KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Genetic Analysis
2.3. Expression Plasmids and Site-Directed Mutagenesis
2.4. Cell Culture, Transfection and Dual-Luciferase Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics of the Study Subjects
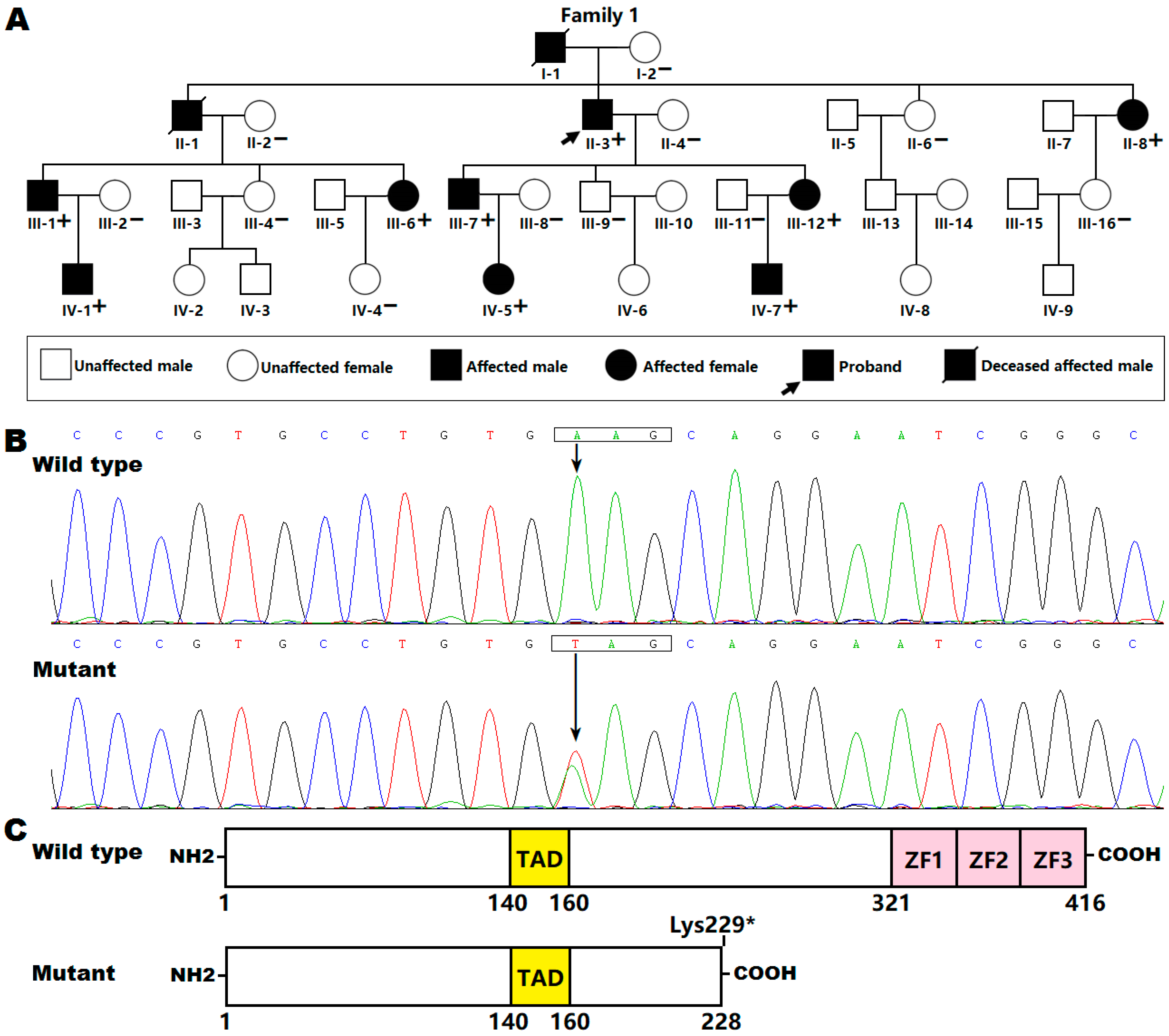
3.2. Identification of a New KLF15 Variation
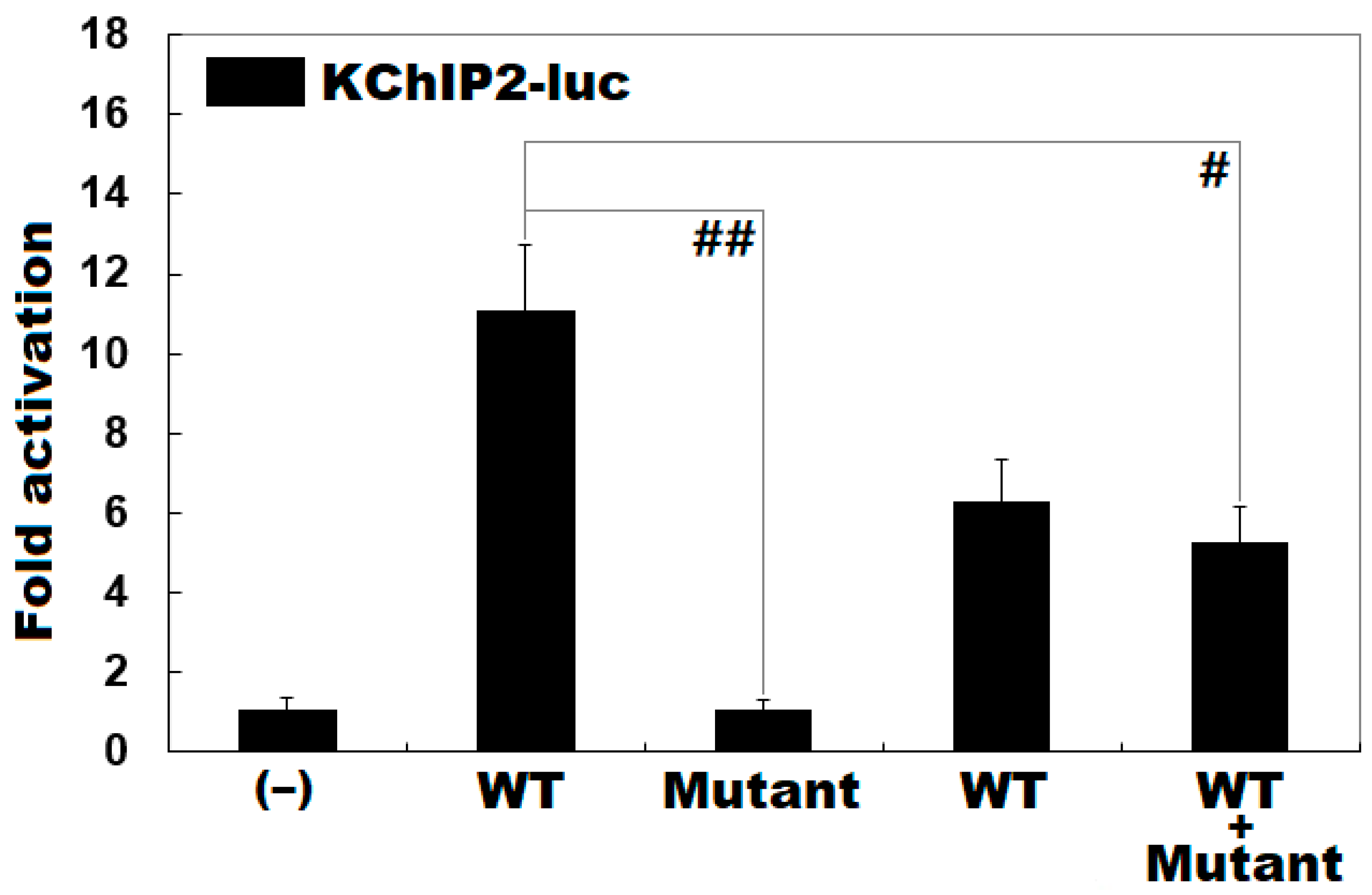
3.3. Functional Loss of the Mutant KLF15 Protein
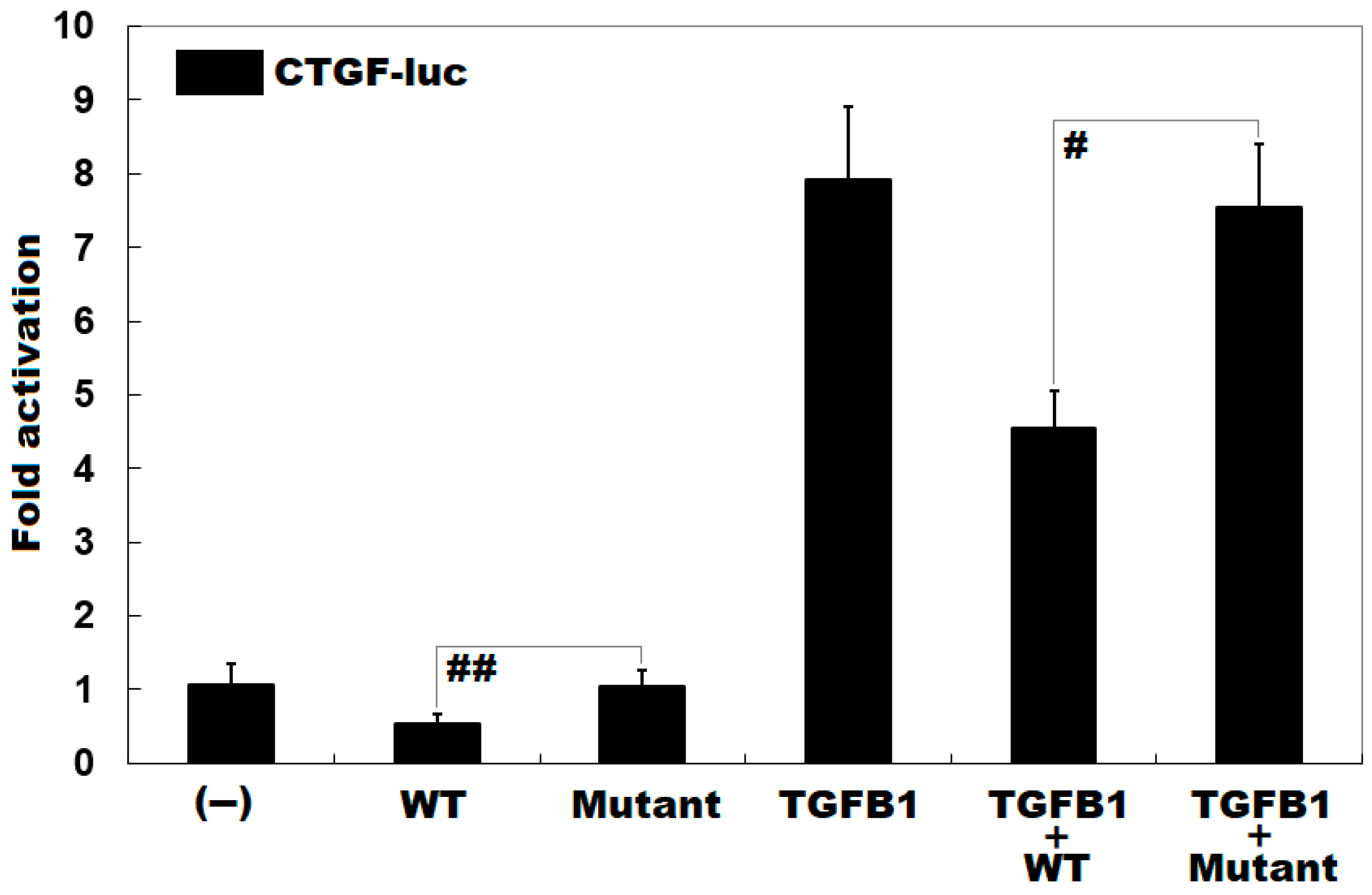
3.4. Diminished Inhibitory Effect of KLF15 on CTGF by the Mutation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Alkhouli, M.; Friedman, P.A. Ischemic stroke risk in patients with nonvalvular atrial fibrillation: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 3050–3065. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Kajiyama, T.; Yamao, K.; Hada, M.; Yamaguchi, M.; Nakamura, H.; Hachiya, H.; Tada, H.; Hirao, K.; Iesaka, Y. Silent cerebral events/lesions after second-generation cryoballoon ablation: How can we reduce the risk of silent strokes? Heart Rhythm 2019, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Graves, K.G.; May, H.T.; Jacobs, V.; Knowlton, K.U.; Muhlestein, J.B.; Lappe, D.L.; Anderson, J.L.; Horne, B.D.; Bunch, T.J. CHA(2)DS(2)-VASc scores and Intermountain Mortality Risk Scores for the joint risk stratification of dementia among patients with atrial fibrillation. Heart Rhythm 2019, 16, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Bunch, T.J.; Bair, T.L.; Crandall, B.G.; Cutler, M.J.; Day, J.D.; Graves, K.G.; Jacobs, V.; Mallender, C.; Osborn, J.S.; Weiss, J.P.; et al. Stroke and dementia risk in patients with and without atrial fibrillation and carotid arterial disease. Heart Rhythm 2020, 17, 20–26. [Google Scholar] [CrossRef]
