Craniofacial Diseases Caused by Defects in Intracellular Trafficking
Abstract
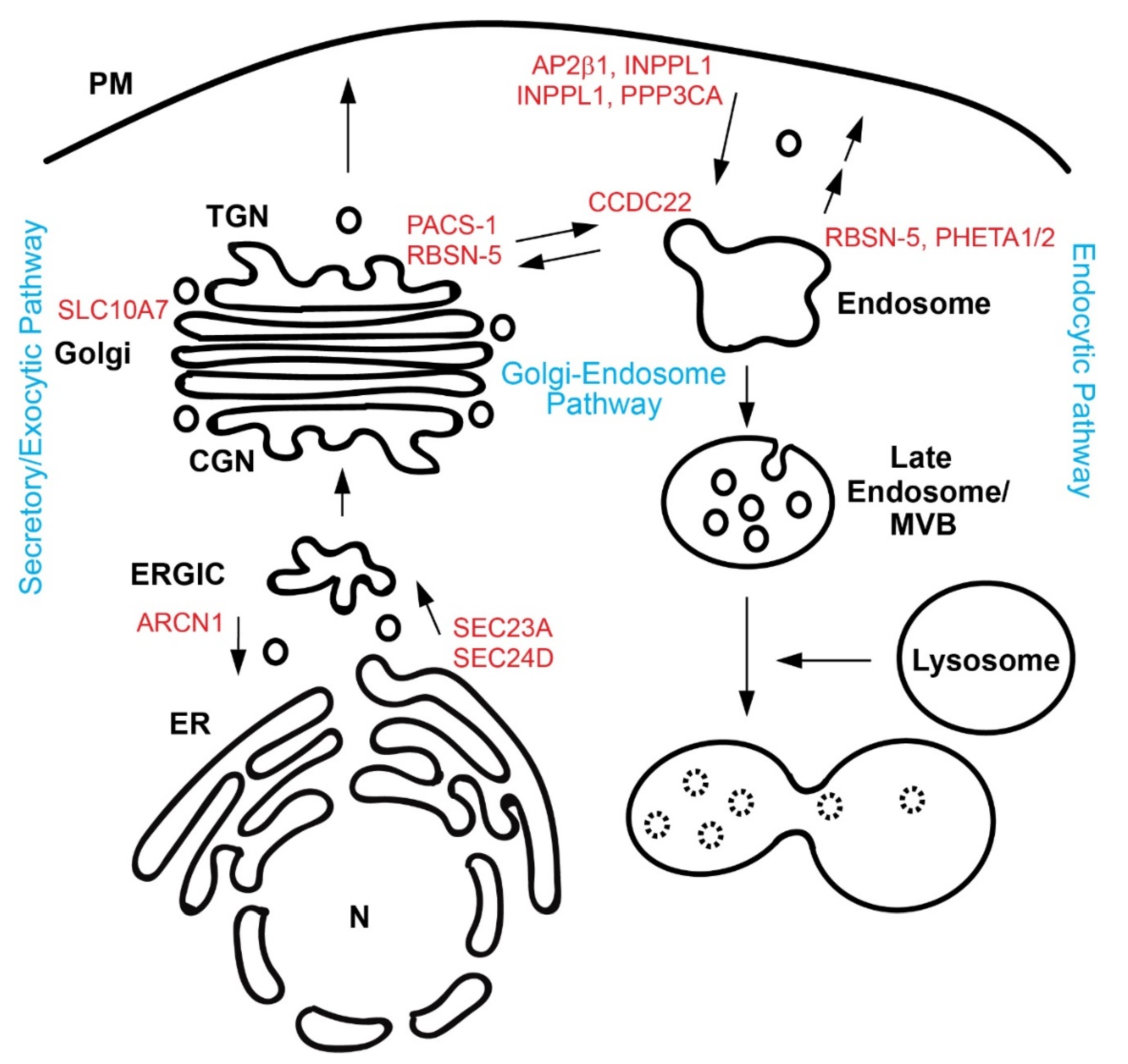
:1. Introduction
2. Craniofacial Diseases Arising from Intracellular Trafficking Defects
2.1. The Secretory/Exocytic Pathway
2.1.1. SEC23A
2.1.2. SEC24D
2.1.3. Archain 1 (ARCN1)
2.1.4. Solute Carrier Family 10, Member 7 (SLC10A7)
2.2. Golgi-Endosomes/Lysosome Pathways
2.2.1. Phosphofurin Acidic Cluster Sorting Protein-1 (PACS-1)
2.2.2. Coiled-Coil Domain-Containing Protein 22 (CCDC22)
2.3. Endocytic Pathways
2.3.1. Adaptor-Related Protein Complex 2, β-1 Subunit (AP2β1)
2.3.2. Inositol Polyphosphate Phosphatase-Like 1 (INPPL1)
2.3.3. PH Domain-Containing Endocytic Trafficking Adaptor 1 and 2 (PHETA1/2, a.k.a. FAM109A/B, SES1/2, IPIP27A/B)
2.3.4. Calcineurin (PPP3CA)
2.3.5. Rabenosyn-5 (RBSN-5)
2.4. Remaining Questions
2.5. Therapeutic Strategies
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chai, Y.; Maxson, R.E. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef]
- Yoon, A.J.; Pham, B.N.; Dipple, K.M. Genetic Screening in Patients with Craniofacial Malformations. J. Pediatr. Genet. 2016, 5, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Constam, D.B. Intracellular trafficking and signaling in development. F1000 Biol. Rep. 2009, 1, 59. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y.; Ito, S.; Nagata, K.; Sakai, L.Y.; Bächinger, H.P. Intracellular mechanisms of molecular recognition and sorting for transport of large extracellular matrix molecules. Proc. Natl. Acad. Sci. USA 2016, 113, E6036–E6044. [Google Scholar] [CrossRef] [Green Version]
- Venditti, R.; Wilson, C.; De Matteis, M.A. Exiting the ER: What we know and what we don’t. Trends Cell Biol. 2014, 24, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Progida, C.; Bakke, O. Bidirectional traffic between the Golgi and the endosomes-machineries and regulation. J. Cell Sci. 2016, 129, 3971–3982. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Van Weering, J.; Sharp, T.; Mantell, J.; Verkade, P. Capturing endocytic segregation events with HPF-CLEM. Methods Cell Biol. 2012, 111, 175–201. [Google Scholar] [CrossRef]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Jensen, D.; Schekman, R. COPII-mediated vesicle formation at a glance. J. Cell Sci. 2011, 124, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Boyadjiev, S.A.; Justice, C.M.; Eyaid, W.; McKusick, V.A.; Lachman, R.S.; Chowdry, A.B.; Jabak, M.; Zwaan, J.; Wilson, A.F.; Jabs, E.W. A novel dysmorphic syndrome with open calvarial sutures and sutural cataracts maps to chromosome 14q13-q21. Hum. Genet. 2003, 113, 1–9. [Google Scholar] [CrossRef]
- Boyadjiev, S.A.; Fromme, J.C.; Ben, J.; Chong, S.S.; Nauta, C.; Hur, D.J.; Zhang, G.; Hamamoto, S.; Schekman, R.; Ravazzola, M.; et al. Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat. Genet. 2006, 38, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Boyadjiev, S.A.; Kim, S.D.; Hata, A.; Haldeman-Englert, C.; Zackai, E.H.; Naydenov, C.; Hamamoto, S.; Schekman, R.W.; Kim, J. Cranio-lenticulo-sutural dysplasia associated with defects in collagen secretion. Clin. Genet. 2011, 80, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanetti, G.; Pahuja, K.B.; Studer, S.; Shim, S.; Schekman, R. COPII and the regulation of protein sorting in mammals. Nat. Cell Biol. 2011, 14, 20–28. [Google Scholar] [CrossRef]
