Expansion and Diversification of Fluorescent Protein Genes in Fifteen Acropora Species during the Evolution of Acroporid Corals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genomic Data and Surveys of Fluorescent Protein (FP) Genes
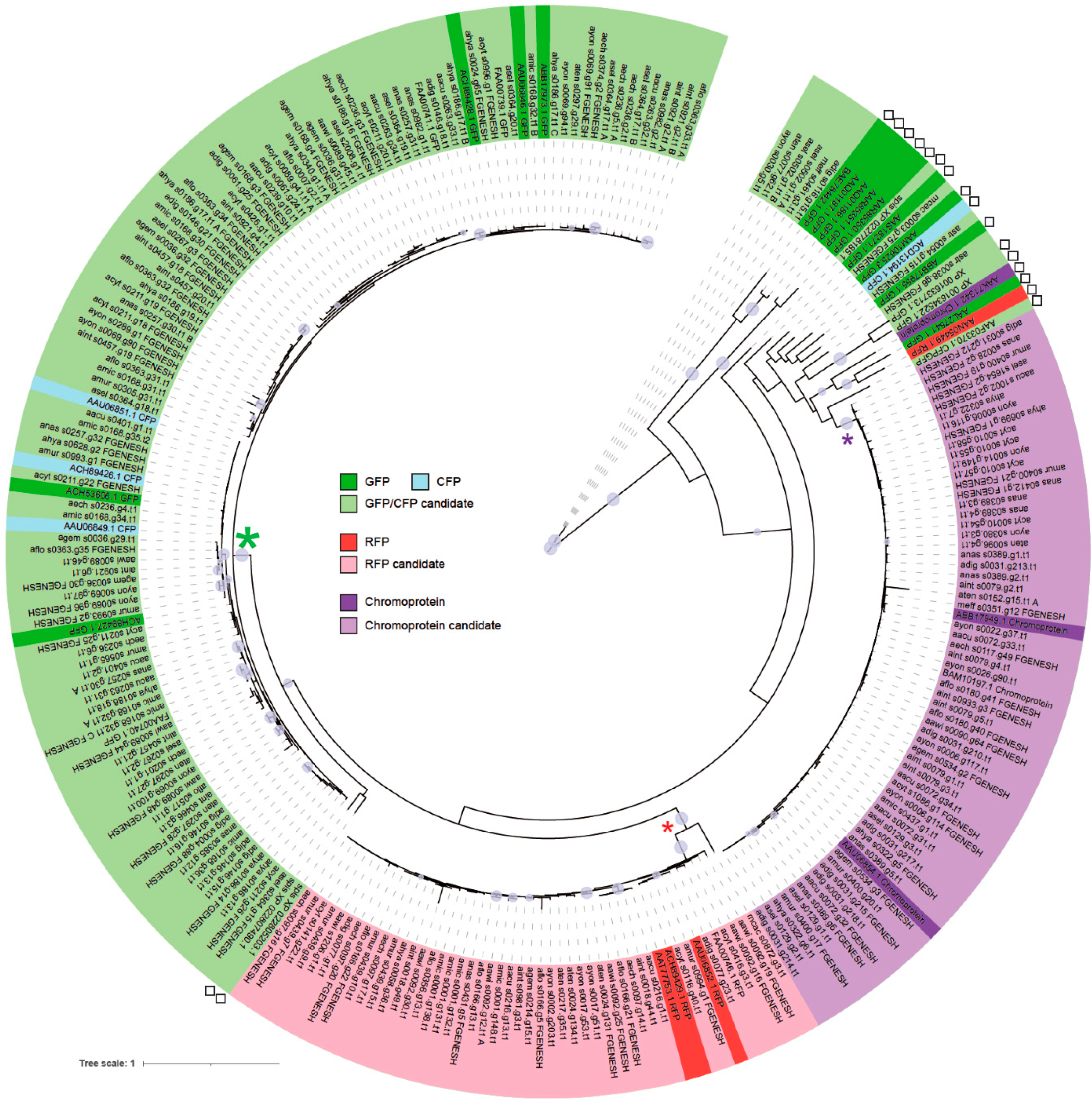
2.2. Molecular Phylogenetic Analysis
| Accession Number and Definition in GenBank | Colored Light Emitted in Spectroscopic Analysis |
|---|---|
| AAU06846.1 green fluorescent protein [Acropora millepora] | Green [1] |
| FAA00739.1 TPA: fluorescent protein 2 [Acropora digitifera] | |
| ABB17973.1 green fluorescent GFP-like protein [Acropora millepora] | Green [1] |
| ACH89428.1 green fluorescent protein FP512 [Acropora millepora] | Green [43] |
| FAA00741.1 TPA: fluorescent protein 4 [Acropora digitifera] | |
| FAA00743.1 TPA: fluorescent protein 6, partial [Acropora digitifera] | |
| FAA00742.1 TPA: fluorescent protein 5, partial [Acropora digitifera] | |
| AAU06851.1 cyan fluorescent protein 2 [Acropora robusta] | Cyan [1] |
| FAA00738.1 TPA: fluorescent protein 1 [Acropora digitifera] | |
| ACH53606.1 green fluorescent-like protein, partial [Acropora millepora] | Green [44] |
| ACH89426.1 cyan fluorescent protein FP484 [Acropora millepora] | Cyan [1] |
| AAU06849.1 cyan fluorescent protein [Acropora millepora] | Cyan [1] |
| ACH89427.1 green fluorescent protein FP497 [Acropora millepora] | Green [44] |
| FAA00740.1 TPA: fluorescent protein 3 [Acropora digitifera] | |
| AAS18271.1 green fluorescent protein 2 [Astrangia lajollaensis] | Green [45] |
| AAT77753.1 colorless GFP-like protein [Acropora millepora] | Red [1] |
| ACH53607.1 red fluorescent-like protein, partial [Acropora millepora] | Red [1] |
| ACH89429.1 red fluorescent protein FP597 [Acropora millepora] | Red [1] |
| AAU06852.1 red fluorescent protein [Acropora millepora] | Red [1] |
| FAA00746.1 TPA: fluorescent protein 10 [Acropora digitifera] | |
| ACD13194.1 green fluorescent GFP-like protein [Platygyra lamellina] | Cyan [1] |
| ABB17955.1 cyan fluorescent GFP-like protein [Mycedium elephantotus] | Green [1] |
| AAM10625.3 green fluorescent protein [Dendronephthya sp. SSAL-2002] | Green [46] |
| ABB17949.1 GFP-like chromoprotein [Goniopora djiboutiensis] | Non-fluorescent [1] |
| FAA00744.1 TPA: fluorescent protein 7, partial [Acropora digitifera] | |
| FAA00745.1 TPA: fluorescent protein 9, partial [Acropora digitifera] | |
| BAM10197.1 fluorescent protein 8 [Acropora digitifera] | |
| AAU06854.1 chromoprotein [Acropora millepora] | Non-fluorescent [1] |
| AAG16224.1 red fluorescent protein [Discosoma sp. SSAL-2000] | Red [47] |
| AAF03370.1 fluorescent protein FP483 [Discosoma striata] | |
| XP_001634522.1 predicted protein [Nematostella vectensis] | |
| XP_001633713.1 predicted protein [Nematostella vectensis] | |
