Clinical Evidence for the Importance of the Wild-Type PRPF31 Allele in the Phenotypic Expression of RP11
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. DNA Analysis and Pathogenicity Assessment
2.4. Statistical Analysis
3. Results
3.1. DNA Analysis and Pathogenicity Assessment
3.2. Baseline Clinical Features
3.2.1. Small Deletion/Deletion-Insertion
3.2.2. Splice Site Mutations
3.2.3. Large Deletions
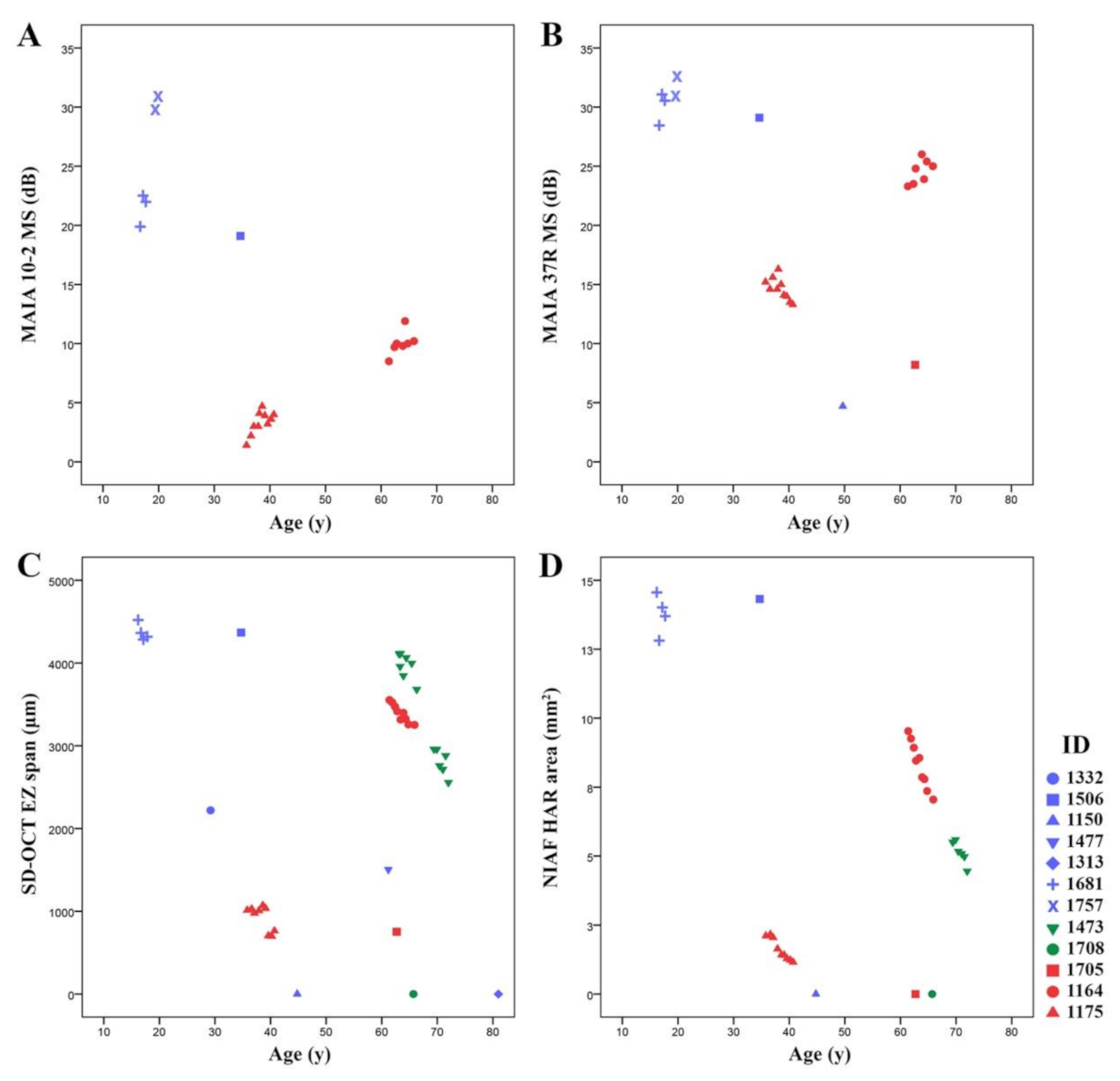
3.3. Natural History of Disease Progression
3.4. Phenotype Patterns
4. Discussion
4.1. RP11 Phenotype Varies in Patients with Identical or Similar Mutations
4.2. RP11 Progression May Not Follow a First-Order Exponential Curve
4.3. Implication of Wild-Type vs. Mutant Allele in RP11 Phenotype
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berson, E.L.; Gouras, P.; Gunkel, R.D.; Myrianthopoulos, N.C. Dominant Retinitis Pigmentosa with Reduced Penetrance. Arch. Ophthalmol. 1969, 81, 226–234. [Google Scholar] [CrossRef]
- Al-Maghtheh, M.; Inglehearn, C.F.; Jeffrey, T.K.; Evans, K.; Moore, A.T.; Jay, M.; Bird, A.C.; Bhattacharya, S.S. Identification of a sixth locus for autosomal dominant retinitis pigmentosa on chromosome. Hum. Mol. Genet. 1994, 3, 351–354. [Google Scholar] [CrossRef]
- Evans, K.; Al-Maghtheh, M.; Fitzke, F.W.; Moore, A.T.; Jay, M.; Inglehearn, C.F.; Arden, G.; Bird, A.C. Bimodal expressivity in dominant retinitis pigmentosa genetically linked to chromosome 19q. Br. J. Ophthalmol. 1995, 79, 841–846. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, M.; Xu, S.; Gal, A.; Wada, Y.; Tamai, M. Variable Expressivity in a Japanese Family with Autosomal Dominant Retinitis Pigmentosa Closely Linked to Chromosome 19q. Arch. Ophthalmol. 1996, 114, 318–322. [Google Scholar] [CrossRef]
