Intracytoplasmic Sperm Injection in Cattle
Abstract
:1. Introduction
2. Applications of Bovine ICSI
3. Potential Reasons for Failure of ICSI in Cattle
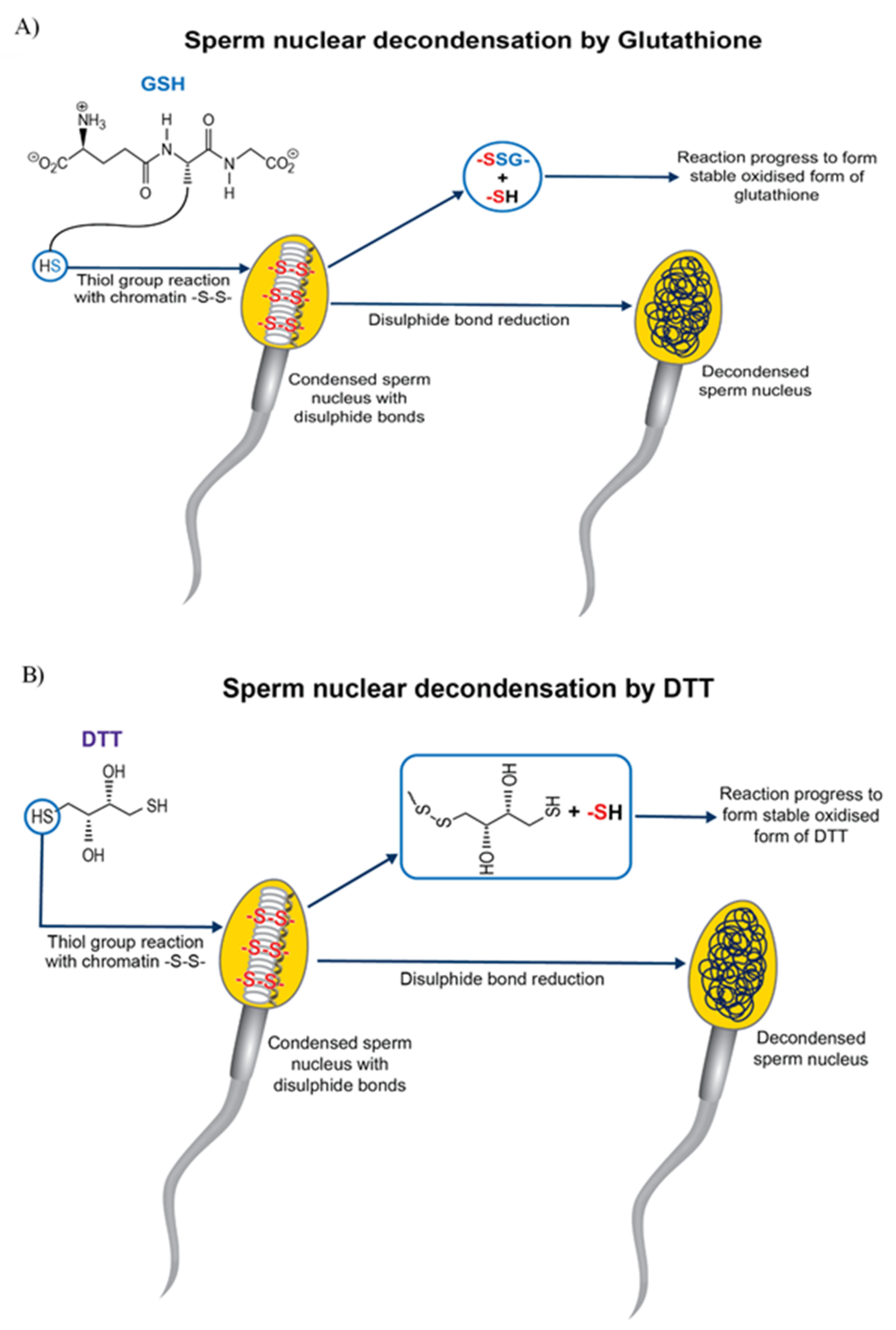
4. Pre-Treatment of Sperm for Improving the Success of Bovine ICSI
| Pre-Treatment | Effect on Sperm | Reference |
|---|---|---|
| Mechanical pre-treatment | ||
| Tail scoring | Removal of sperm membrane | [80] |
| Piezo pulses | [81] | |
| Chemical pre-treatment | ||
| DTT | Reduction of disulphide bond (sperm head decondensation) and involved in microtuble organisation | [85,86,87,88,89] |
| NaOH + DTT | Sperm decondensation and DNA fragmentation | [106] |
| DTBA | Disulphide bond reduction | [74] |
| LL + TX-100 | Membrane destabilization | [104] |
| LL + TX + glutathione | Membrane destabilization and disulphide bond reduction | [107] |
| Pre-treatment with capacitating agents | ||
| MβCD | Capacitation-associated changes | [93] |
| Heparin | Capacitation-associated changes and Sperm decondensation | [80,94] |
| Heparin + Glutathione | Capacitation-associated changes and disulphide bond reduction Sperm decondensation. Enhanced mitochondrial function | [95,96] |
| Heparin + Caffeine | Capacitation associated changes and acrosome reaction, Sperm decondensation | [80,108] |
| Glutathione | Disulphide bond reduction (sperm head decondensation). Enhanced Mitochondrial function | [91,92] |
| Cumulus oocyte complexes (COCs) | Acrosome reaction of sperm | [105] |
5. Artificial Activation of Oocyte for Improving the Success of Bovine ICSI
6. Sperm Oocyte Activation Factors (SOAFs)
7. PLC ζ as a Sperm-Specific Oocyte Activating Factor
8. A Hypothetical Model for PLC ζ Activation during Sperm Capacitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Transforming Food and Agriculture to Acheive the SDGs; FAO: Rome, Italy, 2018; p. 71. Available online: http://www.fao.org/fao-stories/article/en/c/1184363/ (accessed on 1 September 2020).