- Bunch, T.J. Atrial Fibrillation and Dementia. Circulation 2020, 142, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Ochi, H.; Sairaku, A.; Onohara, Y.; Tokuyama, T.; Motoda, C.; Matsumura, H.; Tomomori, S.; Amioka, M.; Hi-ronobe, N.; et al. HCN4 gene polymorphisms are associated with occurrence of tachycardia-induced cardiomyopathy in patients with atrial fibrillation. Circ. Genom. Precis. Med. 2018, 11, e001980. [Google Scholar] [CrossRef]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Hickey, K.T.; Colombo, P.C.; Naka, Y.; Garan, A.R.; Yuzefpolskaya, M.; Garan, H.; Wan, E.Y.; Sciacca, R.R.; Goldenthal, I.; Biviano, A.B. Atrial Fibrillation Is Associated with Recurrent Ventricular Arrhythmias After LVAD Implant: Incidence and Impact in a Consecutive Series. J. Cardiovasc. Transl. Res. 2020, 13, 199–203. [Google Scholar] [CrossRef]
- Bassand, J.-P.; Virdone, S.; Goldhaber, S.Z.; Camm, A.J.; Fitzmaurice, D.A.; Fox, K.A.; Goto, S.; Haas, S.; Hacke, W.; Kayani, G.; et al. Early Risks of Death, Stroke/Systemic Embolism, and Major Bleeding in Patients With Newly Diagnosed Atrial Fibrillation. Circulation 2019, 139, 787–798. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Wenzel, P.; Münzel, T.; Espinola-Klein, C.; Ostad, M.A. Impact of atrial fibrillation/flutter on the in-hospital mortality of ischemic stroke patients. Heart Rhythm 2020, 17, 383–390. [Google Scholar] [CrossRef]
- Keller, K.; Hobohm, L.; Engelhardt, M. Impact of atrial fibrillation/flutter on the in-hospital mortality of surgical patients—Results from the German nationwide cohort. Thromb. Res. 2020, 196, 526–535. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.; Nattel, S.; Kalman, J.M.; Sanders, P. Modifiable Risk Factors and Atrial Fibrillation. Circulation 2017, 136, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Rosian, A.N.; Rosian, S.H.; Kiss, B.; Stefan, M.G.; Trifa, A.P.; Ober, C.D.; Anchidin, O.; Buzoianu, A.D. Interindividual varia-bility of apixaban plasma concentrations: Influence of clinical and genetic factors in a real-life cohort of atrial fibrillation patients. Genes 2020, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Rienstra, M.; Ellinor, P.T. Genetics of atrial fibrillation in 2020: GWAS, genome sequencing, polygenic risk, and beyond. Circ. Res. 2020, 127, 21–33. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Zhang, J.; Wang, X.; Li, Z.; Li, G. The molecular genetic basis of atrial fibrillation. Qual. Life Res. 2020, 139, 1–14. [Google Scholar] [CrossRef]