| AAN05449.1 red fluorescent protein FP611 [Entacmaea quadricolor] | Red [48] |
| AAL27541.1 GFP-like chromoprotein [Condylactis passiflora] | Green [49] |
| AAK71342.1 cgigFP-g [Condylactis gigantea] | Non-fluorescent [46] |
| AAQ01187.1 green fluorescent protein 2 [Pontella meadi] | Green [50] |
| AAQ01186.1 green fluorescent protein 1 [Pontella meadi] | Green [50] |
| BAE78442.1 green fluorescent protein [Chiridius poppei] | Green [51] |
| AAR85351.1 green fluorescent protein 2 [Anthomedusae sp. SL-2003] | Green [50] |
| AAR85350.1 green fluorescent protein 1 [Anthomedusae sp. SL-2003] | Green [50] |
2.3. Classification into GFP/CFP, RFP, and ChrP Groups and Chromophore Sequences
2.4. Gene Duplication and Gene Loss Analysis
2.5. Synteny Analysis

3. Results
3.1. Fluorescent Protein Genes in 18 Coral Species
3.2. Emission Color Prediction of Fluorescent Proteins by Molecular Phylogenetic Analysis
3.3. Expansion of the Number of Fluorescent Protein Genes in Acropora
3.4. Localization of Fluorescent Protein Genes in Acropora Genomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alieva, N.O.; Konzen, K.A.; Field, S.F.; Meleshkevitch, E.A.; Hunt, M.E.; Beltran-Ramirez, V.; Miller, D.J.; Wiedenmann, J.; Salih, A.; Matz, M.V. Diversity and evolution of coral fluorescent proteins. PLoS ONE 2008, 3. [Google Scholar] [CrossRef] [Green Version]
- Dove, S.G.; Hoegh-Guldberg, O.; Ranganathan, S. Major colour patterns of reef-building corals are due to a family of GFP-like proteins. Coral Reefs 2001, 19, 197–204. [Google Scholar] [CrossRef]
- Mazel, C.H.; Lesser, M.P.; Gorbunov, M.Y.; Barry, T.M.; Farrell, J.H.; Wyman, K.D.; Falkowski, P.G. Green-fluorescent proteins in Caribbean corals. Limnol. Oceanogr. 2003, 48, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Matz, M.V.; Marshall, N.J.; Vorobyev, M. Are Corals Colorful? Photochem. Photobiol. 2006, 82, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, C.; Shoguchi, E.; Tanaka, M.; Satoh, N. Fluorescent protein candidate genes in the coral Acropora digitifera genome. Zool. Sci. 2012, 29, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef]
- Kelmanson, I.V.; Matz, M.V. Molecular basis and evolutionary origins of color diversity in great star coral montastraea cavernosa (Scleractinia: Faviida). Mol. Biol. Evol. 2003, 20, 1125–1133. [Google Scholar] [CrossRef] [Green Version]
- Field, S.F.; Bulina, M.Y.; Kelmanson, I.V.; Bielawski, J.P.; Matz, M.V. Adaptive evolution of multicolored fluorescent proteins in reef-building corals. J. Mol. Evol. 2006, 62, 332–339. [Google Scholar] [CrossRef]
- Prasher, D.C.; Eckenrode, V.K.; Ward, W.W.; Prendergast, F.G.; Cormier, M.J. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 1992, 111, 229–233. [Google Scholar] [CrossRef]