- Taylor, J.F.; Schnabel, R.D.; Sutovsky, P. Genomics of bull fertility. Animal 2018, 12, s172–s183. [Google Scholar] [CrossRef] [Green Version]
- Gasparrini, B. In vitro embryo production in buffalo species: State of the art. Theriogenology 2002, 57, 237–256. [Google Scholar] [CrossRef]
- Kumar, V. Genetic and breeding aspects of lactation. In Trends in Advance Veterinary Genetics, 1st ed.; Abubakar, M., Ed.; Intech Open: London, UK, 2017; p. 13. [Google Scholar]
- Chesnais, J.P. How is the AI industry using genomic tools in practice? Interbull Bull. 2010, 41, 59–62. [Google Scholar]
- Kasinathan, P.; Wei, H.; Xiang, T.; Molina, J.A.; Metzger, J.; Broek, D.; Kasinathan, S.; Faber, D.C.; Allan, M.F. Acceleration of genetic gain in cattle by reduction of generation interval. Sci. Rep. 2015, 5, 8674. [Google Scholar] [CrossRef] [Green Version]
- Vanraden, P.M. Selection on Net Merit to Improve Lifetime Profit. J. Dairy Sci. 2004, 87, 3125–3131. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, I.; Misztal, I.; Johnson, D.L.; Legarra, A.; Tsuruta, S.; Lawlor, T.J. Hot topic: A unified approach to utilize phenotypic, full pedigree, and genomic information for genetic evaluation of Holstein final score. J. Dairy Sci. 2010, 93, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.F.; Geisert, R.D.; Parrish, J.J. Reproduction in domestic ruminants during the past 50 yr: Discovery to application. J. Anim. Sci. 2018, 96, 2952–2970. [Google Scholar] [CrossRef]
- Rawlings, N.; Evans, A.C.O.; Chandolia, R.K.; Bagu, E.T. Sexual maturation in the bull. Reprod. Domest. Anim. 2008, 43, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kinoshita, A.; Nakanishi, Y.; Ogawa, K. Blastocyst formation following intracytoplasmic injection of in-vitro derived spermatids into bovine oocytes. Hum. Reprod. 1996, 11, 824–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Baldassarre, H.; Pierson, J.; Cote, F.; Rao, K.M.; Karatzas, C.N. The in vitro and in vivo development of goat embryos produced by intracytoplasmic sperm injection using tail-cut spermatozoa. Zygote 2003, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Schultz, R.M. ICSI in the mouse. Method Enzymol. 2010, 476, 251–262. [Google Scholar]
- Uehara, T.; Yanagimachi, R. Microsurgical injection of spermatozoa into hamster eggs with subsequent transformation of sperm nuclei into male pronuclei. Biol. Reprod. 1976, 15, 467–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanzendorf, S.E.; Maloney, M.K.; Veeck, L.L.; Slusser, J.; Hodgen, G.D.; Rosenwaks, Z. A preclinical evaluation of pronuclear formation by microinjection of human spermatozoa into human oocytes. Fertil. Steril. 1988, 49, 835–842. [Google Scholar] [CrossRef]
- De Abreu Santos, D.J.; Cole, J.B.; Liu, G.E.; VanRaden, P.M.; Ma, L. Gamevar.f90: A software package for calculating individual gametic diversity. BMC Bioinf. 2020, 21, 100. [Google Scholar] [CrossRef] [PubMed]
- Lacham-kaplan, O.; Trounson, A. Intracytoplasmic sperm injection in mice: Increased fertilization and development to term after induction of the acrosome reaction. Hum. Reprod. 1995, 10, 2642–2649. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ng, S.C.; Liow, S.L.; Ali, J.; Bongso, A.; Ratnam, S.S. Intracytoplasmic sperm injection of mouse oocytes with 5 mM Ca 2+ at different intervals. Hum. Reprod. 1995, 10, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Colleoni, S.; Barbacini, S.; Necchi, D.; Ecar, D.; Duchi, R.; Lazzari, G. Application of ovum pick-up, intracytoplasmic sperm injection and embryo culture in equine practice. Reproduction 2007, 53, 554–559. [Google Scholar]
- Kolbe, T.; Holtz, W. Birth of a piglet derived from an oocyte fertilized by intracytoplasmic sperm injection (ICSI). Anim. Reprod. Sci. 2000, 64, 97–101. [Google Scholar] [CrossRef]
- Kurome, M.; Ueda, H.; Tomii, R.; Naruse, K.; Nagashima, H. Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgen. Res. 2006, 15, 229–240. [Google Scholar] [CrossRef]
- Graham, J.K.; Carnevale, E.M. Validation of a heterologous fertilization assay and comparison of fertilization rates of equine oocytes using in vitro fertilization, perivitelline, and intracytoplasmic sperm injections. Theriogenology 2014, 82, 274–282. [Google Scholar]
- Zhang, J.J.; Muzs, L.Z.; Boyle, M.S. In vitro fertilization of horse follicular oocytes matured in vitro. Mol. Reprod. Dev. 1990, 26, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kinoshita, A.; Takuma, Y.; Ogawa, K. Fertilisation of bovine oocytes by the injection of immobilised, killed spermatozoa. Vet. Rec. 1990, 127, 517–520. [Google Scholar] [PubMed]
- Wei, H.; Fukui, Y. Births of calves derived from embryos produced by intracytoplasmic sperm injection without exogenous oocyte activation. Zygote 2002, 10, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Yanagita, K. Normality of calves obtained by intracytoplasmic sperm injection. Hum. Reprod. 1995, 10, 1554–1556. [Google Scholar] [CrossRef] [PubMed]
- Keskintepe, L.; Brackett, B.G. Cryopreservation of bovine blastocyst obtained by intracytoplasmic sperm injection. Theriogenology 2000, 53, 1041–1052. [Google Scholar] [PubMed]
- Abdalla, H.; Shimoda, M.; Hara, H.; Morita, H.; Kuwayama, M. Vitrification of ICSI- and IVF-derived bovine blastocysts by minimum volume cooling procedure: Effect of developmental stage and age. Theriogenology 2010, 74, 1028–1035. [Google Scholar] [CrossRef] [Green Version]
- Ock, S.; Kwack, D.; Lee, S.; Cho, S. In vitro development of bovine oocytes reconstructed with round spermatids. Theriogenology 2006, 65, 1242–1253. [Google Scholar] [CrossRef]
