Sensory Reactivity Symptoms Are a Core Feature of ADNP Syndrome Irrespective of Autism Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Declaration
2.3. Clinical Evaluation
2.4. Analysis
3. Results
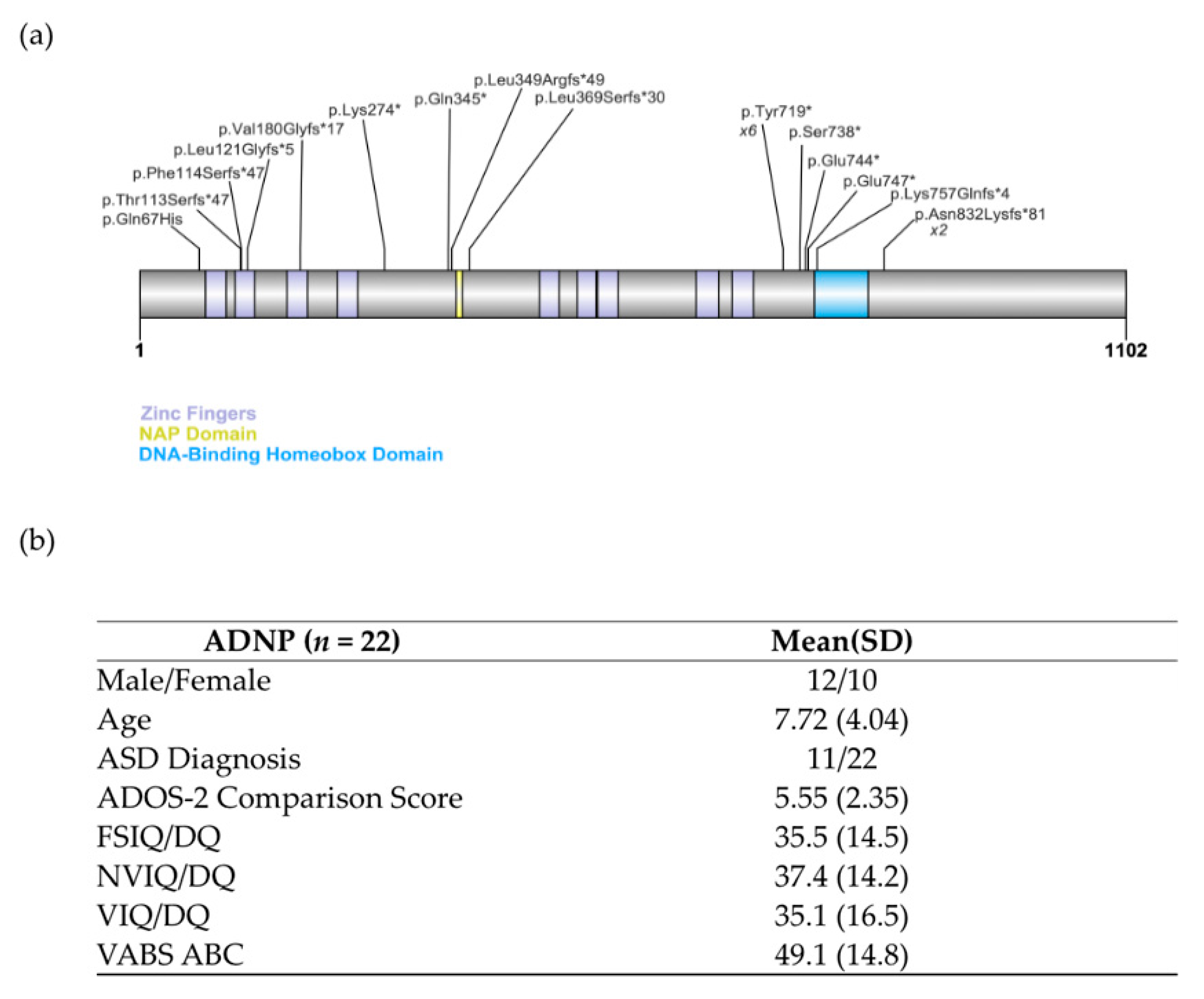
3.1. Genetic Testing
3.2. Participant Characteristics
3.3. Frequency and Type of Sensory Symptoms by Measure
3.3.1. SAND
3.3.2. SSP
3.3.3. ADOS-2
3.3.4. ADI-R
3.4. Group Differences and Correlations with Clinical, Demographic, and Genetic Factors
3.4.1. ASD
3.4.2. Cognitive and Adaptive Functioning
3.4.3. Age and Sex
3.4.4. Genotype-Phenotype
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnett, A.B.; Rhoads, C.L.; Hoekzema, K.; Turner, T.N.; Gerdts, J.; Wallace, A.S.; Bedrosian-Sermone, S.; Eichler, E.E.; Bernier, R.A. The autism spectrum phenotype in ADNP syndrome. Autism Res. 2018, 11, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Helsmoortel, C.; Vulto-van Silfhout, A.T.; Coe, B.P.; Vandeweyer, G.; Rooms, L.; van den Ende, J.; Schuurs-Hoeijmakers, J.H.; Marcelis, C.L.; Willemsen, M.H.; Vissers, L.E.; et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nat. Genet. 2014, 46, 380–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dijck, A.; Vulto-van Silfhout, A.T.; Cappuyns, E.; van der Werf, I.M.; Mancini, G.M.; Tzschach, A.; Bernier, R.; Gozes, I.; Eichler, E.E.; Romano, C.; et al. Clinical Presentation of a Complex Neurodevelopmental Disorder Caused by Mutations in ADNP. Biol. Psychiatry 2019, 85, 287–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostapcuk, V.; Mohn, F.; Carl, S.H.; Basters, A.; Hess, D.; Iesmantavicius, V.; Lampersberger, L.; Flemr, M.; Pandey, A.; Thomä, N.H.; et al. Activity-dependent neuroprotective protein recruits HP1 and CHD4 to control lineage-specifying genes. Nature 2018, 557, 739–743. [Google Scholar] [CrossRef]
- Kaaij, L.J.T.; Mohn, F.; van der Weide, R.H.; de Wit, E.; Bühler, M. The ChAHP Complex Counteracts Chromatin Looping at CTCF Sites that Emerged from SINE Expansions in Mouse. Cell 2019, 178, 1437–1451.e14. [Google Scholar] [CrossRef]
- Hacohen-Kleiman, G.; Sragovich, S.; Karmon, G.; Gao, A.Y.L.; Grigg, I.; Pasmanik-Chor, M.; Le, A.; Korenková, V.; McKinney, R.A.; Gozes, I. Activity-dependent neuroprotective protein deficiency models synaptic and developmental phenotypes of autism-like syndrome. J. Clin. Investig. 2018, 128, 4956–4969. [Google Scholar] [CrossRef] [Green Version]
- Sragovich, S.; Malishkevich, A.; Piontkewitz, Y.; Giladi, E.; Touloumi, O.; Lagoudaki, R.; Grigoriadis, N.; Gozes, I. The autism/neuroprotection-linked ADNP/NAP regulate the excitatory glutamatergic synapse. Transl. Psychiatr. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oz, S.; Kapitansky, O.; Ivashco-Pachima, Y.; Malishkevich, A.; Giladi, E.; Skalka, N.; Rosin-Arbesfeld, R.; Mittelman, L.; Segev, O.; Hirsch, J.A.; et al. The NAP motif of activity-dependent neuroprotective protein (ADNP) regulates dendritic spines through microtubule end binding proteins. Mol. Psychiatry 2014, 19, 1115–1124. [Google Scholar] [CrossRef] [Green Version]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Gozes, I.; Van Dijck, A.; Hacohen-Kleiman, G.; Grigg, I.; Karmon, G.; Giladi, E.; Eger, M.; Gabet, Y.; Pasmanik-Chor, M.; Cappuyns, E.; et al. Premature primary tooth eruption in cognitive/motor-delayed ADNP-mutated children. Transl. Psychiatry 2017, 7, e1043. [Google Scholar] [CrossRef]
- Siper, P.M.; De Rubeis, S.; Trelles, M.d.P.; Durkin, A.; Di Marino, D.; Muratet, F.; Frank, Y.; Lozano, R.; Eichler, E.E.; Kelly, M.; et al. Prospective investigation of FOXP1 syndrome. Mol. Autism 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rubeis, S.; Siper, P.M.; Durkin, A.; Weissman, J.; Muratet, F.; Halpern, D.; Trelles, M.D.P.; Frank, Y.; Lozano, R.; Wang, A.T.; et al. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol. Autism 2018, 9, 31. [Google Scholar] [CrossRef]
- Rais, M.; Binder, D.K.; Razak, K.A.; Ethell, I.M. Sensory Processing Phenotypes in Fragile X Syndrome. ASN Neuro 2018, 10, 1759091418801092. [Google Scholar] [CrossRef] [Green Version]
- Mieses, A.M.; Tavassoli, T.; Li, E.; Soorya, L.; Lurie, S.; Wang, A.T.; Siper, P.M.; Kolevzon, A. Brief Report: Sensory Reactivity in Children with Phelan-McDermid Syndrome. J. Autism Dev. Disord. 2016, 46, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Lansing, MI, USA, 2013. [Google Scholar]
- Sacrey, L.A.; Bennett, J.A.; Zwaigenbaum, L. Early Infant Development and Intervention for Autism Spectrum Disorder. J. Child. Neurol. 2015, 30, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.J.; Dimian, A.F.; Botteron, K.N.; Dager, S.R.; Elison, J.T.; Estes, A.M.; Hazlett, H.C.; Schultz, R.T.; Zwaigenbaum, L.; Piven, J. A longitudinal study of parent-reported sensory responsiveness in toddlers at-risk for autism. J. Child. Psychol. Psychiatry 2019, 60, 314–324. [Google Scholar] [CrossRef]
- Lane, A.E.; Dennis, S.J.; Geraghty, M.E. Brief report: Further evidence of sensory subtypes in autism. J. Autism Dev. Disord. 2011, 41, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Tomchek, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Dellapiazza, F.; Vernhet, C.; Blanc, N.; Miot, S.; Schmidt, R.; Baghdadli, A. Links between sensory processing, adaptive behaviours, and attention in children with autism spectrum disorder: A systematic review. Psychiatry Res. 2018, 270, 78–88. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Cermak, S.A.; Orsmond, G.I.; Tager-Flusberg, H.; Kadlec, M.B.; Carter, A.S. Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. J. Child. Psychol. Psychiatry 2008, 49, 817–825. [Google Scholar] [CrossRef]