- Garbes, L.; Kim, K.; Rieß, A.; Hoyer-Kuhn, H.; Beleggia, F.; Bevot, A.; Kim, M.J.; Huh, Y.H.; Kweon, H.S.; Savarirayan, R.; et al. Mutations in SEC24D, encoding a component of the COPII machinery, cause a syndromic form of osteogenesis imperfecta. Am. J. Hum. Genet. 2015, 96, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeyari, S.; Kubota, T.; Miyata, K.; Yamamoto, K.; Nakayama, H.; Ohata, Y.; Kitaoka, T.; Yanagi, K.; Kaname, T.; Ozono, K. Japanese patient with Cole-carpenter syndrome with compound heterozygous variants of SEC24D. Am. J. Med. Genet. A 2018, 176, 2882–2886. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, H.; Wang, C.; Gu, J.; He, J.; Fu, W.; Hu, W.; Zhang, Z. Novel mutations in the SEC24D gene in Chinese families with autosomal recessive osteogenesis imperfecta. Osteoporos Int. 2017, 28, 1473–1480. [Google Scholar] [CrossRef]
- Adolf, F.; Rhiel, M.; Hessling, B.; Gao, Q.; Hellwig, A.; Béthune, J.; Wieland, F.T. Proteomic Profiling of Mammalian COPII and COPI Vesicles. Cell Rep. 2019, 26, 250–265.e255. [Google Scholar] [CrossRef] [Green Version]
- Ohisa, S.; Inohaya, K.; Takano, Y.; Kudo, A. sec24d encoding a component of COPII is essential for vertebra formation, revealed by the analysis of the medaka mutant, vbi. Dev. Biol. 2010, 342, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Sarmah, S.; Barrallo-Gimeno, A.; Melville, D.B.; Topczewski, J.; Solnica-Krezel, L.; Knapik, E.W. Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS ONE 2010, 5, e10367. [Google Scholar] [CrossRef] [Green Version]
- Arakel, E.C.; Schwappach, B. Correction: Formation of COPI-coated vesicles at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Izumi, K.; Brett, M.; Nishi, E.; Drunat, S.; Tan, E.S.; Fujiki, K.; Lebon, S.; Cham, B.; Masuda, K.; Arakawa, M.; et al. ARCN1 Mutations Cause a Recognizable Craniofacial Syndrome Due to COPI-Mediated Transport Defects. Am. J. Hum. Genet. 2016, 99, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Kedlaya, R.; Higuchi, H.; Ikeda, S.; Justice, M.J.; Setaluri, V.; Ikeda, A. Mutation in archain 1, a subunit of COPI coatomer complex, causes diluted coat color and Purkinje cell degeneration. PLoS Genet. 2010, 6, e1000956. [Google Scholar] [CrossRef] [Green Version]
- Ashikov, A.; Abu Bakar, N.; Wen, X.Y.; Niemeijer, M.; Rodrigues Pinto Osorio, G.; Brand-Arzamendi, K.; Hasadsri, L.; Hansikova, H.; Raymond, K.; Vicogne, D.; et al. Integrating glycomics and genomics uncovers SLC10A7 as essential factor for bone mineralization by regulating post-Golgi protein transport and glycosylation. Hum. Mol. Genet. 2018, 27, 3029–3045. [Google Scholar] [CrossRef] [PubMed]
- Dubail, J.; Huber, C.; Chantepie, S.; Sonntag, S.; Tüysüz, B.; Mihci, E.; Gordon, C.T.; Steichen-Gersdorf, E.; Amiel, J.; Nur, B.; et al. SLC10A7 mutations cause a skeletal dysplasia with amelogenesis imperfecta mediated by GAG biosynthesis defects. Nat. Commun. 2018, 9, 3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Molloy, S.S.; Thomas, L.; Liu, G.; Xiang, Y.; Rybak, S.L.; Thomas, G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 1998, 94, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Schuurs-Hoeijmakers, J.H.; Oh, E.C.; Vissers, L.E.; Swinkels, M.E.; Gilissen, C.; Willemsen, M.A.; Holvoet, M.; Steehouwer, M.; Veltman, J.A.; de Vries, B.B.; et al. Recurrent de novo mutations in PACS1 cause defective cranial-neural-crest migration and define a recognizable intellectual-disability syndrome. Am. J. Hum. Genet. 2012, 91, 1122–1127. [Google Scholar] [CrossRef] [Green Version]
