478. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Distelfeld, A.; Avni, R.; Fischer, A.M. Senescence, nutrient remobilization, and yield in wheat and barley. J. Exp. Bot. 2014, 65, 3783–3798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H.; Stoddart, J. Leaf senescence. Annu. Rev. Plant. Physiol. 1980, 31, 83–111. [Google Scholar] [CrossRef]
- Thomas, H.; Ougham, H. The stay-green trait. J. Exp. Bot. 2014, 65, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Hollmann, J.; Blattner, F.R.; Radchuk, V.; Andersch, F.; Steuernagel, B.; Schmutzer, T.; Scholz, U.; Krupinska, K.; Weber, H.; et al. A putative role for amino acid permeases in sink-source communication of barley tissues uncovered by RNA-seq. BMC Plant Biol. 2012, 12, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollmann, J.; Gregersen, P.L.; Krupinska, K. Identification of predominant genes involved in regulation and execution of senescenceassociated nitrogen remobilization in flag leaves of field grown barley. J. Exp. Bot. 2014, 65, 3963–3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by finetuning abscisic acid biosynthesis and directly targeting senescenceassociated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [Green Version]
- Bogard, M.; Jourdan, M.; Allard, V.; Martre, P.; Perretant, M.R.; Ravel, C.; Heumez, E.; Orford, S.; Snape, J.; Griffiths, S.; et al. Anthesis date mainly explained correlations between post-anthesis leaf senescence, grain yield, and grain protein concentration in a winter wheat population segregating for flowering time QTLs. J. Exp. Bot. 2011, 62, 3621–3636. [Google Scholar] [CrossRef] [Green Version]
- Egli, D.B. Time and the productivity of agronomic crops and cropping systems. Agron. J. 2011, 103, 743–750. [Google Scholar] [CrossRef]
- Zhao, D.; Derkx, A.P.; Liu, D.C.; Buchner, P.; Hawkesford, M.J. Overexpression of a NAC transcription factor delays leaf senescence and increases grain nitrogen concentration in wheat. Plant Biol. 2015, 17, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Kibite, S.; Evans, L.E. Causes of negative correlations between grain yield and grain protein concentration in common wheat. Euphytica 1984, 33, 801–810. [Google Scholar] [CrossRef]
- Simmonds, N.W. The relation between yield and protein in cereal grain. J. Sci. Food Agric. 1995, 67, 309–315. [Google Scholar] [CrossRef]
- Oury, F.X.; Godin, C. Yield and grain protein concentration in bread wheat: How to use the negative relationship between the two characters to identify favourable genotypes? Euphytica 2007, 157, 45–57. [Google Scholar] [CrossRef]
- Blanco, A.; Mangini, G.; Giancaspro, A.; Giove, S.; Colasuonno, P.; Simeone, R.; Signorile, A.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L.; et al. Relationships between grain protein content and grain yield components through quantitative trait locus analyses in a recombinant inbred line population derived from two elite durum wheat cultivars. Mol. Breed. 2012, 30, 79–92. [Google Scholar] [CrossRef]
- Gregersen, P.L. Senescence and nutrient remobilization in crop plants. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Hawkesford, M.J., Barraclough, P.B., Eds.; Wiley: Oxford, UK, 2011; pp. 83–102. [Google Scholar]
- Munier-Jolain, N.G.; Salon, C. Are the carbon costs of seed production related to the quantitative and qualitative performance? An appraisal for legumes and other crops. Plant Cell Environ. 2005, 28, 1388–1395. [Google Scholar] [CrossRef]
- Borrell, A.K.; Hammer, G.L.; Douglas, A.C.L. Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Sci. 2000, 40, 1026–1037. [Google Scholar] [CrossRef]
- Borrell, A.K.; Hammer, G.L.; Henzell, R.G. Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Sci. 2000, 40, 1037–1048. [Google Scholar] [CrossRef]
- Antonietta, M.; Fanello, D.D.; Acciaresi, H.A.; Guiamet, J.J. Senescence and yield responses to plant density in stay green and earlier-senescing maize hybrids from Argentina. Field Crops Res. 2014, 155, 111–119. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotech. 2003, 1, 3–22. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Jiang, Z.; Zhao, Y.; Peng, J.; Jin, J.; Guo, H.; Luo, J. LSD: A leaf senescence database. Nucleic Acids Res. 2011, 39, D1103–D1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, C.M. Tansley Review No.64: Gene expression during leaf senescence. New Phytol. 1994, 126, 419–448. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V. The molecular biology of leaf senescence. J. Exp. Bot. 1997, 48, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.G. The molecular genetic analysis of leaf senescence. Curr. Opin. Biotechnol. 1997, 8, 200–207. [Google Scholar] [CrossRef]
- Weaver, L.M.; Gan, S.; Quirino, B.F.; Amasino, R.M. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol. Biol. 1998, 37, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant. Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhao, Y.; Liu, X.; Peng, J.; Guo, H.; Luo, J. LSD 2.0: An update of the leaf senescence database. Nucleic Acids Res. 2014, 42, D1200–D1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, Y.; Zou, D.; Zhao, Y.; Wang, H.L.; Zhang, Y.; Xia, X.; Luo, J.; Guo, H.; Zhang, Z. LSD 3.0: A comprehensive resource for the leaf senescence research community. Nucleic Acids Res. 2020, 48, D1069–D1075. [Google Scholar] [CrossRef]
- Li, Z.; Peng, J.; Wen, X.; Guo, H. Gene network analysis and functional studies of senescence-associated genes reveal novel regulators of Arabidopsis leaf senescence. J. Integr. Plant Biol. 2012, 54, 526–539. [Google Scholar] [CrossRef]
- Noodén, L.D.; Guiamét, J.J.; John, I. Senescence mechanisms. Physiol. Plant. 1997, 101, 746–753. [Google Scholar] [CrossRef]
- Quirino, B.F.; Noh, Y.S.; Himelblau, E.; Amasino, R.M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- Yoshida, S. Molecular regulation of leaf senescence. Curr. Opin. Plant Biol. 2003, 6, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Patterson, S.E. Last exit: Senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell. 1997, 9, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albacete, A.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and metabolic regulation of source–sink relations under salinity and drought: From plant survival to crop yield stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef]
- Ingram, J.; Bartels, D. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 377–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanjulu, S.; Bartels, D. Drought-and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miryeganeh, M. Senescence: The Compromised Time of Death That Plants May Call on Themselves. Genes 2021, 12, 143. https://doi.org/10.3390/genes12020143
Miryeganeh M. Senescence: The Compromised Time of Death That Plants May Call on Themselves. Genes. 2021; 12(2):143. https://doi.org/10.3390/genes12020143
Chicago/Turabian StyleMiryeganeh, Matin. 2021. "Senescence: The Compromised Time of Death That Plants May Call on Themselves" Genes 12, no. 2: 143. https://doi.org/10.3390/genes12020143
APA StyleMiryeganeh, M. (2021). Senescence: The Compromised Time of Death That Plants May Call on Themselves. Genes, 12(2), 143. https://doi.org/10.3390/genes12020143