- Lee, K.; Niwa, K. Fertilization and development in vitro of bovine oocytes following intracytoplasmic injection of heat-dried sperm heads. Biol. Reprod. 2006, 74, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Keskintepe, L.; Pacholczyk, G.; Machnicka, A.; Norris, K.; Curuk, M.A.; Khan, I.; Brackett, B. Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biol. Reprod. 2002, 67, 409–415. [Google Scholar]
- Rho, G.; Lee, S.; Kim, Y.; Yeo, H.; Ock, S.; Balasubramanian, S.; Choe, S.Y. Intracytoplasmic sperm injection of frozen-thawed bovine oocytes and subsequent embryo development. Mol. Reprod. Dev. 2004, 68, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Mavrides, A.; Morroll, D. Bypassing the effect of zona pellucida changes on embryo formation following cryopreservation of bovine oocytes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Ohlweiler, L.U.; Brum, D.S.; Leivas, F.G.; Moyses, A.B.; Ramos, R.S.; Klein, N.; Mezzalira, J.C.; Mezzalira, A. Intracytoplasmic sperm injection improves in vitro embryo production from poor quality bovine oocytes. Theriogenology 2013, 79, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.D.; Fricke, P.M.; Leibfried-rutledge, M.L. In vitro production of bovine embryos using sex-sorted sperm. Theriogenology 2006, 65, 1007–1015. [Google Scholar] [PubMed]
- Wilson, R.D.; Weigel, K.A.; Fricke, P.M.; Rutledge, J.J.; Matthews, D.L.; Schutzkus, V.R. In vitro production of holstein embryos using sex-sorted sperm and oocytes from selected cull cows. J. Dairy Sci. 2005, 88, 776–782. [Google Scholar] [CrossRef]
- Lu, K.H.; Cran, D.G.; Seidel, G.E. In vitro fertilization with flow-cytometrically-sorted bovine sperm. Theriogenology 1999, 52, 1393–1405. [Google Scholar] [CrossRef]
- Jo, H.; Bang, J.; Kim, S.; Choi, B.; Jin, J.; Kim, H.; Jung, I.S.; Suh, T.K.; Ghanem, N.; Wang, Z.; et al. Production of female bovine embryos with sex-sorted sperm using intracytoplasmic sperm injection: Efficiency and in vitro developmental competence. Theriogenology 2014, 81, 675–682.e1. [Google Scholar]
- Hamano, K.; Li, X.; Qian, X.; Funauchi, K.; Furudate, M.; Minato, Y. Gender preselection in cattle with intracytoplasmically injected, flow cytometrically sorted sperm heads. Biol. Reprod. 1999, 60, 1194–1197. [Google Scholar] [CrossRef]
- Bevacqua, R.J.; Pereyra-Bonnet, F.; Fernandez-Martin, R.; Salamone, D.F. High rates of bovine blastocyst development after ICSI-mediated gene transfer assisted by chemical activation. Theriogenology 2010, 74, 922–931. [Google Scholar]
- Sánchez-Villalba, E.; Elena, M.; Loren, P.; Fuentes, F.; Pereyra-Bonnet, F.; Salamone, D.; Felmer, R. Improved expression of green fluorescent protein in cattle embryos produced by ICSI-mediated gene transfer with spermatozoa treated with streptolysin-O. Anim. Reprod. Sci. 2018, 196, 130–137. [Google Scholar] [CrossRef]
- Pereyra-Bonnet, F.; Fernandez-Martin, R.; Olivera, R.A.; Jarazo, J.A.; Vichera, G.A. A unique method to produce transgenic embryos in ovine, porcine, feline, bovine and equine species. Reprod. Fertil. Dev. 2008, 20, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Sekhavati, M.H.; Hosseini, S.M.; Tahmoorespur, M.; Ghaedi, K.; Jafarpour, F.; Hajian, M.; Dormiani, K.; Nasr-Esfahani, M.H. PhiC31-based site-specific transgenesis system for production of transgenic bovine embryos by somatic cell nuclear transfer and intracytoplasmic sperm injection. Cell J. 2018, 21, 98–107. [Google Scholar]
- Terada, Y.; Hasegawa, H.; Takahashi, A.; Ugajin, T.; Yaegashi, N.; Okamura, K. Successful pregnancy after oocyte activation by a calcium ionophore for a patient with recurrent intracytoplasmic sperm injection failure, with an assessment of oocyte activation and sperm centrosomal function using bovine eggs. Fertil. Steril. 2009, 91, e11–e935. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto-Kakoi, T.; Terada, Y.; Tachibana, M.; Murakami, T.; Yaegashi, N.; Okamura, K. Assessing centrosomal function of infertile males assessing centrosomal function of infertile males using heterologous ICSI. Syst. Biol. Reprod. Med. 2008, 54, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, S.; Terada, Y.; Horiuchi, T.; Emuta, C.; Murakami, T.; Yaegashi, N.; Okamura, K. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a piezo-driven pipette: A novel assay for human sperm centrosomal function. Biol. Reprod. 2001, 65, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Terada, Y.; Horiuchi, T.; Emuta, C.; Murakami, T.; Yaegashi, N.; Okamura, K. Analysis of the human sperm centrosomal function and the oocyte activation ability in a case of globozoospermia, by ICSI into bovine oocytes. Hum. Reprod. 2002, 17, 2930–2934. [Google Scholar] [PubMed]
- Rawe, V.Y.; Terada, Y.; Nakamura, S.; Chillik, C.F.; Olmedo, S.B.; Chemes, H.E. A pathology of the sperm centriole responsible for defective sperm aster formation, syngamy and cleavage. Hum. Reprod. 2002, 17, 2344–2349. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Fukui, Y. Fertilisability of ovine, bovine or minke whale (Balaenoptera acutorostrata) spermatozoa intracytoplasmically injected into bovine oocytes. Zygote 2000, 8, 267–274. [Google Scholar] [CrossRef]
- Kato, Y.; Nagao, Y. Changes in sperm motility and capacitation induce chromosomal aberration of the bovine embryo following intracytoplasmic sperm injection. PLoS ONE 2015, 10, e0129285. [Google Scholar] [CrossRef]
- Terada, Y.; Nakamura, S.I.; Hewitson, L.; Simerly, C.; Horiuchi, T.; Murakami, T.; Okamura, K.; Schatten, G. Human sperm aster formation after intracytoplasmic sperm injection with rabbit and bovine eggs. Fertil. Steril. 2002, 77, 1283–1284. [Google Scholar] [CrossRef]