- Phillips-Krawczak, C.A.; Singla, A.; Starokadomskyy, P.; Deng, Z.; Osborne, D.G.; Li, H.; Dick, C.J.; Gomez, T.S.; Koenecke, M.; Zhang, J.S.; et al. COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol. Biol. Cell 2015, 26, 91–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolanczyk, M.; Krawitz, P.; Hecht, J.; Hupalowska, A.; Miaczynska, M.; Marschner, K.; Schlack, C.; Emmerich, D.; Kobus, K.; Kornak, U.; et al. Missense variant in CCDC22 causes X-linked recessive intellectual disability with features of Ritscher-Schinzel/3C syndrome. Eur. J. Hum. Genet. 2015, 23, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, A.M.; Simard, L.R.; Coghlan, G.; Chudley, A.E.; Chodirker, B.N.; Greenberg, C.R.; Burch, T.; Ly, V.; Hatch, G.M.; Zelinski, T. A novel mutation in KIAA0196: Identification of a gene involved in Ritscher-Schinzel/3C syndrome in a First Nations cohort. J. Med. Genet. 2013, 50, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Bonifacino, J.S. Adaptins: The final recount. Mol. Biol. Cell 2001, 12, 2907–2920. [Google Scholar] [CrossRef]
- Traub, L.M. Clathrin-associated adaptor proteins-putting it all together. Trends Cell Biol. 1997, 7, 43–46. [Google Scholar] [CrossRef]
- Li, W.; Puertollano, R.; Bonifacino, J.S.; Overbeek, P.A.; Everett, E.T. Disruption of the murine Ap2β1 gene causes nonsyndromic cleft palate. Cleft Palate Craniofac. J. 2010, 47, 566–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.M.; Shuler, C.F. The TGF-beta type III receptor is localized to the medial edge epithelium during palatal fusion. Int. J. Dev. Biol. 2000, 44, 397–402. [Google Scholar]
- Below, J.E.; Earl, D.L.; Shively, K.M.; McMillin, M.J.; Smith, J.D.; Turner, E.H.; Stephan, M.J.; Al-Gazali, L.I.; Hertecant, J.L.; Chitayat, D.; et al. Whole-genome analysis reveals that mutations in inositol polyphosphate phosphatase-like 1 cause opsismodysplasia. Am. J. Hum. Genet. 2013, 92, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Huber, C.; Faqeih, E.A.; Bartholdi, D.; Bole-Feysot, C.; Borochowitz, Z.; Cavalcanti, D.P.; Frigo, A.; Nitschke, P.; Roume, J.; Santos, H.G.; et al. Exome sequencing identifies INPPL1 mutations as a cause of opsismodysplasia. Am. J. Hum. Genet. 2013, 92, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Hunter, S.; Hwang, Y.; Chen, J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J. Biol. Chem. 2007, 282, 2683–2694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noakes, C.J.; Lee, G.; Lowe, M. The PH domain proteins IPIP27A and B link OCRL1 to receptor recycling in the endocytic pathway. Mol. Biol. Cell 2011, 22, 606–623. [Google Scholar] [CrossRef]