- Vithana, E.N.; Abu-Safieh, L.; Allen, M.J.; Carey, A.; Papaioannou, M.; Chakarova, C.; Al-Maghtheh, M.; Ebenezer, N.D.; Willis, C.; Moore, A.T.; et al. A Human Homolog of Yeast Pre-mRNA Splicing Gene, PRP31, Underlies Autosomal Dominant Retinitis Pigmentosa on Chromosome 19q13.4 (RP11). Mol. Cell 2001, 8, 375–381. [Google Scholar] [CrossRef]
- Sullivan, L.S.; Bowne, S.J.; Birch, D.G.; Hughbanks-Wheaton, D.; Heckenlively, J.R.; Lewis, R.A.; Garcia, C.A.; Ruiz, R.S.; Blanton, S.H.; Northrup, H.; et al. Prevalence of Disease-Causing Mutations in Families with Autosomal Dominant Retinitis Pigmentosa: A Screen of Known Genes in 200 Families. Investig. Opthalmol. Vis. Sci. 2006, 47, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Audo, I.; Bujakowska, K.; Mohand-Saïd, S.; Lancelot, M.-E.; Moskova-Doumanova, V.; Waseem, N.H.; Antonio, A.; Sahel, J.-A.; Bhattacharya, S.S.; Zeitz, C. Prevalence and novelty of PRPF31 mutations in French autosomal dominant rod-cone dystrophy patients and a review of published reports. BMC Med. Genet. 2010, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tian, D.; Lee, J.; Zeng, J.; Zhang, H.; Chen, S.; Guo, H.; Xiong, Z.; Xia, K.; Hu, Z.; et al. Clinical and Genetic Identification of a Large Chinese Family with Autosomal Dominant Retinitis Pigmentosa. Ophthalmic Genet. 2013, 36, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Waseem, N.H.; Vaclavik, V.; Webster, A.; Jenkins, S.A.; Bird, A.C.; Bhattacharya, S.S. Mutations in the Gene Coding for the Pre-mRNA Splicing Factor,PRPF31, in Patients with Autosomal Dominant Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2007, 48, 1330–1334. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Robinson, P.; Singh, J.R.; Vanita, V. A novel 7 bp deletion in PRPF31 associated with autosomal dominant retinitis pigmentosa with incomplete penetrance in an Indian family. Exp. Eye Res. 2012, 104, 82–88. [Google Scholar] [CrossRef]