- Salamone, D.F.; Canel, N.G.; Rodríguez, M.B. Intracytoplasmic sperm injection in domestic and wild mammals. Reproduction 2017, 154, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Águila, L.; Felmer, R.; Arias, M.E.; Navarrete, F.; Martin-Hidalgo, D.; Lee, H.C.; Visconti, P.; Fissore, R. Defective sperm head decondensation undermines the success of ICSI in the bovine. Reproduction 2017, 154, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Abdalla, H.; Morita, H.; Kuwayama, M. Procedure for Bovine ICSI, not sperm freeze-drying, impairs the function of the microtubule-organizing center. J. Reprod. Dev. 2011, 57, 10–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcuit, C.; Maserati, M.; Takahashi, Y.; Page, R.; Fissore, R.A. Intracytoplasmic sperm injection in the bovine induces abnormal [Ca 2+] i responses and oocyte activation. Reprod. Fertil. Dev. 2006, 18, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morozumi, K.; Yanagimachi, R. Incorporation of the acrosome into the oocyte during intracytoplasmic sperm injection could be potentially hazardous to embryo development. Proc. Natl. Acad. Sci. USA 2005, 102, 14209–14214. [Google Scholar] [CrossRef] [Green Version]
- Garcı-Rosello, E.; Gracia-Mengual, E.; Coy, P.; Alfonso, J.; Silvestre, M.A. Intracytoplasmic sperm injection in livestock species: An update. Reprod. Domest. Anim. 2009, 44, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M. Novel signalling mechanism and clinical applications of sperm-specific PLC ζ. Biochem. Soc. Trans. 2015, 43, 371–376. [Google Scholar] [CrossRef]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Blayney, L.M.; Swann, K.; Lai, F.A. PLC ζ: A sperm-specific trigger of Ca 2+ oscillations in eggs and embryo development. Development 2002, 129, 3533–3544. [Google Scholar]
- Machaty, Z. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res. 2016, 363, 169–183. [Google Scholar]
- Berridge, J.M.; Galione, A. Cytosolic calcium oscillators. FASEB J. 1988, 2, 3074–3082. [Google Scholar] [CrossRef]
- Fujimoto, S.; Yoshida, N.; Fukui, T.; Amanai, M.; Isobe, T.; Itagaki, C.; Izumi, T.; Perry, A.C. Mammalian phospholipase Czeta induces oocyte activation from the sperm perinuclear matrix. Dev. Biol. 2004, 274, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, L.; Dominko, T.; Takahashi, D.; Martinovich, C.; Ramalho-Santos, J.; Sutovsky, P.; Fanton, J.; Jacob, D.; Monteith, D.; Neuringer, M.; et al. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat. Med. 1999, 5, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Ramalho-Santos, J.; Sutovsky, P.; Simerly, C.; Oko, R.; Wessel, G.M.; Hewitson, L.; Schatten, G. ICSI choreography: Fate of sperm structures after monospermic rhesus ICSI and first cell cycle implications. Hum. Reprod. 2000, 15, 2610–2620. [Google Scholar] [CrossRef] [Green Version]
- Sutovsky, P.; Manandhar, G.; Wu, A.; Oko, R. Interactions of sperm perinuclear theca with the oocyte: Implications for oocyte activation, anti-polyspermy defense, and assisted reproduction. Microsc. Res. Tech. 2003, 61, 362–378. [Google Scholar] [CrossRef] [PubMed]
- Sutovsky, P.; Oko, R.; Hewitson, L.; Schatten, G. The removal of the sperm perinuclear theca and its association with the bovine oocyte surface during fertilization. Dev. Biol. 1997, 188, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Perreault, S.D.; Barbee, R.R.; Elstein, K.H.; Zucker, R.M.; Keefer, C.L. Interspecies differences in the stability of mammalian sperm nuclei assessed in vivo by sperm microinjection and in vitro by flow cytometry. Biol. Reprod. 1988, 39, 157–167. [Google Scholar] [CrossRef]
- Yanagimachi, R. Mammalian Fertilisation. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, J., Eds.; Raven Press: New York, NY, USA, 1994; Volume 1, pp. 189–299. [Google Scholar]
- Jager, S.; Wijchman, J.; Kremer, J. Studies on the decondensation of human, mouse, and bull sperm nuclei by heparin and other polyanions. J. Exp. Zool. 1990, 256, 315–322. [Google Scholar]
- Maier, W.; Nussbaum, G.; Domenjoud, L.; Klemm, U.; Engel, W. The lack of protamine 2 (P2) in boar and bull spermatozoa is due to mutations within the P2 gene. Nucl. Acids Res. 1990, 18, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Brewer, L.; Corzett, M.; Lau, E.Y.; Balhorn, R. Dynamics of protamine 1 binding to single DNA molecules. J. Biol. Chem. 2003, 278, 42403–42408. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, J.M.; Rau, D.C.; Derouchey, J.E. Role of Disulfide Bonds on DNA Packaging Forces in Bull Sperm Chromatin. Biophys. J. 2017, 113, 1925–1933. [Google Scholar] [CrossRef] [Green Version]
- Tayita, S.; Tamas, S.; Satoko, M.; Takashi, N.; Parnpai, R.; Geshi, M. Pretreatment of bovine sperm with dithiobutylamine (DTBA) significantly improves embryo development after ICSI. J. Reprod. Dev. 2016, 62, 577–585. [Google Scholar]
- Sanchez, M.C.; Sedo, C.A.; Julianelli, V.L.; Romanato, M.; Calvo, L.; Calvo, J.C.; Fontana, V.A. Dermatan sulfate synergizes with heparin in murine sperm chromatin decondensation. Syst. Biol. Reprod. Med. 2013, 59, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Hochi, S. Microtubule assembly crucial to bovine embryonic development in assisted reproductive technologies. Anim. Sci. J. 2016, 87, 1076–1083. [Google Scholar] [PubMed] [Green Version]
- Ushijima, H.; Nakane, T. Present status and prospects for bovine vitro-matured oocytes and frozen semen. J. Mamm. Ova Res. 2006, 23, 107–113. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Moulavi, F.; TanhaieVash, N.; Shams-Esfandabadi, N.; Nasr-Esfahani, M.H.; Shirazi, A. Evidence of oocyte polarity in bovine; implications for intracytoplasmic sperm injection and somatic cell nuclear transfer. Cell J. 2017, 19, 482–491. [Google Scholar]