- Mazel, C.H. Coral fluorescence characteristics: Excitation/emmission spectra, fluorescence efficiences, and ontributi to apparent reflectance. In Proceedings of the one Ocean Optics XIII; International Society for Optics and Photonics: Bellingham, WA, USA, 1997; Volume 2963, pp. 240–245. [Google Scholar]
- Morin, J.G.; Hastings, J.W. Energy transfer in a bioluminescent system. J. Cell Physiol. 1971, 77, 313–318. [Google Scholar] [CrossRef]
- Ward, W.W.; Cody, C.W.; Hart, R.C.; Cormier, M.J. Spectrophotometric identity of the energy transfer chromophores in renilla and aequorea green-fluorescent proteins. Photochem. Photobiol. 1980, 31, 611–615. [Google Scholar] [CrossRef]
- Henderson, J.N.; Remington, S.J. Crystal structures and mutational analysis of amFP486, a cyan fluorescent protein from Anemonia majano. Proc. Natl. Acad. Sci. USA 2005, 102, 12712–12717. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Nakamura, T. Mass coral bleaching event in Sekisei lagoon observed in the summer of 2016. J. Jpn. Coral Reef Soc. 2017, 19, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Head, C.E.I.; Bayley, D.T.I.; Rowlands, G.; Roche, R.C.; Tickler, D.M.; Rogers, A.D.; Koldewey, H.; Turner, J.R.; Andradi-Brown, D.A. Coral bleaching impacts from back-to-back 2015–2016 thermal anomalies in the remote central Indian Ocean. Coral Reefs 2019, 38, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Perry, C.T.; Alvarez-Filip, L.; Graham, N.A.J.; Mumby, P.J.; Wilson, S.K.; Kench, P.S.; Manzello, D.P.; Morgan, K.M.; Slangen, A.B.A.; Thomson, D.P.; et al. Loss of coral reef growth capacity to track future increases in sea level. Nature 2018, 558, 396–400. [Google Scholar] [CrossRef]
- Wallace, C.C. Staghorn Corals of the World: A Revision of the Coral Genus Acropora (Scleractinia; Astrocoeniina; Acroporidae) Worldwide, with Emphasis on Morphology, Phylogeny and Biogeography; CSIRO Publishing: Collingwood, Australia, 1999; ISBN 978-0-643-06391-4. [Google Scholar]
- Loya, Y.; Sakai, K.; Yamazato, K.; Nakano, Y.; Sambali, H.; Woesik, R. van Coral bleaching: The winners and the losers. Ecol. Lett. 2001, 4, 122–131. [Google Scholar] [CrossRef]
- Shinzato, C.; Shoguchi, E.; Kawashima, T.; Hamada, M.; Hisata, K.; Tanaka, M.; Fujie, M.; Fujiwara, M.; Koyanagi, R.; Ikuta, T.; et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 2011, 476, 320–323. [Google Scholar] [CrossRef]
- Shinzato, C.; Khalturin, K.; Inoue, J.; Zayasu, Y.; Kanda, M.; Kawamitsu, M.; Yoshioka, Y.; Yamashita, H.; Suzuki, G.; Satoh, N. Eighteen coral genomes reveal the evolutionary origin of Acropora strategies to accommodate environmental changes. Mol. Biol. Evol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bollati, E.; D’Angelo, C.; Alderdice, R.; Pratchett, M.; Ziegler, M.; Wiedenmann, J. Optical Feedback Loop Involving Dinoflagellate Symbiont and Scleractinian Host Drives Colorful Coral Bleaching. Curr. Biol. 2020, 30, 2433–2445.e3. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.; Larkum, A.; Cox, G.; Kühl, M.; Hoegh-Guldberg, O. Fluorescent pigments in corals are photoprotective. Nature 2000, 408, 850–853. [Google Scholar] [CrossRef]
