Retinal Oxygenation in Inherited Diseases of the Retina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Retinal Vessel Imaging
2.3. Optical Coherence Tomography Imaging
Statistical Procedures
3. Results
3.1. Increased A-SO2, V-SO2, O2-C and Decreased A-V SO2 Values in RCD Patients
3.2. Attenuated Retinal Vessel Diameters in RCD Patients
3.3. Peripapillary Retinal Nerve Fiber Layer Thickness Results: Thickest Peripapillary RNFL in RCD Patients
3.4. Central Retinal Thickness in RCD
3.5. Oxygen Exposure of the Retina in Patients with IRDs
4. Discussion
4.1. Altered Structural and Metabolic Function in IRDs
4.2. Altered Central Structure and Metabolic Function in IRDs
4.3. Altered Peripapillary Metabolic Function in IRDs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammann, F.; Klein, D.; Franceschetti, A. Genetic and epidemiological investigations on pigmentary degeneration of the retina and allied disorders in Switzerland. J. Neurol. Sci. 1965, 2, 183–196. [Google Scholar] [CrossRef]
- Michaelides, M.; Hunt, D.M.; Moore, A.T. The genetics of inherited macular dystrophies. J. Med. Genet. 2003, 40, 641–650. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Fishman, G. Electrophysiology and inherited retinal disorders. Doc. Ophthalmol. 1985, 60, 107–119. [Google Scholar] [CrossRef]
- Gouras, P.; Carr, R.E. Electrophysiological studies in early retinitis pigmentosa. Arch. Ophthalmol. 1964, 72, 104–110. [Google Scholar]
- Birch, D.G.; Sandberg, M.A. Dependence of cone b-wave implicit time on rod amplitude in retinitis pigmentosa. Vision Res. 1987, 27, 1105–1112. [Google Scholar] [CrossRef]
- Sandberg, M.A.; Weigel-DiFranco, C.; Rosner, B.; Berson, E.L. The relationship between visual field size and electroretinogram amplitude in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1693–1698. [Google Scholar]
- Tzekov, R.T.; Locke, K.G.; Hood, D.C.; Birch, D.G. Cone and rod ERG phototransduction parameters in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3993–4000. [Google Scholar] [CrossRef] [Green Version]
- Milam, A.H.; Li, Z.Y.; Fariss, R.N. Histopathology of the human retina in retinitis pigmentosa. Prog. Retin. Eye Res. 1998, 17, 175–205. [Google Scholar]
- Marc, R.E.; Jones, B.W. Retinal remodeling in inherited photoreceptor degenerations. Mol. Neurobiol. 2003, 28, 139–147. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B.W.; Watt, C.B.; Strettoi, E. Neural remodeling in retinal degeneration. Prog. Retin. Eye Res. 2003, 22, 607–655. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.W.; Watt, C.B.; Frederick, J.M.; Baehr, W.; Chen, C.K.; Levine, E.M.; Milam, A.H.; Lavail, M.M.; Marc, R.E. Retinal remodeling triggered by photoreceptor degenerations. J. Comp. Neurol. 2003, 464, 1–16. [Google Scholar] [CrossRef]