- Ashibe, S.; Miyamoto, R.; Kato, Y.; Nagao, Y. Detrimental effects of oxidative stress in bovine oocytes during intracytoplasmic sperm injection (ICSI). Theriogenology 2019, 133, 71–78. [Google Scholar] [CrossRef]
- Wei, H.; Fukui, Y. Effects of bull, sperm type and sperm pretreatment on male pronuclear formation after intracytoplasmic sperm injaection in cattle. Reprod. Fertil. Dev. 1999, 11, 59–65. [Google Scholar] [CrossRef]
- Katayose, H.; Yanagida, K.; Shinoki, T.; Kawahara, T.; Horiuchis, T.; Satol, A. Efficient injection of bull spermatozoa into oocytes using a piezo-driven pipette. Theriogenology 1999, 52, 1215–1224. [Google Scholar]
- Yanagimachi, R. Intracytoplasmic sperm injection experiments using the mouse as a model. Hum. Reprod. 1998, 13, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Yanagimachi, R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: Its biology and applications in humans and animals. Reprod. Biomed. Online 2005, 10, 247–288. [Google Scholar] [CrossRef]
- Kasai, T.; Hoshi, K.; Yanagimachi, R. Effect of sperm immobilisation and demembranation on the oocyte. Zygote 1999, 7, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Suttner, R.; Zakhartchenko, V.; Stojkovic, P.; Muller, S.; Alberio, R.; Medjugorac, I.; Brem, G.; Wolf, E.; Stojkovic, M. Intracytoplasmic sperm injection in bovine: Effects of oocyte activation, sperm preteatment and injection technique. Theriogenology 2000, 54, 935–946. [Google Scholar] [CrossRef]
- Oikawa, T.; Itahashi, T.; Numabe, T. Improved embryo development in Japanese black cattle by in vitro fertilisation using ovum pick-up plus intracytoplasmic sperm injection with dithiothreitol. J. Reprod. Dev. 2016, 62, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, C.; Vassiliev, I.; Lagutina, I.; Galli, A.; Lazzari, G. Bovine embryo development following ICSI: Effect of activation, sperm capacitation and pre-treatment with dithiothreitol. Theriogenology 2003, 60, 1467–1480. [Google Scholar] [CrossRef]
- Rho, G.; Kawarsky, S.; Johnson, W.H.; Kochhar, K.; Betteridge, K.J. Sperm and oocyte treatments to improve the formation of male and female pronuclei and subsequent development following intracytoplasmic sperm injection into bovine oocytes. Biol. Reprod. 1998, 59, 918–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.; Keefer, C.; Downey, B. Activation of bovine oocytes following intracytoplasmic sperm injection. Theriogenology 1999, 53, 1273–1284. [Google Scholar]
- Perreault, S.D.; Wolff, R.A.; Zirkin, B.R. The role of disulfide bond reduction during mammalian sperm nuclear decondensation in vivo. Dev. Biol. 1984, 101, 160–167. [Google Scholar] [CrossRef]
- Oikawa, T.; Itahashi, T.; Yajima, R.; Numabe, T. Glutathione treatment of Japanese Black bull sperm prior to intracytoplasmic sperm injection promotes embryo development. J. Reprod. Dev. 2018, 64, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Chang, H.C.; Wu, H.Y.; Liu, S.S.; Wang, C.H.; Chu, C.Y.; Shen, P.C. Effects of sperm pretreatment and embryo activation methods on the development of bovine embryos produced by intracytoplasmic sperm injection. Reprod. Biol. 2015, 15, 154–162. [Google Scholar] [CrossRef]
- Águila, L.; Zambrano, F.; Arias, M.E.; Felmer, R. Sperm capacitation pretreatment positively impacts bovine intracytoplasmic sperm injection. Mol. Reprod. Dev. 2017, 84, 649–659. [Google Scholar]
- Li, G.; Seidel, G.E.; Squires, E.L. Improved cleavage of bovine ICSI ova cultured in heparin-containing medium. Theriogenology 2004, 61, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Sekhavati, M.H.; Shadanloo, F.; Hosseini, M.S.; Tahmoorespur, M.; Nasiri, M.R.; Hajian, M.; Nasr-Esfahani, M.H. Improved bovine ICSI outcomes by sperm selected after combined heparin-glutathione treatment. Cell Reprogram. 2012, 14, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canel, N.G.; Bevacqua, R.J.; Hiriart, M.I.; Rabelo, N.C.; de Almeida Camargo, L.S.; Romanato, M.; de Calvo, L.P.; Salamone, D.F. Sperm pretreatment with heparin and L-glutathione, sex-sorting, and double cryopreservation to improve intracytoplasmic sperm injection in bovine. Theriogenology 2017, 93, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vázquez, M.L.; Flores-Alonso, J.C.; Merchant-Larios, H.; Reyes, R. Presence and release of bovine sperm histone H1 during chromatin decondensation by heparin-glutathione. Syst. Biol. Reprod. Med. 2008, 54, 221–230. [Google Scholar]
- Lane, M.; Thérien, I.; Moreau, R.; Manjunath, P. Heparin and high-density lipoprotein mediate bovine sperm capacitation by different mechanisms. Biol. Reprod. 1999, 60, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handrow, R.R.; Lenz, R.W. Structural comparison among glycosaminoglycans to promote an acrosome reaction in bovine spermatozoa. Biochem. Biophys. Res. Commun. 1982, 107, 1326–1332. [Google Scholar] [CrossRef]
- Delgado, N.M.; Reyes, R.; Huacuja, L.; Merchant, H.; Rosado, A. Heparin binding sites in the human spermatozoa membrane. Arch. Androl. 1982, 8, 87–95. [Google Scholar]
- Romanato, M.; Cameo, M.S.; Bertolesi, G.; Baldini, C.; Calvo, J.C.; Calvo, L. Heparan sulphate: A putative decondensing agent for human spermatozoa in vivo. Hum. Reprod. 2003, 18, 1868–1873. [Google Scholar]
- Romanato, M.; Regueira, E.; Cameo, M.S.; Baldini, C.; Calvo, L.; Calvo, J.C. Further evidence on the role of heparan sulfate as protamine acceptor during the decondensation of human spermatozoa. Hum. Reprod. 2005, 20, 2784–2789. [Google Scholar] [CrossRef] [Green Version]