- Green, S.A.; Ben-Sasson, A.; Soto, T.W.; Carter, A.S. Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. J. Autism Dev. Disord. 2012, 42, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Ermer, J.; Dunn, W. The sensory profile: A discriminant analysis of children with and without disabilities. Am. J. Occup. Ther. 1998, 52, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watling, R.L.; Deitz, J.; White, O. Comparison of Sensory Profile scores of young children with and without autism spectrum disorders. Am. J. Occup. Ther. 2001, 55, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Charman, T.; Loth, E.; Tillmann, J.; Crawley, D.; Wooldridge, C.; Goyard, D.; Ahmad, J.; Auyeung, B.; Ambrosino, S.; Banaschewski, T.; et al. The EU-AIMS Longitudinal European Autism Project (LEAP): Clinical characterisation. Mol. Autism 2017, 8, 27. [Google Scholar] [CrossRef]
- Mullen, E.M. Mullen Scales of Early Learning; AGS Publishing: Circle Pines, MN, USA, 1995. [Google Scholar]
- Roid, G.H.; Pomplun, M. The Stanford-Binet Intelligence Scales; The Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Hill, T.L.; Saulnier, C.A.; Cicchetti, D.; Gray, S.A.O.; Carter, A.S. Vineland III. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer: New York, NY, USA, 2017; pp. 1–4. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L. Autism Diagnostic Observation Schedule—Generic (ADOS-G) Manual (Part I): Modules 1–4; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Lord, C.; Rutter, M.; Le Couteur, A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994, 24, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Gotham, K.; Risi, S.; Pickles, A.; Lord, C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. J. Autism Dev. Disord. 2007, 37, 613–627. [Google Scholar] [CrossRef]
- Siper, P.M.; Kolevzon, A.; Wang, A.T.; Buxbaum, J.D.; Tavassoli, T. A clinician-administered observation and corresponding caregiver interview capturing DSM-5 sensory reactivity symptoms in children with ASD. Autism Res. 2017, 10, 1133–1140. [Google Scholar] [CrossRef]
- Dunn, W.; Westman, K. The sensory profile: The performance of a national sample of children without disabilities. Am. J. Occup. Ther. 1997, 51, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Feise, R.J. Do multiple outcome measures require p-value adjustment? BMC Med. Res. Methodol. 2002, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, S.S. Vineland Adaptive Behavior Scales. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 2618–2621. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales Vineland-II: Survey Forms Manual; Pearson Minneapolis, MN, USA, 2005. [Google Scholar]
- Proffitt, J.; Osann, K.; McManus, B.; Kimonis, V.E.; Heinemann, J.; Butler, M.G.; Stevenson, D.A.; Gold, J.A. Contributing factors of mortality in Prader–Willi syndrome. Am. J. Med Genet. Part A 2019, 179, 196–205. [Google Scholar] [CrossRef]
- Luchsinger, K.; Lau, H.; Hedlund, J.L.; Friedman, D.; Krushel, K.; Devinsky, O. Parental-reported pain insensitivity in Dup15q. Epilepsy Behav. 2016, 55, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Géranton, S.M.; Bebbington, A.; Jacoby, P.; Bahi-Buisson, N.; Ravine, D.; Leonard, H. Linking MECP2 and pain sensitivity: The example of Rett syndrome. Am. J. Med Genet. Part A 2010, 152A, 1197–1205. [Google Scholar] [CrossRef] [Green Version]