- Swan, L.E.; Tomasini, L.; Pirruccello, M.; Lunardi, J.; De Camilli, P. Two closely related endocytic proteins that share a common OCRL-binding motif with APPL1. Proc. Natl. Acad. Sci. USA 2010, 107, 3511–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ates, K.M.; Wang, T.; Moreland, T.; Veeranan-Karmegam, R.; Ma, M.; Jeter, C.; Anand, P.; Wenzel, W.; Kim, H.G.; Wolfe, L.A.; et al. Deficiency in the endocytic adaptor proteins PHETA1/2 impairs renal and craniofacial development. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Peralta, M.S.; Mouguelar, V.S.; Sdrigotti, M.A.; Ishiy, F.A.; Fanganiello, R.D.; Passos-Bueno, M.R.; Coux, G.; Calcaterra, N.B. Cnbp ameliorates Treacher Collins Syndrome craniofacial anomalies through a pathway that involves redox-responsive genes. Cell Death Dis. 2016, 7, e2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Wu, X.S.; Xu, J.; McNeil, B.D.; Pang, Z.P.; Yang, W.; Bai, L.; Qadri, S.; Molkentin, J.D.; Yue, D.T.; et al. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J. Neurosci. 2010, 30, 11838–11847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.S.; Zhang, Z.; Zhao, W.D.; Wang, D.; Luo, F.; Wu, L.G. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 2014, 7, 982–988. [Google Scholar] [CrossRef] [Green Version]
- Mizuguchi, T.; Nakashima, M.; Kato, M.; Okamoto, N.; Kurahashi, H.; Ekhilevitch, N.; Shiina, M.; Nishimura, G.; Shibata, T.; Matsuo, M.; et al. Loss-of-function and gain-of-function mutations in PPP3CA cause two distinct disorders. Hum. Mol. Genet. 2018, 27, 1421–1433. [Google Scholar] [CrossRef]
- Stockler, S.; Corvera, S.; Lambright, D.; Fogarty, K.; Nosova, E.; Leonard, D.; Steinfeld, R.; Ackerley, C.; Shyr, C.; Au, N.; et al. Single point mutation in Rabenosyn-5 in a female with intractable seizures and evidence of defective endocytotic trafficking. Orphanet. J. Rare Dis. 2014, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Naslavsky, N.; McKenzie, J.; Altan-Bonnet, N.; Sheff, D.; Caplan, S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J. Cell Sci. 2009, 122, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.W.; Wang, H.; Bajaj, K.; Zhang, P.; Meng, Z.X.; Ma, D.; Bai, Y.; Liu, H.H.; Adams, E.; Baines, A.; et al. SEC24A deficiency lowers plasma cholesterol through reduced PCSK9 secretion. eLife 2013, 2, e00444. [Google Scholar] [CrossRef] [PubMed]
- Merte, J.; Jensen, D.; Wright, K.; Sarsfield, S.; Wang, Y.; Schekman, R.; Ginty, D.D. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat. Cell Biol. 2010, 12, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.C.; Adams, E.J.; Zhang, B.; Ginsburg, D. Disruption of the Sec24d gene results in early embryonic lethality in the mouse. PLoS ONE 2013, 8, e61114. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-L.; Cain, J.; Brudvig, J.; Ortmeier, S.; Boyadjiev, S.A.; Weimer, J.M.; Kim, J. Collagen has a unique SEC24 preference for efficient export from the endoplasmic reticulum. bioRxiv 2021. [Google Scholar] [CrossRef]