- Romanato, M.; Julianelli, V.; Zappi, M.; Calvo, L.; Calvo, J.C. The presence of heparan sulfate in the mammalian oocyte provides a clue to human sperm nuclear decondensation in vivo. Hum. Reprod. 2008, 23, 1145–1150. [Google Scholar] [CrossRef]
- Zambrano, F.; Aguila, L.; Arias, M.E.; Sánchez, R.; Felmer, R. Improved preimplantation development of bovine ICSI embryos generated with spermatozoa pretreated with membrane-destabilizing agents lysolecithin and Triton X-100. Theriogenology 2016, 86, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Canel, N.G.; Suvá, M.; Bevacqua, R.J.; Arias, M.E.; Felmer, R.; Salamone, D.F. Improved embryo development using high cysteamine concentration during IVM and sperm co-culture with COCs previous to ICSI in bovine. Theriogenology 2018, 117, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aris, M.E.; Sanchez, R.; Risopatron, J.; Perez, L.; Felmer, R. Effect of sperm pretreatment with sodium hydroxide and dithiothreitol on the efficiency of bovine intracytoplasmic sperm injection. Reprod. Fertil. Dev. 2014, 26, 847–854. [Google Scholar] [CrossRef]
- Zambrano, F.; Aguila, L.; Arias, M.E.; Sanchez, R.; Felmer, R. Effect of sperm pretreatment with glutathione and membrane destabilizing agents lysolecithin and Triton X-100, on the efficiency of bovine intracytoplasmic sperm injection. Reprod. Domest. Anim. 2017, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Tajik, P.; Niwa, K. Effects of caffine and/or heparin in a chemically defined medium with or without glucose on in vitro penetration of bovine oocytes and their subsequent development. Theriogenology 1998, 49, 771–777. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, E.; Yoon, J.; Yoon, B.K.; Lee, J.H.; Choi, D. Effects of electric stimulation on bovine oocyte activation and embryo development in intracytoplasmic sperm injection procedure. J. Assist. Reprod. Genet. 2000, 17, 310–314. [Google Scholar] [CrossRef]
- Fujinami, N.; Hosoi, Y.; Kato, H.; Matsumoto, K.; Saeki, K.; Iritani, A. Activation with ethanol improves embryo development of ICSI-derived oocytes by regulation of kinetics of mpf activity. J. Reprod. Dev. 2004, 50, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Vichera, G.; Alfonso, J.; Duque, C.C.; Silvestre, M.A.; Pereyra-Bonnet, F.; Fernández-Martín, R.; Salamone, D. Chemical activation with a combination of ionomycin and dehydroleucodine for production of parthenogenetic, ICSI and cloned bovine embryos. Reprod. Domest. Anim. 2010, 45, e306–e312. [Google Scholar] [CrossRef]
- Ikumi, S.; Asada, M.; Sawai, K.; Fukui, Y. Effect of activation methods for bovine oocytes after intracytoplasmic injection. J. Reprod. Dev. 2003, 49, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Presicce, G.A.; Moraghan, L.; Jiang, S.; Foote, R.H. Synergistic effect of ethanol and cyclohexidine on activation of freshly matured bovine oocytes. Theriogenology 1994, 41, 395–403. [Google Scholar] [CrossRef]
- Abdalla, H.; Shimoda, M.; Hirabayashi, M.; Hochi, S. A combined treatment of ionomycin with ethanol improves blastocyst development of bovine oocytes harvested from stored ovaries and microinjected with spermatozoa. Theriogenology 2009, 72, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiuchi, T.; Emuta, C.; Yamauchi, Y.; Oikawa, T. Birth of normal calves after intracytoplasmic sperm injection of bovine oocytes: A methodological approach. Theriogenology 2002, 57, 1013–1024. [Google Scholar] [CrossRef]
- Horiuchi, T. Application study of intracytoplasmic sperm injection for golden hamster and cattle production. J. Reprod. Dev. 2006, 52, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, T.; Takada, N.; Kikuchi, T.; Numabe, T. Evaluation of activation treatments for blastocyst production and birth of viable calves following bovine intracytoplasmic sperm injection. Anim. Reprod. Sci. 2005, 86, 187–194. [Google Scholar] [CrossRef]
- Cuthbertson, K.S.; Whittingham, D.G.; Cobbold, P.H. Free calcium increases in exponential phases during mouse oocyte activation. Nature 1981, 294, 754–757. [Google Scholar] [CrossRef]
- Masui, Y. From oocyte maturation to the in vitro cell cycle: The history of discoveries of maturation-promoting factor (MPF) and cytostatic factor (CSF). Differentiation 2001, 69, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Dunphy, W.G.; Brizuela, L.; Beach, D.; Newport, J. The xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell 1988, 54, 423–431. [Google Scholar] [CrossRef]
- Labbe, J.C.; Picard, A.; Peaucellier, G. Purification of MPF from starfish: Identification as the H1 histone kinase p34 cdc2 and a possible mechanism for its periodic activation. Cell 1989, 57, 253–263. [Google Scholar] [CrossRef]
- Clarke, P.R.; Karsenti, E. Regulation of p34 cdc2 protein kinase: New insights into protein phosphorylation and the cell cycle. J. Cell Sci. 1991, 100, 409–414. [Google Scholar]
- Sagata, N.; Oskarsson, M.; Copeland, T.; Brumbaugh, J.; Vande Woude, G.F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature 1986, 335, 519–525. [Google Scholar] [CrossRef]
- Watanabe, N.; Vande Woude, G.F.; Ikawa, Y.; Sagata, N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature 1989, 342, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ock, S.A.; Bhak, J.S.; Balasubramanian, S.; Lee, H.J.; Choe, S.Y.; Rho, G.J. Different activation treatments for successful development of bovine oocytes following intracytoplasmic sperm injection. Zygote 2003, 11, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Rho, G.J.; Wu, B.; Kawarsky, S.; Leibo, S.P.; Betteridge, K.J. Activation regimens to prepare bovine oocytes for intracytoplasmic sperm injection. Mol. Reprod. Dev. 1998, 50, 485–492. [Google Scholar] [CrossRef]