- Sancho-Pelluz, J.; Arango-Gonzalez, B.; Kustermann, S.; Romero, F.J.; van Veen, T.; Zrenner, E.; Ekström, P.; Paquet-Durand, F. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol. Neurobiol. 2008, 38, 253–269. [Google Scholar] [CrossRef]
- Cottet, S.; Schorderet, D.F. Mechanisms of apoptosis in retinitis pigmentosa. Curr. Mol. Med. 2009, 9, 375–383. [Google Scholar] [CrossRef]
- Panfoli, I.; Calzia, D.; Ravera, S.; Morelli, A.M.; Traverso, C.E. Extra-mitochondrial aerobic metabolism in retinal rod outer segments: New perspectives in retinopathies. Med. Hypotheses. 2012, 78, 423–427. [Google Scholar] [CrossRef]
- Vanderkooi, J.M.; Erecińska, M.; Silver, I.A. Oxygen in mammalian tissue: Methods of measurement and affinities of various reactions. Am. J. Physiol. 1991, 260, C1131–C1150. [Google Scholar] [CrossRef]
- Stefánsson, E.; Olafsdottir, O.B.; Eliasdottir, T.S.; Vehmeijer, W.; Einarsdottir, A.B.; Bek, T.; Torp, T.L.; Grauslund, J.; Eysteinsson, T.; Karlsson, R.A.; et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog. Retin. Eye Res. 2019, 70, 1–22. [Google Scholar] [CrossRef]
- Bill, A.; Sperber, G. Control of retinal and choroidal blood flow. Eye (Lond) 1990, 4, 319–325. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, C.S.; Su, E.N.; Yu, P.K. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3999–4006. [Google Scholar] [PubMed]
- Yu, D.Y.; Cringle, C.S.; Valter, K.; Walsh, N.; Lee, D.; Stone, J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2013–2019. [Google Scholar] [CrossRef]
- Yu, D.Y.; Cringle, C.S. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Padnick-Silver, L.; Kang Derwelt, J.; Giuliano, E.; Narfström, K.; Linsenmeier, R.A. Retinal oxygenation and oxygen metabolism in Abyssinian cats with a hereditary retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Haugh, L.M.; Linsenmeier, R.A.; Goldstick, T.K. Mathematical models of the spatial distribution of retinal oxygen tension and consumption, including changes upon illumination. Ann. Biomed Eng. 1990, 18, 19–36. [Google Scholar] [CrossRef]
- Cringle, S.J.; Yu, D. A multi-layer model of retinal oxygen supply and consumption helps explain the muted rise in inner retinal PO(2) during systemic hyperoxia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 61–66. [Google Scholar] [CrossRef]
- Cringle, S.J.; Yu, D.; Yu, P.K.; Su, E.N. Intraretinal oxygen consumption in the rat in vivo. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1922–1927. [Google Scholar]