- Balch, W.E.; Morimoto, R.I.; Dillin, A.; Kelly, J.W. Adapting proteostasis for disease intervention. Science 2008, 319, 916–919. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Goldberg, E.; Goldberg, J. ER retention is imposed by COPII protein sorting and attenuated by 4-phenylbutyrate. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, R.C.; Zeitlin, P.L. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: Partial restoration of nasal epithelial CFTR function. Am. J. Respir. Crit. Care Med. 1998, 157, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.P.; Krishnakumar, R.; Timlin, J.A.; Carney, J.P.; Butler, K.S. Gene editing and CRISPR in the clinic: Current and future perspectives. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matharu, N.; Rattanasopha, S.; Tamura, S.; Maliskova, L.; Wang, Y.; Bernard, A.; Hardin, A.; Eckalbar, W.L.; Vaisse, C.; Ahituv, N. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 2019, 363. [Google Scholar] [CrossRef]
- Safary, A.; Akbarzadeh Khiavi, M.; Mousavi, R.; Barar, J.; Rafi, M.A. Enzyme replacement therapies: What is the best option? Bioimpacts 2018, 8, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Kakkis, E.D.; McEntee, M.F.; Schmidtchen, A.; Neufeld, E.F.; Ward, D.A.; Gompf, R.E.; Kania, S.; Bedolla, C.; Chien, S.L.; Shull, R.M. Long-term and high-dose trials of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. Biochem. Mol. Med. 1996, 58, 156–167. [Google Scholar] [CrossRef]
- Turner, C.T.; Hopwood, J.J.; Brooks, D.A. Enzyme replacement therapy in mucopolysaccharidosis I: Altered distribution and targeting of alpha-L-iduronidase in immunized rats. Mol. Genet. Metab. 2000, 69, 277–285. [Google Scholar] [CrossRef]
- Anson, D.S.; McIntyre, C.; Byers, S. Therapies for neurological disease in the mucopolysaccharidoses. Curr. Gene Ther. 2011, 11, 132–143. [Google Scholar] [CrossRef]
- Gelfond, D.; Heltshe, S.L.; Skalland, M.; Heubi, J.E.; Kloster, M.; Leung, D.H.; Ramsey, B.W.; Borowitz, D.; Investigators, B.S. Pancreatic Enzyme Replacement Therapy Use in Infants with Cystic Fibrosis Diagnosed by Newborn Screening. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 657–663. [Google Scholar] [CrossRef]
- Braun, R.; Wang, Z.; Mack, D.L.; Childers, M.K. Gene therapy for inherited muscle diseases: Where genetics meets rehabilitation medicine. Am. J. Phys. Med. Rehabil. 2014, 93, S97–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esrick, E.B.; Lehmann, L.E.; Biffi, A.; Achebe, M.; Brendel, C.; Ciuculescu, M.F.; Daley, H.; MacKinnon, B.; Morris, E.; Federico, A.; et al. Post-Transcriptional Genetic Silencing of. N. Engl. J. Med. 2021, 384, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
| Gene | Craniofacial Features | Extra Cranial Features |
|---|---|---|
| Sec23A | Wide-open calvarial sutures with large and late-closing anterior fontanels, frontal bossing, hyperpigmentation with capillary hemangioma of the forehead, significant hypertelorism, a broad and prominent nose, and Y-shaped sutural cataracts | Short stature, coarse brittle and scarce hair, dorsal wedging of the vertebral bodies, and high and narrow iliac wings |
| Sec24D | Ocular proptosis with orbital craniosynostosis, hydrocephalus, frontal bossing, midface hypoplasia, and micrognathia | Multiple bone fractures, usually resulting from minimal trauma, bone deformity |
| ARCN1 | Severe micrognathia and microcephalic dwarfism. | Rhizomelic shortening and mild developmental delay. |
| SCL10A7 | Defective enamel formation (amelogenesis imperfecta), coarse/dysmorphic face, teeth anomalies, and mandibular hypoplasia | Short stature, chest deformity, moderate hearing impairment, and mildly impaired intellectual development |