- Devito, L.G.; Fernandes, C.B.; Blanco, I.D.; Tsuribe, P.M.; Landim-Alvarenga, F.C. Use of a piezo drill for intracytoplasmic sperm injection into cattle oocytes activated with ionomycin associated with roscovitine. Reprod. Domest. Anim. 2010, 45, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.B.; Devito, L.G.; Martins, L.R.; Blanco, I.D.; de Lima Neto, J.F.; Tsuribe, P.M.; Gonçalves, C.G.; da Cruz Landim-Alvarenga, F. Artificial activation of bovine and equine oocytes with cycloheximide, roscovitine, strontium, or 6-dimethylaminopurine in low or high calcium concentrations. Zygote 2013, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Szöllösi, M.S.; Kubiak, J.Z.; Debey, P.; de Pennart, H.; Szöllösi, D.; Maro, B. Inhibition of protein kinases by 6-dimethylaminopurine accelerates the transition to interphase in activated mouse oocytes. J. Cell Sci. 1993, 104, 861–872. [Google Scholar] [PubMed]
- Coy, P.; Romar, R.; Payton, R.R.; McCann, L.; Saxton, A.M.; Edwards, J.L. Maintenance of meiotic arrest in bovine oocytes using the S-enantiomer of roscovitine: Effects on maturation, fertilization and subsequent embryo development in vitro. Reproduction 2005, 129, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.E.; Sánchez, R.; Felmer, R. Effect of anisomycin, a protein synthesis inhibitor, on the in vitro developmental potential, ploidy and embryo quality of bovine ICSI embryos. Zygote 2016, 24, 724–732. [Google Scholar] [CrossRef]
- Felmer, R.; Arias, M.E. Activation treatment of recipient oocytes affects the subsequent development and ploidy of bovine parthenogenetic and somatic cell nuclear transfer (SCNT) embryos. Mol. Reprod. Dev. 2015, 82, 441–449. [Google Scholar] [CrossRef]
- Joiakim, A.; Mathieu, P.A.; Elliott, A.A.; Reiners, J.J., Jr. Superinduction of CYP1A1 in MCF10A cultures by cycloheximide, anisomycin, and puromycin: A process independent of effects on protein translation and unrelated to suppression of aryl hydrocarbon receptor proteolysis by the proteasome. Mol. Pharmacol. 2004, 66, 936–947. [Google Scholar] [CrossRef]
- Ross, P.J.; Beyhan, Z.; Iager, A.E.; Yoon, S.Y.; Malcuit, C.; Schellander, K.; Fissore, R.A.; Cibelli, J.B. Parthenogenetic activation of bovine oocytes using bovine and murine phospholipase C zeta. BMC Dev. Biol. 2008, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Okitsu, O.; Yamano, S.; Aono, T. Activation of bovine oocytes matured in vitro by injection of bovine and human spermatozoa or their cytosolic fractions. Zygote 2001, 9, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Parrington, J.; Swann, K.; Shevchenko, V.I.; Sesay, A.K.; Lai, F.A. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature 1996, 379, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Swann, K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 1990, 110, 1295–1302. [Google Scholar] [PubMed]
- Swann, K.; Igusa, Y.; Miyazaki, S. Evidence for an inhibitory effect of protein kinase C on G-protein-mediated repetitive calcium transients in hamster eggs. EMBO J. 1989, 8, 3711–3718. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.M.; Swann, K.; Lai, F.A. PLCzeta, a sperm-specific PLC and its potential role in fertilization. Biochem. Soc. Symp. 2007, 74, 23–36. [Google Scholar]
- Swann, K.; Saunders, C.M.; Rogers, N.T.; Lai, F.A. PLC zeta: A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin. Cell Dev. Biol. 2006, 17, 264–273. [Google Scholar] [CrossRef]
- Parrington, J.; Lai, F.A.; Swann, K. A novel protein for Ca2+ signaling at fertilization. Curr. Top. Dev. Biol. 1998, 39, 215–243. [Google Scholar]
- Amdani, S.N.; Yeste, M.; Jones, C.; Coward, K. Sperm factors and oocyte activation: Current controversies and considerations. Biol. Reprod. 2015, 93, 50. [Google Scholar] [CrossRef]
- Miyazaki, S.; Shirakawa, H.; Nakada, K.; Honda, Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev. Biol. 1993, 158, 62–78. [Google Scholar] [CrossRef]
- Swann, K.; Yu, Y. The dynamics of calcium oscillations that activate mammalian eggs. Int. J. Dev. Biol. 2008, 52, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M.; Kashir, J.; Swann, K.; Lai, F.A. Sperm PLCζ: From structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett. 2013, 587, 3609–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.T.; Sutovsky, P.; Manandhar, G.; Xu, W.; Katayama, M.; Day, B.N.; Park, K.W.; Yi, Y.J.; Xi, Y.W.; Prather, R.S.; et al. PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development. J. Biol. Chem. 2007, 282, 12164–12175. [Google Scholar] [CrossRef] [Green Version]
- Aarabi, M.; Balakier, H.; Bashar, S.; Moskovtsev, S.I.; Sutovsky, P.; Librach, C.L.; Oko, R. Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014, 28, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.; Qin, Z.; Xu, W.; Mewburn, J.; Oko, R. Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol. Reprod. Dev. 2010, 77, 249–256. [Google Scholar] [CrossRef]
- Nomikos, M.; Sanders, J.R.; Theodoridou, M.; Kashir, J.; Matthews, E.; Nounesis, G.; Lai, F.A.; Swann, K. Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol. Hum. Reprod. 2014, 20, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.Y.; Fissore, R.A.; Hamer, R.; Deane, C.M.; Ruas, M.; et al. Reduced amounts and abnormal forms of phospholipase C zeta (PLC z) in spermatozoa from infertile men. Hum. Reprod. 2009, 24, 2417–2428. [Google Scholar] [CrossRef] [Green Version]