- Stefánsson, E.; Wolbarsht, M.L.; Landers, M.B. In vivo O2 consumption in rhesus monkeys in light and dark. Exp. Eye Res. 1983, 37, 251–256. [Google Scholar] [CrossRef]
- Linsenmeier, R. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J. Gen. Physiol. 1986, 88, 521–542. [Google Scholar] [CrossRef] [Green Version]
- Usui, S.; Brian, C.O.; Lee, S.Y.; Jo, Y.J.; Yoshida, T.; Akiko, M.; Katsuaki, M.; Iwase, T.; Lu, L.; Campochiaro, P.A. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J. Neurochem. 2009, 110, 1028–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.W.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, D.; Thamm, E.; Hammer, M.; Kraft, J. A new method for the measurement of oxygen saturation at the human ocular fundus. Int. Ophthalmol. 2001, 23, 347–353. [Google Scholar] [PubMed]
- Hammer, M.; Thamm, E.; Schweitzer, D. A simple algorithm for in vivo ocular fundus oximetry compensating for non-haemoglobin absorption and scattering. Phys. Med. Biol. 2002, 47, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardarson, S. Retinal oximetry. Acta Ophthalmol. 2013, 91, 1–47. [Google Scholar] [CrossRef]
- Geirsdottir, A.; Palsson, O.; Hardarson, S.H.; Olafsdottir, O.B.; Kristjansdottir, J.V.; Stefánsson, E. Retinal vessel oxygen saturation in healthy individuals. Invest. Ophthalmol. Vis. Sci. 2012, 13, 5433–5442. [Google Scholar] [CrossRef] [Green Version]
- Türksever, C.; Orgül, S.; Todorova, M.G. Reproducibility of retinal oximetry measurements in healthy and diseased retinas. Acta Ophthalmol. 2015, 93, e439–e445. [Google Scholar] [CrossRef]
- Türksever, C.; Valmaggia, C.; Orguel, S.; Schorderet, D.F.; Todorova, M.G. Retinal vessel oxygen saturation in retinitis pigmentosa patients. Acta Ophthalmol. 2013, 91, eTO23. [Google Scholar] [CrossRef]
- Todorova, M.G.; Türksever, C.; Schorderet, D.F.; Valmaggia, C. Retinal vessel Oxygen Saturation in patients suffering from inherited diseases of the retina. Klin. Monbl. Augenheilkd. 2014, 231, 447–452. [Google Scholar]
- Bojinova, R.I.; Türksever, C.; Schötzau, A.; Valmaggia, C.; Schorderet, D.F.; Todorova, M.G. Reduced metabolic function and structural alterations in inherited retinal dystrophies: Investigating the effect of peripapillary vessel oxygen saturation and vascular diameter on the retinal nerve fibre layer thickness. Acta Ophthalmol. 2017, 95, 252–261. [Google Scholar] [CrossRef]