- Palmer, C.V.; Modi, C.K.; Mydlarz, L.D. Coral fluorescent proteins as antioxidants. PLoS ONE 2009, 4, e7298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, M.S. The engine of the reef: Photobiology of the coral–algal symbiosis. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Bou-Abdallah, F.; Chasteen, N.D.; Lesser, M.P. Quenching of Superoxide Radicals by Green Fluorescent Protein. Biochim. Biophys. Acta 2006, 1760, 1690–1695. [Google Scholar] [CrossRef] [Green Version]
- Aihara, Y.; Maruyama, S.; Baird, A.H.; Iguchi, A.; Takahashi, S.; Minagawa, J. Green fluorescence from cnidarian hosts attracts symbiotic algae. Proc. Natl. Acad. Sci. USA 2019, 116, 2118–2123. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Kariyazono, S.; Sakai, K.; Terai, Y. Presence–absence polymorphisms of highly expressed fp sequences contribute to fluorescent polymorphisms in acropora digitifera. Genome Biol. Evol. 2018, 10, 1715–1729. [Google Scholar] [CrossRef]
- Takahashi-Kariyazono, S.; Gojobori, J.; Satta, Y.; Sakai, K.; Terai, Y. Acropora digitifera encodes the largest known family of fluorescent proteins that has persisted during the evolution of Acropora species. Genome Biol. Evol. 2016, 8, 3271–3283. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, V.; Talke, I.N.; Weber, M.; Eils, R.; Brors, B.; Clemens, S.; Krämer, U. Between-species differences in gene copy number are enriched among functions critical for adaptive evolution in arabidopsis halleri. BMC Genom. 2016, 17, 1034. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Kariyazono, S.; Satta, Y.; Terai, Y. Genetic diversity of fluorescent protein genes generated by gene duplication and alternative splicing in reef-building corals. Zool. Lett. 2015, 1, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, N.; Kinjo, K.; Shintaku, K.; Kezuka, D.; Ishimori, H.; Yokokura, A.; Hagiwara, K.; Hisata, K.; Kawamitsu, M.; Koizumi, K.; et al. Color morphs of the coral, Acropora tenuis, show different responses to environmental stress and different expression profiles of fluorescent-protein genes. G3 Genes|Genomes|Genetics 2021, 11, jkab018. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, R.; Takeuchi, T.; Hisata, K.; Gyoja, F.; Shoguchi, E.; Satoh, N.; Kawashima, T. MarinegenomicsDB: An integrated genome viewer for community-based annotation of genomes. Zool. Sci. 2013, 30, 797–800. [Google Scholar] [CrossRef] [PubMed]
- OIST Marine Genomics Unit Genome Browser. Available online: https://marinegenomics.oist.jp/aten/viewer/download?project_id=97 (accessed on 10 February 2020).