- Kashir, J.; Konstantinidis, M.; Jones, C.; Lemmon, B.; Lee, H.C.; Hamer, R.; Heindryckx, B.; Deane, C.M.; De Sutter, P.; Fissore, R.A.; et al. A maternally inherited autosomal point mutation in human phospholipase C zeta (PLC z) leads to male infertility. Hum. Reprod. 2012, 27, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Knott, J.G.; Kurokawa, M.; Fissore, R.A.; Schultz, R.M.; Williams, C.J. Transgenic RNA interference reveals role for mouse sperm phospholipase Czeta in triggering Ca2+ oscillations during fertilization. Biol. Reprod. 2005, 72, 992–996. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.Y.; Jellerette, T.; Salicioni, A.M.; Lee, H.C.; Yoo, M.S.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.B.; Visconti, P.E.; et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca 2+ release and are unable to initiate the first step of embryo development. J. Clin. Investig. 2008, 118, 3671–3681. [Google Scholar] [CrossRef] [Green Version]
- Wassarman, P.M.; Albertini, D.F. The Mammalian Ovum. In The Physiology of Reproduction, 2nd ed.; Knobil, E., Neill, J., Eds.; Raven Press: New York, NY, USA, 1988; Volume 1, pp. 79–122. [Google Scholar]
- Anifandis, G.; Messini, C.I.; Dafopoulos, K.; Daponte, A.; Messinis, I.E. Sperm contributions to oocyte activation: More that meets the eye. J. Assist. Reprod. Genet. 2016, 33, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomikos, M.; Swann, K.; Lai, F. Starting a new life: Sperm PLC zeta mobilizes the calcium signal that induces egg activation and embryo development: An essential phospholipase C with implication for male infertility. Bioessays 2011, 34, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Shikano, T.; Oda, S.; Horiguchi, T.; Tanimoto, S.; Awaji, T.; Mitani, H.; Miyazaki, S. Difference in Ca2+ oscillation-inducing activity and nuclear translocation ability of PLCZ1, an egg-activating sperm factor candidate, between mouse, rat, human, and medaka fish. Biol. Reprod. 2008, 78, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, A.; Kashima, M.; Yoshida, S.; Terada, K.; Nakagawa, S.; Sakamoto, A.; Hayakawa, K.; Suzuki, K.; Ueda, J.; Watanabe, T. Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Czeta. Reproduction 2006, 132, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Cox, L.J.; Larman, M.G.; Saunders, C.M.; Hashimoto, K.; Swann, K.; Lai, F.A. Sperm phospholipase Czeta from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 2002, 124, 611–623. [Google Scholar] [CrossRef]
- Tomes, C.N. Activation of mouse sperm phosphatidylinositol-4,5 bisphosphate-phospholipase C by zona pellucida is modulated by tyrosine phosphorylation. Mol. Reprod. Dev. 1996, 43, 196–204. [Google Scholar] [CrossRef]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Content of testis-specific isoform of Na/K-ATPase (ATP1A4) is increased during bovine sperm capacitation through translation in mitochondrial ribosomes. Cell Tissue Res. 2017, 368, 187–200. [Google Scholar] [CrossRef]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Testis-specific isoform of Na/K-ATPase (ATP1A4) interactome in raft and non-raft membrane fractions from capacitated bovine sperm. Int. J. Mol. Sci. 2019, 20, 3159. [Google Scholar]
- Thundathil, J.C.; Rajamanickam, G.D.; Kastelic, J.P. Na/K-ATPase and regulation of sperm function. Anim. Reprod. 2018, 15, 711–720. [Google Scholar] [CrossRef]
- Flesch, F.M.; Colenbrander, B.; van Golde, L.M.; Gadella, B.M. Capacitation induces tyrosine phosphorylation of proteins in the boar sperm plasma membrane. Biochem. Biophys. Res. Commun. 1999, 262, 787–792. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef]
- Salicioni, A.M.; Platt, M.D.; Wertheimer, E.V.; Arcelay, E.; Allaire, A.; Sosnik, J.; Visconti, P.E. Signalling pathways involved in sperm capacitation. Soc. Reprod. Fertil. Suppl. 2007, 65, 245–259. [Google Scholar]
- Thundathil, J.C.; Anzar, M.; Buhr, M.M. Na/K-ATPase as a signaling molecule during bovine sperm capacitation. Biol. Reprod. 2006, 75, 308–317. [Google Scholar] [CrossRef]
- Newton, L.D.; Krishnakumar, S.; Menon, A.G.; Kastelic, J.P.; van der Hoorn, F.A.; Thundathil, J.C. Na/K-ATPase regulates sperm capacitation through a mechanism involving kinases and redistribution of its testis-specific isoform. Mol. Reprod. Dev. 2010, 77, 136–148. [Google Scholar] [PubMed] [Green Version]
- Anapalakan, A. Sodium-Potassium-ATPase Signalling Mechanism Inducing Capacitation in Bull Sperm. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2010. [Google Scholar]
- Yuan, Z.; Cai, T.; Tian, J.; Ivanov, A.V.; Giovannucci, D.R.; Xie, Z.Y. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 2005, 16, 4034–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thundathil, J.C.; Rajamanickam, G.D.; Kastelic, J.P.; Newton, L.D. The effects of increased testicular temperature on testis-specific isoform of Na ⁄ K -ATPase in sperm and its role in spermatogenesis and sperm function. Reprod. Domest. Anim. 2012, 47, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Na/K-ATPase regulates bovine sperm capacitation through raft- and non-raft-mediated signaling mechanisms. Mol. Reprod. Dev. 2017, 84, 1168–1182. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnikrishnan, V.; Kastelic, J.; Thundathil, J. Intracytoplasmic Sperm Injection in Cattle. Genes 2021, 12, 198. https://doi.org/10.3390/genes12020198
Unnikrishnan V, Kastelic J, Thundathil J. Intracytoplasmic Sperm Injection in Cattle. Genes. 2021; 12(2):198. https://doi.org/10.3390/genes12020198
Chicago/Turabian StyleUnnikrishnan, Veena, John Kastelic, and Jacob Thundathil. 2021. "Intracytoplasmic Sperm Injection in Cattle" Genes 12, no. 2: 198. https://doi.org/10.3390/genes12020198
APA StyleUnnikrishnan, V., Kastelic, J., & Thundathil, J. (2021). Intracytoplasmic Sperm Injection in Cattle. Genes, 12(2), 198. https://doi.org/10.3390/genes12020198