- Della-Volpe, W.M.; Scholl, H.P.N.; Valmaggia, C.; Todorova, M.G. Retinal vessel oximetry in children with inherited retinal diseases. Acta Ophthalmol. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Türksever, C.; Valmaggia, C.; Orgül, S.; Schorderet, D.F.; Flammer, J.; Todorova, M.G. Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol. 2014, 92, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bojinova, R.I.; Schorderet, D.F.; Valmaggia, C.; Türksever, C.; Schoetzau, A.; Todorova, M.G. Higher retinal vessel oxygen saturation: Investigating its relationship with macular oedema in retinitis pigmentosa patients. Eye (Lond) 2018, 32, 1209–1219. [Google Scholar] [CrossRef] [Green Version]
- Todorova, M.G.; Türksever, C.; Schötzau, A.; Schorderet, D.F.; Valmaggia, C. Metabolic and functional changes in retinitis pigmentosa: Comparing retinal vessel oximetry to full-field electroretinography, electrooculogram and multifocal electroretinography. Acta Ophthalmol. 2016, 94, e231–e241. [Google Scholar] [CrossRef]
- Lopez-Torres, L.T.; Türksever, C.; Schötzau, A.; Orgül, S.; Todorova, M.G. Peripapillary retinal vessel diameter correlates with mfERG alterations in retinitis pigmentosa. Acta Ophthalmol. 2015, 93, e527–e533. [Google Scholar] [CrossRef]
- Waizel, M.; Türksever, C.; Todorova, M.G. The effect of autoimmune retinopathy on retinal vessel oxygen saturation. Eye (Lond) 2018, 32, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- Walia, S.; Fishman, G.A.; Edward, D.P.; Lindeman, M. Retinal nerve fiber layer defects in RP patients. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4748–4752. [Google Scholar] [CrossRef]
- Walia, S.; Fishman, G.A. Retinal nerve fiber layer analysis in RP patients using Fourier-domain OCT. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3525–3528. [Google Scholar] [CrossRef]
- Hood, D.C.; Lin, C.E.; Lazow, M.A.; Locke, K.G.; Zhang, X.; Birch, D.G. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2009, 50, 2328–2336. [Google Scholar]
- Anastasakis, A.; Genead, M.A.; McAnany, J.J.; Fishman, G.A. Evaluation of retinal nerve fiber layer thickness in patients with retinitis pigmentosa using spectral-domain optical coherence tomography. Retina 2012, 32, 358–363. [Google Scholar] [CrossRef]
- Xue, K.; Wang, M.; Chen, J.; Huang, X.; Xu, G. Retinal nerve fiber layer analysis with scanning laser polarimetry and RTVue-OCT in patients of retinitis pigmentosa. Ophthalmologica 2013, 229, 38–42. [Google Scholar] [CrossRef]
- Hajali, M.; Fishman, G.A.; Anderson, R.J. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br. J. Ophthalmol. 2008, 92, 1065–1068. [Google Scholar]
- Testa, F.; Rossi, S.; Colucci, R.; Gallo, B.; Di Iorio, V.; della Corte, M.; Azzolini, C.; Melillo, P.; Simonelli, F. Macular abnormalities in Italian patients with retinitis pigmentosa. Br. J. Ophthalmol. 2014, 98, 946–950. [Google Scholar] [CrossRef] [Green Version]