- Bhatia, S.; Goyal, S.; Singh, I.R.; Singh, D.; Vanita, V. A novel mutation in the PRPF31 in a North Indian adRP family with incomplete penetrance. Doc. Ophthalmol. 2018, 137, 103–119. [Google Scholar] [CrossRef]
- Villanueva, A.; Willer, J.R.; Bryois, J.; Dermitzakis, E.T.; Katsanis, N.; Davis, E.E. Whole Exome Sequencing of a Dominant Retinitis Pigmentosa Family Identifies a Novel Deletion in PRPF. Investig. Opthalmol. Vis. Sci. 2014, 55, 2121–2129. [Google Scholar] [CrossRef] [Green Version]
- Vithana, E.N.; Abu-Safieh, L.; Pelosini, L.; Winchester, E.; Hornan, D.; Bird, A.C.; Hunt, D.M.; Bustin, S.A.; Bhattacharya, S.S. Expression of PRPF31 mRNA in Patients with Autosomal Dominant Retinitis Pigmentosa: A Molecular Clue for Incomplete Penetrance? Investig. Opthalmol. Vis. Sci. 2003, 44, 4204–4209. [Google Scholar] [CrossRef]
- Rivolta, C.; McGee, T.L.; Frio, T.R.; Jensen, R.V.; Berson, E.L.; Dryja, T.P. Variation in retinitis pigmentosa-11 (PRPF31 or RP11) gene expression between symptomatic and asymptomatic patients with dominant RP11 mutations. Hum. Mutat. 2006, 27, 644–653. [Google Scholar] [CrossRef]
- Rose, A.M.; Bhattacharya, S.S. Variant haploinsufficiency and phenotypic non-penetrance inPRPF31-associated retinitis pigmentosa. Clin. Genet. 2016, 90, 118–126. [Google Scholar] [CrossRef]
- Brydon, E.M.; Bronstein, R.; Buskin, A.; Lako, M.; Pierce, E.A.; Fernandez-Godino, R. AAV-Mediated Gene Augmentation Therapy Restores Critical Functions in Mutant PRPF31+/− iPSC-Derived RPE Cells. Mol. Ther. Methods Clin. Dev. 2019, 15, 392–402. [Google Scholar] [CrossRef]
- Valdés-Sánchez, L.; Calado, S.; De La Cerda, B.; Aramburu, A.; García-Delgado, A.B.; Massalini, S.; Montero-Sánchez, A.; Bhatia, V.; Rodríguez-Bocanegra, E.; Diez-Lloret, A.; et al. Retinal pigment epithelium degeneration caused by aggregation of PRPF31 and the role of HSP70 family of proteins. Mol. Med. 2019, 26, 1–22. [Google Scholar] [CrossRef]