- Breen, M.S.; Garg, P.; Tang, L.; Mendonca, D.; Levy, T.; Barbosa, M.; Arnett, A.B.; Kurtz-Nelson, E.; Agolini, E.; Battaglia, A.; et al. Episignatures Stratifying Helsmoortel-Van Der Aa Syndrome Show Modest Correlation with Phenotype. Am. J. Human Genet. 2020, 107, 555–563. [Google Scholar] [CrossRef]
- Perez Repetto, L.; Jasmin, E.; Fombonne, E.; Gisel, E.; Couture, M. Longitudinal Study of Sensory Features in Children with Autism Spectrum Disorder. Autism Res. Treat. 2017, 2017, 1934701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, P.; Saron, C.D.; Rivera, S.M. Identification of Longitudinal Sensory Subtypes in Typical Development and Autism Spectrum Development Using Growth Mixture Modelling. Res. Autism Spectr. Disord. 2020, 78, 101645. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Carlson, M.; Sideris, J.; Kirby, A.V.; Watson, L.R.; Williams, K.L.; Bulluck, J. Longitudinal assessment of stability of sensory features in children with autism spectrum disorder or other developmental disabilities. Autism Res. 2019, 12, 100–111. [Google Scholar] [CrossRef] [Green Version]


| Item | Domain | % Endorsed |
|---|---|---|
| Does your child appear fascinated with certain textures (e.g., the feel of certain objects, water, a person’s skin)? | Tactile Seeking | 95.24% |
| Does your child enjoy seeking pressure or bump or crush into objects (e.g., walls, furniture) or people? | Tactile Seeking | 85.71% |
| Does your child use objects or his/her voice to create sounds outside of the context of functional play (e.g., banging toys together, repetitive sounds)? | Auditory Seeking | 85.71% |
| Does your child notice hot or cold temperatures (e.g., hot bath, ice) and pain (e.g., getting a shot, hurting self)? (reverse coded) | Tactile Hyporeactivity | 80.95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siper, P.M.; Layton, C.; Levy, T.; Lurie, S.; Benrey, N.; Zweifach, J.; Rowe, M.; Tang, L.; Guillory, S.; Halpern, D.; et al. Sensory Reactivity Symptoms Are a Core Feature of ADNP Syndrome Irrespective of Autism Diagnosis. Genes 2021, 12, 351. https://doi.org/10.3390/genes12030351
Siper PM, Layton C, Levy T, Lurie S, Benrey N, Zweifach J, Rowe M, Tang L, Guillory S, Halpern D, et al. Sensory Reactivity Symptoms Are a Core Feature of ADNP Syndrome Irrespective of Autism Diagnosis. Genes. 2021; 12(3):351. https://doi.org/10.3390/genes12030351
Chicago/Turabian StyleSiper, Paige M., Christina Layton, Tess Levy, Stacey Lurie, Nurit Benrey, Jessica Zweifach, Mikaela Rowe, Lara Tang, Sylvia Guillory, Danielle Halpern, and et al. 2021. "Sensory Reactivity Symptoms Are a Core Feature of ADNP Syndrome Irrespective of Autism Diagnosis" Genes 12, no. 3: 351. https://doi.org/10.3390/genes12030351