- Adackapara, C.A.; Sunness, J.S.; Dibernardo, C.W.; Melia, B.M.; Dagnelie, G. Prevalence of cystoid macular edema and stability in oct retinal thickness in eyes with retinitis pigmentosa during a 48-week lutein trial. Retina 2008, 28, 103–110. [Google Scholar] [CrossRef]
- Hood, D.C.; Lazow, M.A.; Locke, K.G.; Greenstein, V.C.; Birch, D.G. The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2011, 52, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandberg, M.A.; Brockhurst, R.J.; Gaudio, A.R.; Berson, E.L. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2005, 46, 3349–3354. [Google Scholar] [CrossRef] [Green Version]
- Konieczka, K.; Bojinova, R.J.; Valmaggia, C.; Schorderet, D.F.; Todorova, M.G. Preserved functional and structural integrity of the papillomacular area correlates with better visual acuity in retinitis pigmentosa. Eye (Lond) 2016, 30, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.G.; Scholl, H.P.N.; Della Volpe-Waizel, M. The impact of macular edema on microvascular and metabolic alterations in retinitis pigmentosa. Graefes. Arch. Clin. Exp. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Sutherland, F.S.; Stefansson, E.; Hatchell, D.L.; Reiser, H. Retinal oxygen consumption in vitro. The effect of diabetes mellitus, oxygen and glucose. Acta Ophthalmol. (Copenh) 1990, 68, 715–720. [Google Scholar] [CrossRef]
- Iriyama, A.; Yanagi, Y. Fundus autofluorescence and retinal structure as determined by spectral domain optical coherence tomography, and retinal function in retinitis pigmentosa. Graefes. Arch. Clin. Exp. Ophthalmol. 2012, 250, 333–339. [Google Scholar] [CrossRef]
- Wirtschafter, J.D. Optic nerve axons and acquired alterations in the appearance of the optic disc. Trans. Am. Ophthalmol. Soc. 1983, 81, 1034. [Google Scholar]
- Todorova, M.G.; Bojinova, R.I.; Valmaggia, C.; Schorderet, D.F. Prominent Optic Disc Featured in Inherited Retinopathy. Klin. Monbl. Augenheilkd. 2017, 234, 577–583. [Google Scholar] [CrossRef]
- Funatsu, J.; Murakami, Y.; Nakatake, S.; Akiyama, M.; Fujiwara, K.; Shimokawa, S.M.; Tachibana, T.; Hisatomi, T.; Koyanagi, Y.; Momozawa, Y.; et al. Direct comparison of retinal structure and function in retinitis pigmentosa by co-registering microperimetry and optical coherence tomography. PLoS ONE 2019, 14, e0226097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robson, A.G.; Egan, C.A.; Luong, V.A.; Bird, A.C.; Holder, G.E.; Fitzke, F.W. Comparison of fundus autofluorescence with photopic and scotopic fine-matrix mapping in patients with retinitis pigmentosa and normal visual acuity. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4119–4125. [Google Scholar]