- Wheway, G.; Douglas, A.; Baralle, D.; Guillot, E. Mutation spectrum of PRPF31, genotype-phenotype correlation in retinitis pigmentosa, and opportunities for therapy. Exp. Eye Res. 2020, 192, 107950. [Google Scholar] [CrossRef]
- Hafler, B.P.; Comander, J.; DiFranco, C.W.; Place, E.M.; Pierce, E.A. Course of Ocular Function in PRPF31 Retinitis Pigmentosa. Semin. Ophthalmol. 2016, 31, 49–52. [Google Scholar] [CrossRef]
- Kiser, K.; Webb-Jones, K.D.; Bowne, S.J.; Sullivan, L.S.; Daiger, S.P.; Birch, D.G. Time Course of Disease Progression of PRPF31-mediated Retinitis Pigmentosa. Am. J. Ophthalmol. 2019, 200, 76–84. [Google Scholar] [CrossRef]
- Roshandel, D.; Thompson, J.A.; Charng, J.; Zhang, D.; Chelva, E.; Arunachalam, S.; Attia, M.S.; Lamey, T.M.; McLaren, T.L.; De Roach, J.N.; et al. Exploring microperimetry and autofluorescence endpoints for monitoring disease progression in PRPF31-associated retinopathy. Ophthalmic Genet. 2021, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.L.; Marmor, M.F.; Brigell, M.G.; Hamilton, R.; Holder, G.E.; Tzekov, R.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc. Ophthalmol. 2015, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Roach, J.N.; McLaren, T.L.; Paterson, R.L.; O’Brien, E.C.; Hoffmann, L.; Mackey, A.D.; Hewitt, A.W.; Lamey, T.M. Establishment and evolution of the Australian Inherited Retinal Disease Register and DNA Bank. Clin. Exp. Ophthalmol. 2012, 41, 476–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, J.; Pei, W.; Lamey, T.; McLaren, T.; Thompson, A.J.; Montgomery, H.; De Roach, J. Progress and prospects of next-generation sequencing testing for inherited retinal dystrophy. Expert Rev. Mol. Diagn. 