- Ragab, A.A.Y.; Sitorus, G.D.S.; Brundel, B.B.J.J.M.; De Groot, N.M.S. The Genetic Puzzle of Familial Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Brugada, R.; Tapscott, T.; Czernuszewicz, G.Z.; Marian, A.; Iglesias, A.; Mont, L.; Brugada, J.; Girona, J.; Domingo, A.; Bachinski, L.L.; et al. Identification of a Genetic Locus for Familial Atrial Fibrillation. N. Engl. J. Med. 1997, 336, 905–911. [Google Scholar] [CrossRef]
- Chen, Y.H.; Xu, S.J.; Bendahhou, S.; Wang, X.L.; Wang, Y.; Xu, W.Y.; Jin, H.W.; Sun, H.; Su, X.Y.; Zhuang, Q.N.; et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 2003, 299, 251–254. [Google Scholar] [CrossRef]
- Yamada, N.; Asano, Y.; Fujita, M.; Yamazaki, S.; Inanobe, A.; Matsuura, N.; Kobayashi, H.; Ohno, S.; Ebana, Y.; Tsukamoto, O.; et al. Mutant KCNJ3 and KCNJ5 Potassium Channels as Novel Molecular Targets in Bradyarrhythmias and Atrial Fibrillation. Circulation 2019, 139, 2157–2169. [Google Scholar] [CrossRef]
- Van Ouwerkerk, A.F.; Bosada, F.M.; Van Duijvenboden, K.; Hill, M.C.; Montefiori, L.E.; Scholman, K.T.; Liu, J.; De Vries, A.A.F.; Boukens, B.J.; Ellinor, P.T.; et al. Identification of atrial fibrillation associated genes and functional non-coding variants. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Menon, A.; Hong, L.; Savio-Galimberti, E.; Sridhar, A.; Youn, S.-W.; Zhang, M.; Kor, K.; Blair, M.; Kupershmidt, S.; Darbar, D. Electrophysiologic and molecular mechanisms of a frameshift NPPA mutation linked with familial atrial fibrillation. J. Mol. Cell. Cardiol. 2019, 132, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, M.; Cheng, C.; Huang, Y.; Han, S.; Li, W.; Tu, X.; Luo, X.; Yu, X.; Liu, Y.; et al. Lamin A mutation impairs interaction with nucleoporin NUP155 and disrupts nucleocytoplasmic transport in atrial fibrillation. Hum. Mutat. 