- Robson, A.G.; Lenassi, E.; Saihan, Z.; Luong, V.A.; Fitzke, F.W.; Holder, G.E.; Webster, A.R. Comparison of fundus autofluorescence with photopic and scotopic fine matrix mapping in patients with retinitis pigmentosa: 4- to 8-year follow-up. Invest. Ophthalmol. Vis. Sci. 2012, 53, 6187–6195. [Google Scholar] [CrossRef] [Green Version]
- Nakatake, S.; Murakami, Y.; Funatsu, J.; Koyanagi, Y.; Akiyama, M.; Momozawa, Y.; Ishibashi, T.; Sonoda, K.H.; Ikeda, Y. Early detection of cone photoreceptor cell loss in retinitis pigmentosa using adaptive optics scanning laser ophthalmoscopy. Graefes. Arch. Clin. Exp. Ophthalmol. 2019, 257, 1169–1181. [Google Scholar] [CrossRef]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [Green Version]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R. Packing geometry of human cone photoreceptors: Variation with eccentricity and evidence for local anisotropy. Vis. Neurosci. 1992, 9, 169–180. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Packer, O.; Hendrickson, A.E.; Kalina, R.E. Distribution of Cones in human and monkey retina: Individual variability and radial asymmetry. Science 1987, 236, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Jani, P.D.; Mwanza, J.; Billow, K.B.; Waters, A.M.; Moyer, S.; Garg, S. Normative values and predictors of retinal oxygen saturation. Retina 2014, 34, 394–401. [Google Scholar] [CrossRef]

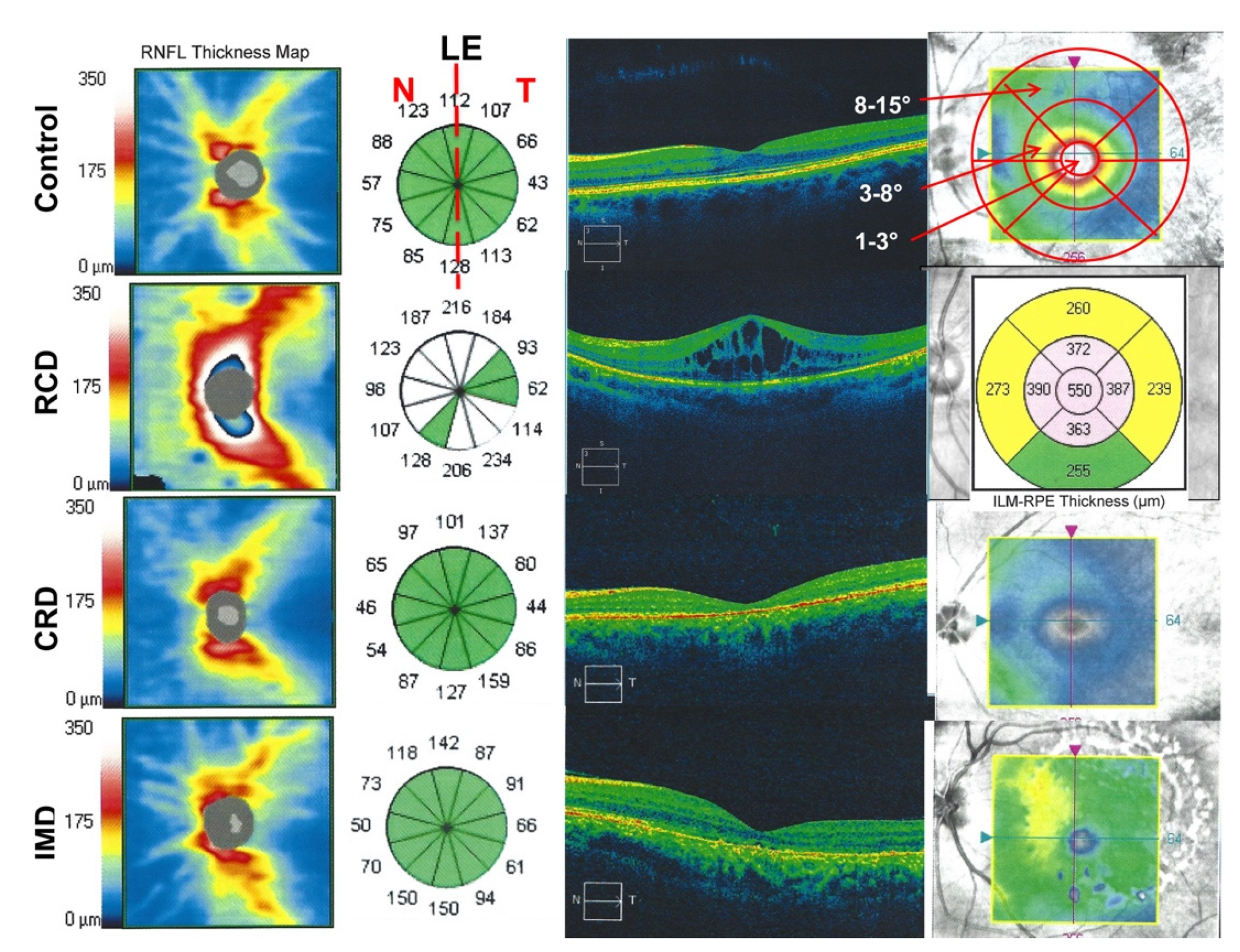
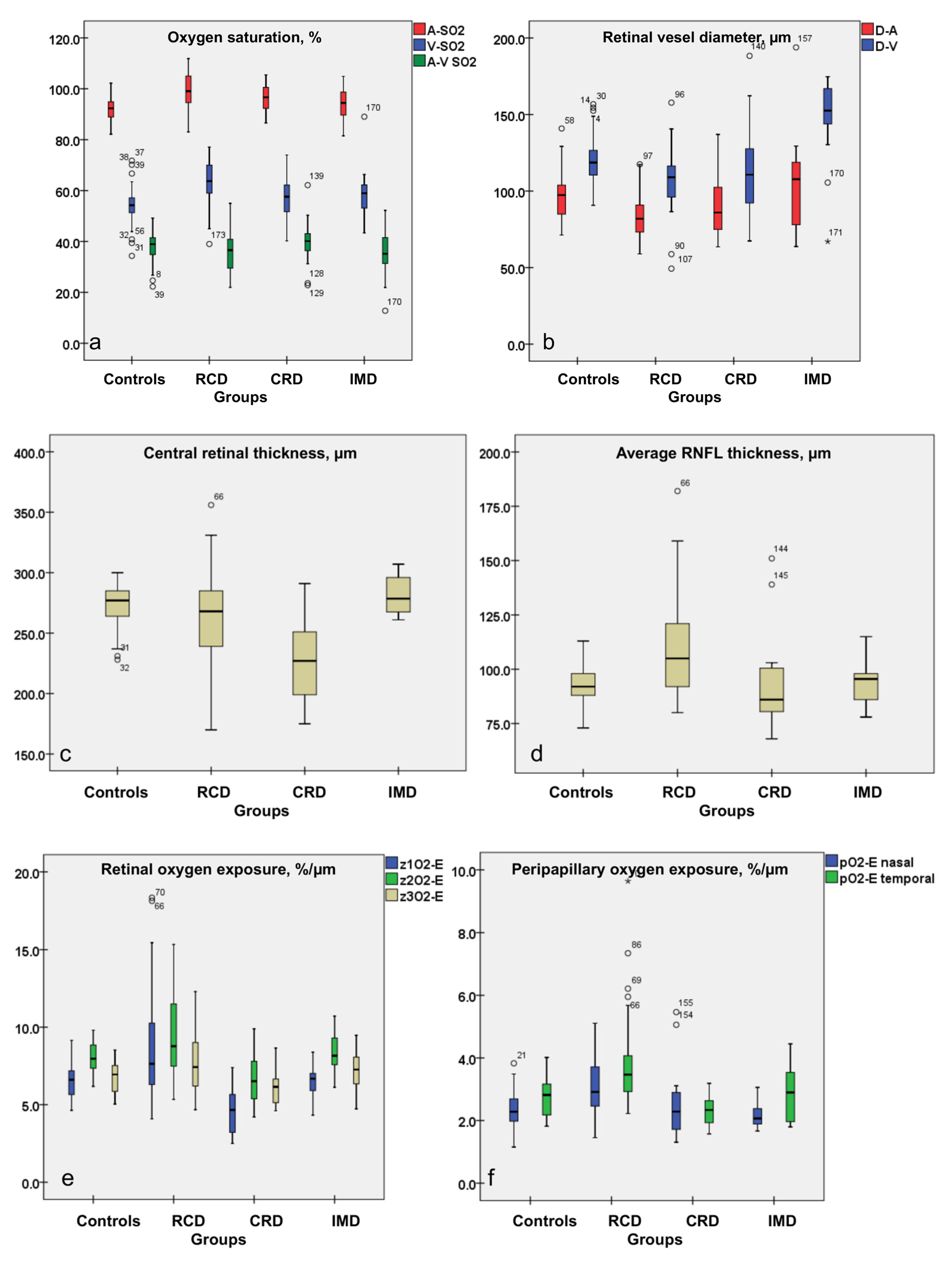
| Groups | Number of Subjects | Evaluated Eyes | Age, Y; Mean (±SD) | Gender (♀:♂) | Mean BCVA, (±SD) (Snellen Charts) | |
|---|---|---|---|---|---|---|
| RE | LE | |||||
| Controls | 30 | 30 | 30 | 46.34 (10.50) | 21:9 | 1.0 (0.03) |
| RCD | 35 | 34 | 35 | 43.88 (13.54) | 18:17 | 0.53 (0.26) |
| CRD | 13 | 13 | 13 | 41.08 (13.02) | 8:6 | 0.47 (0.36) |
| IMD | 13 | 13 | 13 | 54.16 (13.49) | 6:7 | 0.75 (0.30) |
| Adjusted Means in Groups (±SD) | Comparison between Groups | ANOVA, Based on Mixed Effects Model | |||||
|---|---|---|---|---|---|---|---|
| Controls | RCD | CRD | IMD | Group 1 | Group 2 | Mean Difference | p-Values between Groups |
| A-SO2 (%) | |||||||
| 92.08 (4.51) | 99.12 (6.42) | 96.55 (4.95) | 94.30 (6.03) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −7.03 −4.47 −2.21 2.57 4.82 −2.26 | <0.001 0.001 0.102 0.052 <0.001 0.148 |
| V-SO2 (%) | |||||||
| 53.94 (6.65) | 63.55 (7.64) | 56.67 (8.03) | 58.61 (8.63) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −9.60 −2.72 −4.66 6.88 4.94 −1.94 | <0.001 0.138 0.012 <0.001 0.006 0.358 |
| A-V SO2 (%) | |||||||
| 38.21 (5.52) | 35.54 (8.59) | 39.86 (7.74) | 35.69 (9.00) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 2.98 −1.65 2.52 −4.32 −0.15 4.17 | 0.040 0.374 0.177 0.018 0.935 0.053 |
| D-A (μm) | |||||||