2015, 15, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Dunnen, J.T.D.; Dalgleish, R.; Maglott, D.R.; Hart, R.K.; Greenblatt, M.S.; McGowan-Jordan, J.; Roux, A.-F.; Smith, T.; Antonarakis, S.E.; Taschner, P.E.; et al. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016, 37, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.A.; De Roach, J.N.; McLaren, T.L.; Montgomery, H.E.; Hoffmann, L.H.; Campbell, I.R.; Chen, F.K.; Mackey, D.A.; Lamey, T.M. The genetic profile of Leber congenital amaurosis in an Australian cohort. Mol. Genet. Genom. Med. 2017, 5, 652–667. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Jarvik, G.P.; Browning, B.L. Consideration of Cosegregation in the Pathogenicity Classification of Genomic Variants. Am. J. Hum. Genet. 2016, 98, 1077–1081. [Google Scholar] [CrossRef] [Green Version]
- Ellard, S.; Baple, E.L.; Owens, M.; Eccles, D.M.; Abbs, S.; Deans, Z.C.; McMullan, D. ACGS Best Practice Guidelines for Variant Classification ACGS Guidelines. 2017. Available online: https://www.acgs.uk.com/media/11631/uk-practice-guidelines-for-variant-classification-v4-01-2020.pdf (accessed on 10 March 2021).
- ClinGen Sequence Variant Interpretation Work Group Recommendations for ACMG/AMP Guideline Criteria Code Modifications Nomenclature 2017. Available online: https://www.clinicalgenome.org/site/assets/files/3459/svi_criteria_nomenclature_recommendation_v1.pdf (accessed on 10 March 2021).
- Tayoun, A.N.A.; Pesaran, T.; DiStefano, M.T.; Oza, A.; Rehm, H.L.; Biesecker, L.G.; Harrison, S.M. ClinGen Sequence Variant Interpretation Working Group (ClinGen SVI) Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum. Mutat. 2018, 39, 1517–1524. [Google Scholar] [CrossRef]