- Solovyev, V.; Kosarev, P.; Seledsov, I.; Vorobyev, D. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 2006, 7, S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Gouy, M.; Guindon, S.; Gascuel, O. Seaview version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- FigTree. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 28 October 2020).
- Ciccarelli, F.D.; Doerks, T.; von Mering, C.; Creevey, C.J.; Snel, B.; Bork, P. Toward automatic reconstruction of a highly resolved tree of life. Science 2006, 311, 1283–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voolstra, C.R.; Li, Y.; Liew, Y.J.; Baumgarten, S.; Zoccola, D.; Flot, J.-F.; Tambutté, S.; Allemand, D.; Aranda, M. Comparative analysis of the genomes of Stylophora pistillata and Acropora digitifera provides evidence for extensive differences between species of corals. Sci. Rep. 2017, 7, 17583. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, C.; Denzel, A.; Vogt, A.; Matz, M.V.; Oswald, F.; Salih, A.; Nienhaus, G.U.; Wiedenmann, J. Blue light regulation of host pigment in reef-building corals. Mar. Ecol. Prog. Ser. 2008, 364, 97–106. [Google Scholar] [CrossRef]
- Smith-Keune, C.; Dove, S. Gene expression of a green fluorescent protein homolog as a host-specific biomarker of heat stress within a reef-building coral. Mar. Biotechnol. 2008, 10, 166–180. [Google Scholar] [CrossRef]
- Bessette, P.H.; Daugherty, P.S. Flow cytometric screening of cDNA expression libraries for fluorescent proteins. Biotechnol. Prog. 2004, 20, 963–967. [Google Scholar] [CrossRef]
- Labas, Y.A.; Gurskaya, N.G.; Yanushevich, Y.G.; Fradkov, A.F.; Lukyanov, K.A.; Lukyanov, S.A.; Matz, M.V. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA 2002, 99, 4256–4261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fradkov, A.F.; Chen, Y.; Ding, L.; Barsova, E.V.; Matz, M.V.; Lukyanov, S.A. Novel fluorescent protein from Discosoma coral and its mutants possesses a unique far-red fluorescence. FEBS Lett. 2000, 479, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmann, J.; Schenk, A.; Röcker, C.; Girod, A.; Spindler, K.-D.; Nienhaus, G.U. A far-red fluorescent protein with fast maturation and reduced oligomerization tendency from Entacmaea quadricolor (Anthozoa, Actinaria). Proc. Natl. Acad. Sci. USA 2002, 99, 11646–11651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurskaya, N.G.; Fradkov, A.F.; Terskikh, A.; Matz, M.V.; Labas, Y.A.; Martynov, V.I.; Yanushevich, Y.G.; Lukyanov, K.A.; Lukyanov, S.A. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Lett. 2001, 507, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Takenaka, Y.; Yamaguchi, A.; Nishikawa, S.; Mizuno, H. A novel yellowish-green fluorescent protein from the marine copepod, Chiridius poppei, and its use as a reporter protein in HeLa cells. Gene 2006, 372, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shagin, D.A.; Barsova, E.V.; Yanushevich, Y.G.; Fradkov, A.F.; Lukyanov, K.A.; Labas, Y.A.; Semenova, T.N.; Ugalde, J.A.; Meyers, A.; Nunez, J.M.; et al. GFP-like proteins as ubiquitous metazoan superfamily: Evolution of functional features and structural complexity. Mol. Biol. Evol. 2004, 21, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Notung. Available online: http://www.cs.cmu.edu/~durand/Notung/ (accessed on 28 October 2020).
- Chen, K.; Durand, D.; Farach-Colton, M. NOTUNG: A program for dating gene duplications and optimizing gene family trees. J. Comput. Biol. 2000, 7, 429–447. [Google Scholar] [CrossRef]
- Skinner, M.E.; Uzilov, A.V.; Stein, L.D.; Mungall, C.J.; Holmes, I.H. JBrowse: A next-generation genome browser. Genome Res. 2009, 19, 1630–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- RefSeq: NCBI Reference Sequence Database. Available online: https://www.ncbi.nlm.nih.gov/refseq/ (accessed on 28 October 2020).
- Ovcharenko, I.; Loots, G.; Hardison, R.; Miller, W.; Stubbs, L. zPicture: Dynamic alignment and visualization tool for analyzing conservation profiles. Genome Res. 2004, 14, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- zPicture: Dynamic Blastz Alignment Visualization. Available online: https://zpicture.dcode.org/ (accessed on 12 February 2021).