2019, 40, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Goodyer, W.R.; Dunn, K.; Caleshu, C.; Jackson, M.; Wylie, J.; Moscarello, T.; Platt, J.; Reuter, C.; Smith, A.; Trela, A.; et al. Broad genetic testing in a clinical setting uncovers a high prevalence of Titin loss-of-function variants in very early onset atrial fibrillation. Circ. Genom. Precis. Med. 2019, 12, e002713. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, Z.; Kiviniemi, T.; Olafsson, S.; Plotnick, D.; Beerens, M.E.; Zhang, K.; Gillon, L.; Steinbaugh, M.J.; Barrera, V.; Sui, S.H.; et al. Metastable Atrial State Underlies the Primary Genetic Substrate for MYL4 Mutation-Associated Atrial Fibrillation. Circulation 2020, 141, 301–312. [Google Scholar] [CrossRef]
- Choi, S.H.; Jurgens, S.J.; Weng, L.C.; Pirruccello, J.P.; Roselli, C.; Chaffin, M.; Lee, C.J.; Hall, A.W.; Khera, A.V.; Lunetta, K.L.; et al. Monogenic and polygenic contributions to atrial fibrillation risk: Results from a national biobank. Circ. Res. 2020, 126, 200–209. [Google Scholar] [CrossRef]
- Wang, B.; Lunetta, K.L.; Dupuis, J.; Lubitz, S.A.; Trinquart, L.; Yao, L.; Ellinor, P.T.; Benjamin, E.J.; Lin, H. Integrative Omics Approach to Identifying Genes Associated with Atrial Fibrillation. Circ. Res. 2020, 126, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Van Ouwerkerk, A.F.; Bosada, F.M.; Liu, J.; Zhang, J.; van Duijvenboden, K.; Chaffin, M.; Tucker, N.R.; Pijnappels, D.; Ellinor, P.T.; Barnett, P.; et al. Identification of functional variant enhancers associated with atrial fibrillation. Circ. Res. 2020, 127, 229–243. [Google Scholar] [CrossRef]
- Van Ouwerkerk, A.F.; Hall, A.W.; Kadow, Z.A.; Lazarevic, S.; Reyat, J.S.; Tucker, N.R.; Nadadur, R.D.; Bosada, F.M.; Bianchi, V.; Ellinor, P.T.; et al. Epigenetic and transcriptional networks underlying atrial fibrillation. Circ. Res. 2020, 127, 34–50. [Google Scholar] [CrossRef]