- Sullivan, L.S.; Bowne, S.J.; Reeves, M.J.; Blain, D.; Goetz, K.; Ndifor, V.; Vitez, S.; Wang, X.; Tumminia, S.J.; Daiger, S.P. Prevalence of Mutations in eyeGENE Probands With a Diagnosis of Autosomal Dominant Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2013, 54, 6255. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, F.; Wang, H.; Li, Y.; Alexander, S.; Wang, K.; Willoughby, C.; Zaneveld, J.E.; Jiang, L.; Soens, Z.T.; et al. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland. Qual. Life Res. 2015, 134, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Dai, X.; Sheng, J.; Cui, X.; Wang, X.; Jiang, X.; Tu, X.; Tang, Z.; Bai, Y.; Liu, M.; et al. Identification and functional characterization of a novel splicing mutation in RP gene PRPF. Biochem. Biophys. Res. Commun. 2008, 367, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Chakarova, C.F.; Cherninkova, S.; Tournev, I.; Waseem, N.; Kaneva, R.; Jordanova, A.; Veraitch, B.K.; Gill, B.; Colclough, T.; Nakova, A.; et al. Molecular genetics of retinitis pigmentosa in two Romani (Gypsy) families. Mol. Vis. 2006, 12, 909–914. [Google Scholar]
- Abdulridha-Aboud, W.; Kjellström, U.; Andréasson, S.; Ponjavic, V. Characterization of macular structure and function in two Swedish families with genetically identified autosomal dominant retinitis pigmentosa. Mol. Vis. 2016, 22, 362–373. [Google Scholar]
- Jespersgaard, C.; Fang, M.; Bertelsen, M.; Dang, X.; Jensen, H.; Chen, Y.; Bech, N.; Dai, L.; Rosenberg, T.; Zhang, J.; et al. Molecular genetic analysis using targeted NGS analysis of 677 individuals with retinal dystrophy. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.; Xie, Y.; Zhang, X.; Xu, K.; Zhang, X.; Jin, Z.-B.; Li, Y. Variant Profiling of a Large Cohort of 138 Chinese Families With Autosomal Dominant Retinitis Pigmentosa. Front. Cell Dev. Biol. 2021, 8, 629994. [Google Scholar] [CrossRef]