- Olde, K.; Jarvis, I.; Uličný, D.; Pearce, M.A.; Trabucho-Alexandre, J.; Čech, S.; Gröcke, D.R.; Laurin, J.; Švábenická, L.; Tocher, B.A. Geochemical and palynological sea-level proxies in hemipelagic sediments: A critical assessment from the Upper Cretaceous of the Czech Republic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 435, 222–243. [Google Scholar] [CrossRef] [Green Version]
- Cramwinckel, M.J.; Huber, M.; Kocken, I.J.; Agnini, C.; Bijl, P.K.; Bohaty, S.M.; Frieling, J.; Goldner, A.; Hilgen, F.J.; Kip, E.L.; et al. Synchronous tropical and polar temperature evolution in the Eocene. Nature 2018, 559, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Prag, S.; Adams, J.C. Molecular phylogeny of the kelch-repeat superfamily reveals an expansion of BTB/kelch proteins in animals. BMC Bioinform. 2003, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Tautz, D.; Domazet-Lošo, T. The evolutionary origin of orphan genes. Nat. Rev. Genet. 2011, 12, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Gittins, J.R.; D’Angelo, C.; Oswald, F.; Edwards, R.J.; Wiedenmann, J. Fluorescent protein-mediated colour polymorphism in reef corals: Multicopy genes extend the adaptation/acclimatization potential to variable light environments. Mol. Ecol. 2015, 24, 453–465. [Google Scholar] [CrossRef] [Green Version]


| Coral Species | GFP/CFP | RFP | ChrP | FP Total |
|---|---|---|---|---|
| Astrepora myriophthalama | 2 | 0 | 0 | 2 |
| Montipora efflorescens | 1 | 0 | 1 | 2 |
| Montipora cactus | 1 | 1 | 0 | 2 |
| A. tenuis (I) 1 | 4 | 3 | 2 | 9 |
| A. yongei (I) | 8 | 3 | 7 | 18 |
| A. intermedia (II) | 9 | 3 | 6 | 18 |
| A. gemmifera (II) | 6 | 1 | 2 | 9 |
| A. awi (II) | 4 | 6 | 1 | 11 |
| A. florida (II) | 7 | 5 | 2 | 14 |
| A. digitifera (III) | 9 | 2 | 7 | 18 |
| A. nasuta (III) | 7 | 1 | 8 | 16 |
| A. microphthalma (III) | 8 | 4 | 1 | 13 |
| A. acuminata (III) | 7 | 2 | 5 | 14 |
| A. echinata (IV) | 7 | 4 | 1 | 12 |
| A. muricata (IV) | 4 | 5 | 4 | 13 |
| A. selago (IV) | 11 | 0 | 4 | 15 |
| A. cytherea (IV) | 9 | 3 | 5 | 17 |
| A. hyacinthus (IV) | 11 | 1 | 4 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashimoto, R.; Hisata, K.; Shinzato, C.; Satoh, N.; Shoguchi, E. Expansion and Diversification of Fluorescent Protein Genes in Fifteen Acropora Species during the Evolution of Acroporid Corals. Genes 2021, 12, 397. https://doi.org/10.3390/genes12030397
Kashimoto R, Hisata K, Shinzato C, Satoh N, Shoguchi E. Expansion and Diversification of Fluorescent Protein Genes in Fifteen Acropora Species during the Evolution of Acroporid Corals. Genes. 2021; 12(3):397. https://doi.org/10.3390/genes12030397
Chicago/Turabian StyleKashimoto, Rio, Kanako Hisata, Chuya Shinzato, Noriyuki Satoh, and Eiichi Shoguchi. 2021. "Expansion and Diversification of Fluorescent Protein Genes in Fifteen Acropora Species during the Evolution of Acroporid Corals" Genes 12, no. 3: 397. https://doi.org/10.3390/genes12030397
APA StyleKashimoto, R., Hisata, K., Shinzato, C., Satoh, N., & Shoguchi, E. (2021). Expansion and Diversification of Fluorescent Protein Genes in Fifteen Acropora Species during the Evolution of Acroporid Corals. Genes, 12(3), 397. https://doi.org/10.3390/genes12030397