| 96.21 (13.910) | 84.12 (14.43) | 91.20 (19.63) | 106.93 (29.65) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 12.10 5.01 −10.72 −7.09 −22.82 −15.71 | <0.001 0.236 0.027 0.086 <0.001 0.004 |
| D-V (μm) | |||||||
| 121.00 (14.67) | 107.60 (17.88) | 112.23 (28.58) | 147.70 (26.31) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 13.40 8.77 −26.70 −4.63 −40.10 −35.47 | 0.001 0.074 <0.001 0.329 <0.001 <0.001 |
| Central retinal thickness (μm) | |||||||
| 271.67 (19.60) | 263.80 (36.02) | 229.00 (35.84) | 281.88 (15.77) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 7.87 42.68 −10.20 34.80 −18.07 −52.88 | 0.227 <0.001 0.275 <0.001 0.037 <0.001 |
| RNFL thickness (μm) | |||||||
| 92.83 (8.42) | 109.85 (22.91) | 92.50 (20.63) | 92.89 (9.90) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −17.02 0.33 −0.06 17.35 16.96 −0.39 | <0.001 0.946 0.991 <0.001 0.001 0.947 |
| cO2-E (µm/%) | |||||||
| 6.92 (1.05) | 8.08 (2.15) | 5.88 (1.30) | 7.51 (1.14) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −1.16 1.05 −0.60 2.20 0.56 −1.64 | 0.002 0.035 0.242 <0.001 0.005 0.005 |
| pO2-E (µm/%) | |||||||
| 2.35 (0.36) | 3.36 (1.02) | 2.36 (0.60) | 2.58 (0.64) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −1.01 −0.01 −0.23 1.00 0.78 −0.21 | <0.001 0.946 0.320 <0.001 <0.001 0.404 |
| Adjusted Means in Groups (±SD) | Comparison between Groups | ANOVA, Based on Mixed Effects Model | |||||
|---|---|---|---|---|---|---|---|
| Controls | RCD | CRD | IMD | Group 1 | Group 2 | Mean Difference | p-Values between Groups |
| RNFL nasal (μm) | |||||||
| 87.80 (13.97) | 103.88 (28.02) | 90.10 (31.83) | 88.62 (13.10) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −16.07 −4.30 −0.82 11.80 15.25 3.48 | 0.001 0.490 0.904 0.053 0.023 0.649 |
| RNFL temporal (μm) | |||||||
| 96.23 (11.91) | 113.97 (25.35) | 88.36 (17.16) | 96.88 (14.03) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −17.740 7.874 −0.650 25.614 17.090 −8.524 | <0.001 0.121 0.904 <0.001 0.001 0.164 |
| A-V SO2 nasal (%) | |||||||
| 38.20 (5.36) | 37.04 (5.89) | 36.88 (5.30) | 41.62 (6.91) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 1.151 1.313 −3.421 0.162 −4.572 −4.734 | 0.390 0.393 0.039 0.912 0.004 0.008 |
| A-V SO2 temporal (%) | |||||||
| 36.35 (6.45) | 32.60 (6.71) | 38.39 (6.63) | 34.29 (8.61) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 3.743 −2.041 2.058 −5.784 −1.685 −4.099 | 0.019 0.261 0.298 0.001 0.380 0.055 |
| pO2-E nasal (µm/%) | |||||||
| 2.35 (0.58) | 3.03 (0.91) | 2.54 (1.05) | 2.15 (0.42) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −0.681 −0.184 0.200 0.498 0.881 −0.383 | 0.001 0.438 0.457 0.025 0.001 0.177 |