- Powers, K.T.; Szeto, J.-Y.A.; Schaffitzel, C. New insights into no-go, non-stop and nonsense-mediated mRNA decay complexes. Curr. Opin. Struct. Biol. 2020, 65, 110–118. [Google Scholar] [CrossRef]
- Lu, F.; Huang, L.; Lei, C.; Sha, G.; Zheng, H.; Liu, X.; Yang, J.; Shi, Y.; Lin, Y.; Gong, B.; et al. A Novel PRPF31 Mutation in a Large Chinese Family with Autosomal Dominant Retinitis Pigmentosa and Macular Degeneration. PLoS ONE 2013, 8, e78274. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Cao, Y.; Zhang, Z.; Xu, Y.; Zheng, Y.; Chen, L.J.; Pang, C.P.; Chen, H. Novel Mutations in PRPF31 Causing Retinitis Pigmentosa Identified Using Whole-Exome Sequencing. Investig. Opthalmol. Vis. Sci. 2017, 58, 6342. [Google Scholar] [CrossRef] [Green Version]
- Frio, T.R.; Wade, N.M.; Ransijn, A.; Berson, E.L.; Beckmann, J.S.; Rivolta, C. Premature termination codons in PRPF31 cause retinitis pigmentosa via haploinsufficiency due to nonsense-mediated mRNA decay. J. Clin. Investig. 2008, 118, 1519–1531. [Google Scholar] [CrossRef] [Green Version]
- Abu-Safieh, L.; Vithana, E.N.; Mantel, I.; Holder, E.G.; Pelosini, L.; Bird, A.C.; Bhattacharya, S.S. A large deletion in the adRP gene PRPF31: Evidence that haploinsufficiency is the cause of disease. Mol. Vis. 2006, 12, 384–388. [Google Scholar] [PubMed]
- Martin-Merida, I.; Sanchez-Alcudia, R.; Jose, P.F.-S.; Blanco-Kelly, F.; Perez-Carro, R.; Da Silva, L.R.-J.; Almoguera, B.; Garcia-Sandoval, B.; Lopez-Molina, M.I.; Avila-Fernandez, A.; et al. Analysis of the PRPF31 Gene in Spanish Autosomal Dominant Retinitis Pigmentosa Patients: A Novel Genomic Rearrangement. Investig. Opthalmol. Vis. Sci. 2017, 58, 1045–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, K.P.; Yip, S.P.; Cheung, S.C.; Leung, K.W.; Lam, S.T.S.; To, C.H. Novel PRPF31 and PRPH2 Mutations and Co-occurrence of PRPF31 and RHO Mutations in Chinese Patients with Retinitis Pigmentosa. Arch. Ophthalmol. 