| PACS-1 | Hypertelorism with down slanting palpebral fissures, mild synophrys with highly arched eyebrows, long eyelashes, downturned corners of the mouth, and a thin narrow upper lip | Intellectual delay |
| CCDC22 | Ritscher-Schinzel syndrome (RSS, see Table 2), broad forehead, up slanting palpebral fissures, wideset eyes, a short philtrum, and a broad neck with a low posterior hair line | Ventricular septal defect and Dandy-Walker syndrome (see Table 2) |
| AP2β1 | Cleft palate | |
| INPPL1 | Opsismodysplasia, relative macrocephaly with a large anterior fontanel, hypertelorism, high forehead, short nose, long philtrum, large fontanelle, coarse face, mid face hypoplasia, and brachycephaly | Short limbs, and small hands and feet |
| PHETA1/2 | Facial asymmetry, coarse facial features, concave nasal ridge, flat occiput, malar flattening, narrow mouth, sparse scalp hair, relative macrocephaly, abnormality of dental morphology, and widely spaced teeth | Scoliosis, clinodactyly of fourth and fifth digits on both hands, multiple palmar and planar creases, pes planus, short foot and palm, tapered fingers, slow-growing nails, and metatarsus adductus |
| PPP3CA | Trigonocephaly, cleft palate, and micrognathia | West syndrome (see Table 2), brachydactyly, a short stature, and arthrogryposis |
| RBSN-5 | Microcephaly, midfacial bone hypoplasia, deep-set eyes with a hooded appearance, a fullness in the nasal bridge, short nose, and a large mouth with small teeth and tongue protrusion | Developmental delay, macrocytosis, megaloblastoid erythropoiesis, moderate osteopenia involving the pelvis and long bones of both upper and lower limbs, with evidence of undertubulation and hypoplasia of the epiphyses around the knee joint and bilateral coxa valga |
| Syndrome | Craniofacial Features | Extracranial Features |
|---|---|---|
| Dandy-Walker syndrome | Enlargement of the fourth ventricle (a small channel that allows fluid to flow freely between the upper and lower areas of the brain and spinal cord), a partial or complete absence of the cerebellar vermis (the area between the two cerebellar hemispheres), and cyst formation near the internal base of the skull | |
| West syndrome | Axial spasms, psychomotor retardation, and a hypsarrhythmic interictal electroencephalopathy pattern | |
| Ritscher-Schinzel syndrome | Macrocephaly, a prominent forehead and occiput, foramina parietalia, hypertelorism, down slanting palpebral fissures, depressed nasal bridge, narrow palate, and apparently low-set ears | Communicating hydrocephalus, aplasia of the posterior portion of the cerebellar vermis, and high insertion of the confluent sinus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-L.; Kim, J. Craniofacial Diseases Caused by Defects in Intracellular Trafficking. Genes 2021, 12, 726. https://doi.org/10.3390/genes12050726
Lu C-L, Kim J. Craniofacial Diseases Caused by Defects in Intracellular Trafficking. Genes. 2021; 12(5):726. https://doi.org/10.3390/genes12050726
Chicago/Turabian StyleLu, Chung-Ling, and Jinoh Kim. 2021. "Craniofacial Diseases Caused by Defects in Intracellular Trafficking" Genes 12, no. 5: 726. https://doi.org/10.3390/genes12050726