- Hansen, T.H.; Yan, Y.; Ahlberg, G.; Vad, O.B.; Refsgaard, L.; Dos Santos, J.L.; Mutsaers, N.; Svendsen, J.H.; Olesen, M.S.; Bentzen, B.H.; et al. A novel loss-of-function variant in the chloride ion channel gene Clcn2 associates with atrial fibrillation. Sci. Rep. 2020, 10, 1453. [Google Scholar] [CrossRef] [Green Version]
- Vad, O.B.; Paludan-Müller, C.; Ahlberg, G.; Kalstø, S.M.; Ghouse, J.; Andreasen, L.; Haunsø, S.; Tveit, A.; Sajadieh, A.; Christophersen, I.E.; et al. Loss-of-Function Variants in Cytoskeletal Genes Are Associated with Early-Onset Atrial Fibrillation. J. Clin. Med. 2020, 9, 372. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.-H.; Wang, X.-H.; Xu, Y.-J.; Gu, J.-N.; Yang, C.-X.; Qiao, Q.; Guo, X.-J.; Guo, Y.-H.; Qiu, X.-B.; Jiang, W.-F.; et al. ISL1 loss-of-function variation causes familial atrial fibrillation. Eur. J. Med Genet. 2020, 63, 104029. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, S.M.; Benson, C.E.; Khan, M.A.; Berger, R.M.F.; Trembath, R.C.; Machado, R.D.; Southgate, L. Whole exome sequence analysis provides novel insights into the genetic framework of childhood-onset pulmonary arterial hypertension. Genes 2020, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Jorholt, J.; Formicheva, Y.; Vershinina, T.; Kiselev, A.; Muravyev, A.; Demchenko, E.; Fedotov, P.; Zlotina, A.; Rygkov, A.; Vasichkina, E.; et al. Two new cases of hypertrophic cardiomyopathy and skeletal muscle features associated with ALPK3 homozygous and compound heterozygous variants. Genes 2020, 11, 1201. [Google Scholar] [CrossRef]
- Ramzan, K.; Al-Numair, N.S.; Al-Ageel, S.; Elbaik, L.; Sakati, N.; Al-Hazzaa, S.A.F.; Al-Owain, M.; Imtiaz, F. Identification of Novel CDH23 Variants Causing Moderate to Profound Progressive Nonsyndromic Hearing Loss. Genes 2020, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, C.; Wang, Z.; Sun, L.; Li, S.; Zhang, T.; Luo, X.; Ding, X. Mutation Spectrum and De Novo Mutation Analysis in Stickler Syndrome Patients with High Myopia or Retinal Detachment. Genes 2020, 11, 882. [Google Scholar] [CrossRef]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nat. Cell Biol. 2012, 483, 96–99. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-β signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, J.; Ikeda, Y.; Iguchi, H.; Fujino, T.; Tanaka, T.; Asaba, H.; Iwasaki, S.; Ioka, R.X.; Kaneko, I.W.; Magoori, K.; et al. A Kruppel-like factor KLF15 contributes fasting-induced transcriptional activation of mitochondrial acetyl-CoA synthetase gene AceCS2. J. Biol. Chem. 2004, 279, 16954–16962. [Google Scholar] [CrossRef] [Green Version]
- Prosdocimo, D.A.; Anand, P.; Liao, X.; Zhu, H.; Shelkay, S.; Artero-Calderon, P.; Zhang, L.; Kirsh, J.; Moore, D.; Rosca, M.G.; et al. Kruppel-like Factor 15 Is a Critical Regulator of Cardiac Lipid Metabolism. J. Biol. Chem. 2014, 289, 5914–5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, S.; Feinberg, M.W.; Hull, S.; Kuo, C.T.; Watanabe, M.; Sen-Banerjee, S.; DePina, A.; Haspel, R.; Jain, M.K. The Krüp-pel-like factor KLF15 regulates the insulin-sensitive glucose transporter GLUT4. J. Biol. Chem. 2002, 277, 34322–34328. [Google Scholar] [CrossRef] [Green Version]
- Niwa, N.; Nerbonne, J.M. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J. Mol. Cell. Cardiol. 2010, 48, 12–25. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.-C.; Cheng, C.-F.; Clark, R.B.; Lin, J.J.-C.; Lin, J.L.-C.; Hoshijima, M.; Nguyêñ-Trân, V.T.; Gu, Y.; Ikeda, Y.; Chu, P.-H.; et al. A Defect in the Kv Channel-Interacting Protein 2 (KChIP2) Gene Leads to a Complete Loss of Ito and Confers Susceptibility to Ventricular Tachycardia. Cell 2001, 107, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Olesen, M.S.; Refsgaard, L.; Holst, A.G.; Larsen, A.P.; Grubb, S.; Haunsø, S.; Svendsen, J.H.; Olesen, S.-P.; Schmitt, N.; Calloe, K. A novel KCND3 gain-of-function mutation associated with early-onset of persistent lone atrial fibrillation. Cardiovasc. Res. 2013, 98, 488–495. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, S.; Liu, Q. Energy metabolic alterations in the progression of atrial fibrillation: Potential role of AMP-activated protein kinase as a critical regulator. Int. J. Cardiol. 2016, 212, 14–15. [Google Scholar] [CrossRef]
- Harada, M.; Melka, J.; Sobue, Y.; Nattel, S. Metabolic considerations in atrial fibrillation—Mechanistic insights and therapeutic opportunities. Circ. J. 2017, 81, 1749–1757. [Google Scholar] [CrossRef] [Green Version]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Haldar, S.M.; Lu, Y.; Ibrahim, O.A.; Fisch, S.; Gray, S.; Leask, A.; Jain, M.K. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J. Mol. Cell. Cardiol. 2008, 45, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.K.; Ramchand, J.; Crocitti, V.; Burrell, L.M. Kruppel-Like Factor 15 Is Critical for the Development of Left Ventricular Hypertrophy. Int. J. Mol. Sci. 2018, 19, 1303. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.; Wai, B.; Lang, C.C.; Levin, D.; Palmer, C.N.A.; Parry, H.M.; Velkoska, E.; Harrap, S.B.; Srivastava, P.M.; Burrell, L.M. Genetic Variation in Kruppel Like Factor 15 is Associated with Left Ventricular Hypertrophy in Patients with Type 2 Diabetes: Discovery and Replication Cohorts. EBioMedicine 2017, 18, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.K.; Velkoska, E.; Gayed, D.; Ramchand, J.; Lesmana, J.; Burrell, L.M. Left ventricular hypertrophy in experimental chronic kidney disease is associated with reduced expression of cardiac Kruppel-like factor. BMC Nephrol. 2018, 19, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Subject Information | Phenotype | Electrocardiogram | Echocardiogram | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identity (Family 1) | Gender | Age at Study Enrollment (Years) | Age at Initial Diagnosis of AF (Years) | AF (Classification) | Heart Rate (Beats/min) | QRS Interval (ms) | QTc | LAD (mm) | LVEF (%) | IVSD (mm) | LVPWD (mm) | LVEDD (mm) | LVESD (mm) |