| pO2-E temporal (µm/%) | |||||||
| 2.73 (0.66) | 3.82 (1.45) | 2.31 (0.45) | 2.83 (0.91) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −1.095 0.420 −0.104 1.516 0.991 0.525 | <0.001 0.162 0.765 <0.001 0.004 0.159 |
| Adjusted Means in Groups (±SD) | Comparison between Groups | ANOVA, Based on Mixed Effects Model | |||||
|---|---|---|---|---|---|---|---|
| Controls | RCD | CRD | IMD | Group 1 | Group 2 | Mean Difference | p-Values between Groups |
| Retinal thickness, Zone 1 (μm) | |||||||
| 259.56 (28.32) | 272.11 (77.02) | 179.22 (43.32) | 246.69 (15.92) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −13.74 80.34 12.87 94.08 26.61 −64.47 | 0.274 <0.001 0.473 <0.001 0.109 0.001 |
| Retinal thickness, Zone 2 (μm) | |||||||
| 318.79 (12.34) | 303.16 (55.52) | 258.49 (44.85) | 312.26 (27.29) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 15.86 60.30 6.52 44.44 −9.34 −53.78 | 0.094 <0.001 0.628 <0.001 0.452 0.001 |
| Retinal thickness, Zone 3 (μm) | |||||||
| 268.69 (25.47) | 255.92 (33.22) | 239.47 (30.44) | 270.88 (27.36) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | 13.26 29.22 −2.18 15.96 −15.44 −31.40 | 0.043 0.001 0.814 0.053 0.073 0.003 |
| z1O2-E (µm/%) | |||||||
| 6.62 (1.18) | 8.48 (3.06) | 4.66 (1.52) | 6.59 (1.05) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −1.86 1.96 0.03 3.82 1.90 −1.93 | <0.001 0.004 0.961 <0.001 0.004 0.015 |
| z2O2-E (µm/%) | |||||||
| 8.11 (1.03) | 9.36 (2.58) | 6.67 (1.66) | 8.30 (1.20) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −1.26 1.44 −0.19 2.70 1.07 −1.63 | 0.004 0.014 0.755 <0.001 0.059 0.018 |
| z3O2-E (µm/%) | |||||||
| 6.83 (1.03) | 7.81 (1.02) | 6.15 (1.20) | 7.24 (1.36) | Controls Controls Controls RCD RCD CRD | RCD CRD IMD CRD IMD IMD | −0.98 0.68 −0.42 1.66 0.56 −1.10 | 0.006 0.150 0.399 <0.001 0.222 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türksever, C.; López Torres, L.T.; Valmaggia, C.; Todorova, M.G. Retinal Oxygenation in Inherited Diseases of the Retina. Genes 2021, 12, 272. https://doi.org/10.3390/genes12020272
Türksever C, López Torres LT, Valmaggia C, Todorova MG. Retinal Oxygenation in Inherited Diseases of the Retina. Genes. 2021; 12(2):272. https://doi.org/10.3390/genes12020272
Chicago/Turabian StyleTürksever, Cengiz, Lisette T. López Torres, Christophe Valmaggia, and Margarita G. Todorova. 2021. "Retinal Oxygenation in Inherited Diseases of the Retina" Genes 12, no. 2: 272. https://doi.org/10.3390/genes12020272
APA StyleTürksever, C., López Torres, L. T., Valmaggia, C., & Todorova, M. G. (2021). Retinal Oxygenation in Inherited Diseases of the Retina. Genes, 12(2), 272. https://doi.org/10.3390/genes12020272