| I-1 | M | 65 ⁎ | 40 | Permanent | – | – | – | – | – | – | – | – | – |
| II-1 | M | 63 ⁎ | 43 | Permanent | – | – | – | – | – | – | – | – | – |
| II-3 | M | 66 | 32 | Permanent | 63 | 109 | 439 | 39 | 58 | 11 | 11 | 54 | 34 |
| II-8 | F | 61 | 34 | Permanent | 69 | 105 | 461 | 37 | 65 | 22 | 15 | 55 | 36 |
| III-1 | M | 45 | 26 | Persistent | 65 | 92 | 417 | 34 | 60 | 18 | 13 | 51 | 32 |
| III-6 | F | 41 | 37 | Paroxysmal | 110 | 93 | 433 | 31 | 64 | 10 | 10 | 44 | 27 |
| III-7 | M | 43 | 28 | Persistent | 91 | 83 | 447 | 35 | 62 | 11 | 10 | 48 | 31 |
| III-12 | F | 39 | 35 | Persistent | 85 | 95 | 448 | 33 | 68 | 10 | 9 | 46 | 25 |
| IV-1 | M | 22 | 18 | Paroxysmal | 106 | 90 | 416 | 30 | 63 | 9 | 8 | 43 | 23 |
| IV-5 | F | 19 | 19 | Paroxysmal | 83 | 112 | 420 | 28 | 66 | 9 | 7 | 40 | 21 |
| IV-7 | M | 16 | 16 | Paroxysmal | 91 | 103 | 441 | 28 | 62 | 8 | 7 | 38 | 21 |
| Coding Exon | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Amplicon (bp) |
|---|---|---|---|
| 1-a | CTTCTGACCAGGCCTCCTGT | GTCCTTGCTGTTGCCCTCAG | 558 |
| 1-b | AGCCTACCCTGGAGGAGATTGA | CCTGCTGCACACCCAAGTAAG | 743 |
| 2 | GGGGACCCTCCCCTAATCCT | GCGGGTTCGAGGCTCTAAGT | 517 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Xu, Y.-J.; Shi, H.-Y.; Yang, C.-X.; Guo, Y.-H.; Li, R.-G.; Qiu, X.-B.; Yang, Y.-Q.; Zhang, M. KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy. Genes 2021, 12, 408. https://doi.org/10.3390/genes12030408
Li N, Xu Y-J, Shi H-Y, Yang C-X, Guo Y-H, Li R-G, Qiu X-B, Yang Y-Q, Zhang M. KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy. Genes. 2021; 12(3):408. https://doi.org/10.3390/genes12030408
Chicago/Turabian StyleLi, Ning, Ying-Jia Xu, Hong-Yu Shi, Chen-Xi Yang, Yu-Han Guo, Ruo-Gu Li, Xing-Biao Qiu, Yi-Qing Yang, and Min Zhang. 2021. "KLF15 Loss-of-Function Mutation Underlying Atrial Fibrillation as well as Ventricular Arrhythmias and Cardiomyopathy" Genes 12, no. 3: 408. https://doi.org/10.3390/genes12030408