2009, 127, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, G.; Collins, R.A.; Leavitt, B.R.; Andrews, D.F.; Hayden, M.; Lumsden, C.J.; McInnes, R.R. A one-hit model of cell death in inherited neuronal degenerations. Nat. Cell Biol. 2000, 406, 195–199. [Google Scholar] [CrossRef]
- Frio, T.R.; Civic, N.; Ransijn, A.; Beckmann, J.S.; Rivolta, C. Two trans-acting eQTLs modulate the penetrance of PRPF31 mutations. Hum. Mol. Genet. 2008, 17, 3154–3165. [Google Scholar] [CrossRef] [Green Version]
- Venturini, G.; Rose, A.M.; Shah, A.Z.; Bhattacharya, S.S.; Rivolta, C. CNOT3 Is a Modifier of PRPF31 Mutations in Retinitis Pigmentosa with Incomplete Penetrance. PLoS Genet. 2012, 8, e1003040. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.M.; Shah, A.Z.; Venturini, G.; Rivolta, C.; Rose, G.E.; Bhattacharya, S.S. Dominant PRPF31 Mutations Are Hypostatic to a Recessive CNOT3 Polymorphism in Retinitis Pigmentosa: A Novel Phenomenon of “Linked Trans-Acting Epistasis”. Ann. Hum. Genet. 2013, 78, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.M.; Shah, A.Z.; Venturini, G.; Krishna, A.; Chakravarti, A.; Rivolta, C.; Bhattacharya, S.S. Transcriptional regulation of PRPF31 gene expression by MSR1 repeat elements causes incomplete penetrance in retinitis pigmentosa. Sci. Rep. 2016, 6, 19450. [Google Scholar] [CrossRef]
- McLenachan, S.; Zhang, D.; Zhang, X.; Chen, S.-C.; Lamey, T.; Thompson, J.A.; McLaren, T.; De Roach, J.N.; Fletcher, S.; Chen, F.K. Generation of two induced pluripotent stem cell lines from a patient with dominant PRPF31 mutation and a related non-penetrant carrier. Stem Cell Res. 2019, 34, 101357. [Google Scholar] [CrossRef]

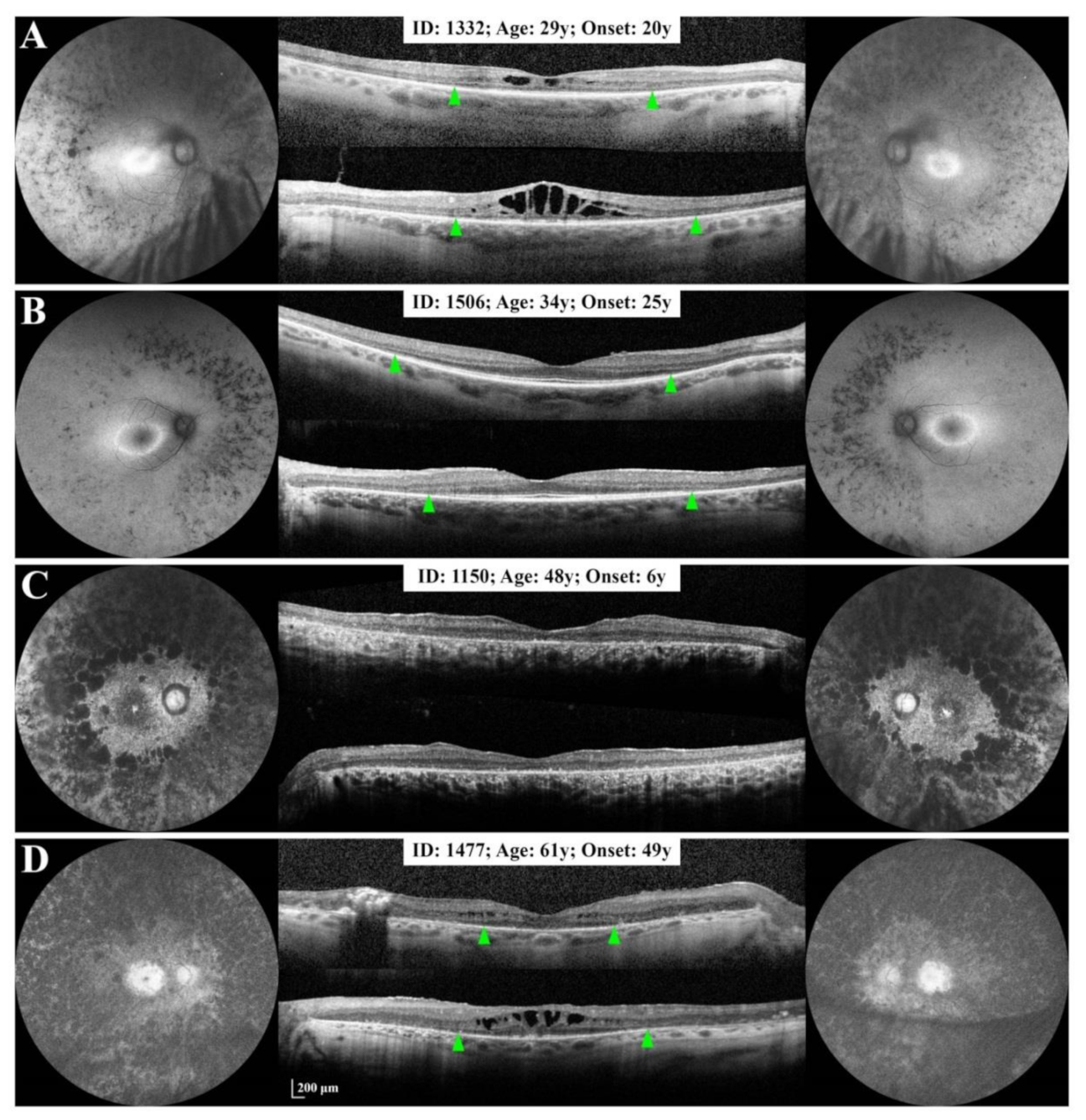
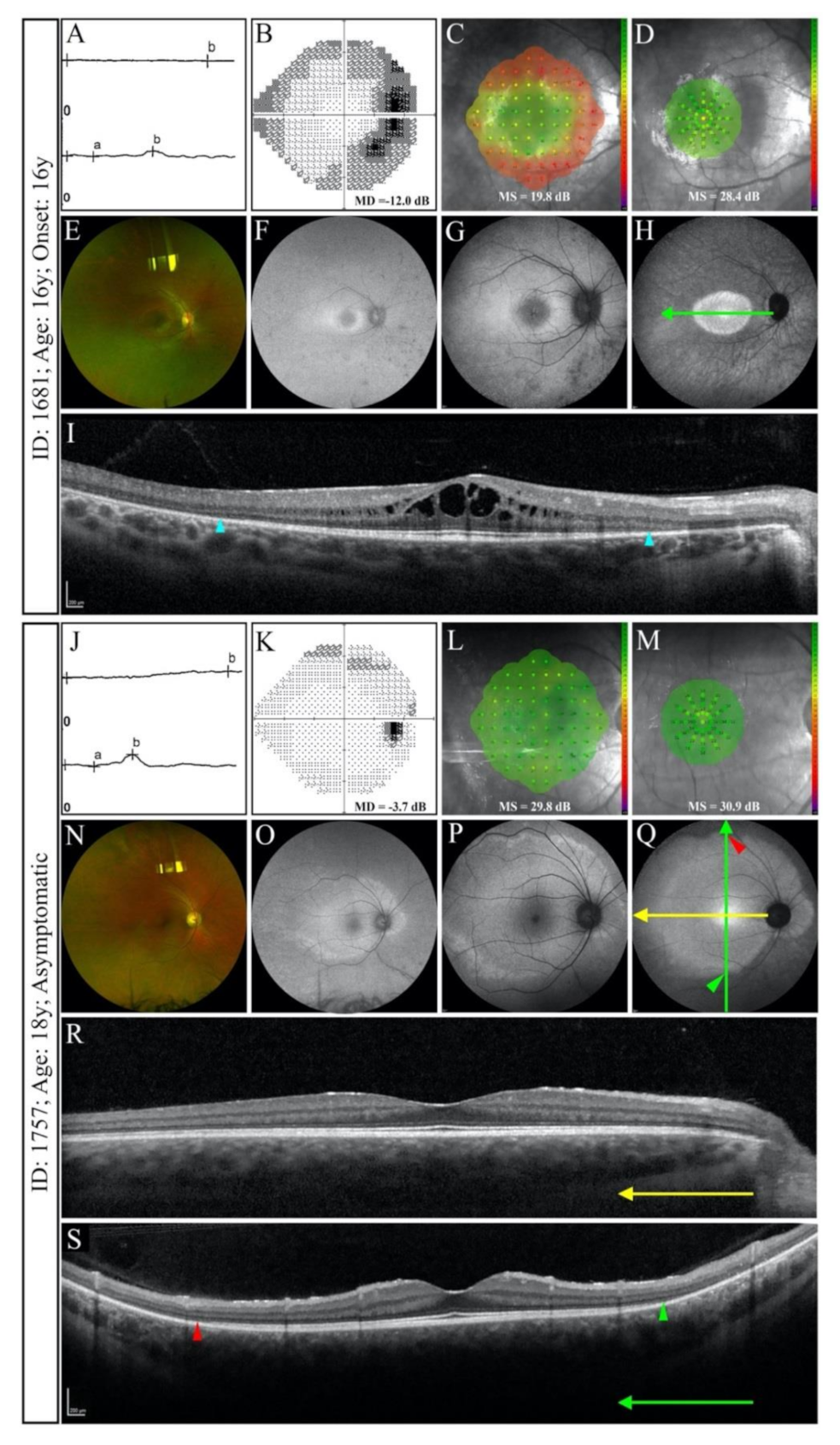

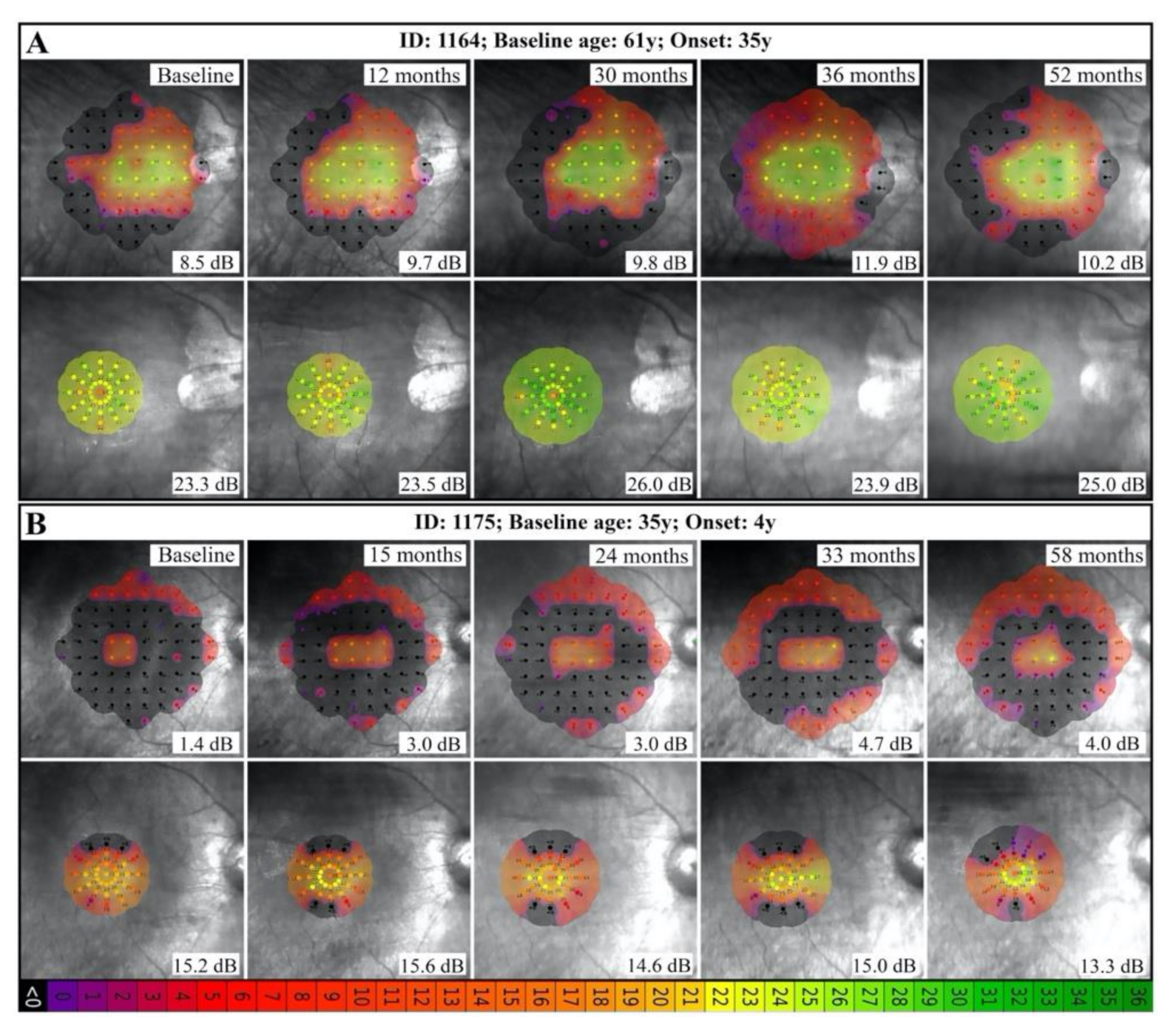
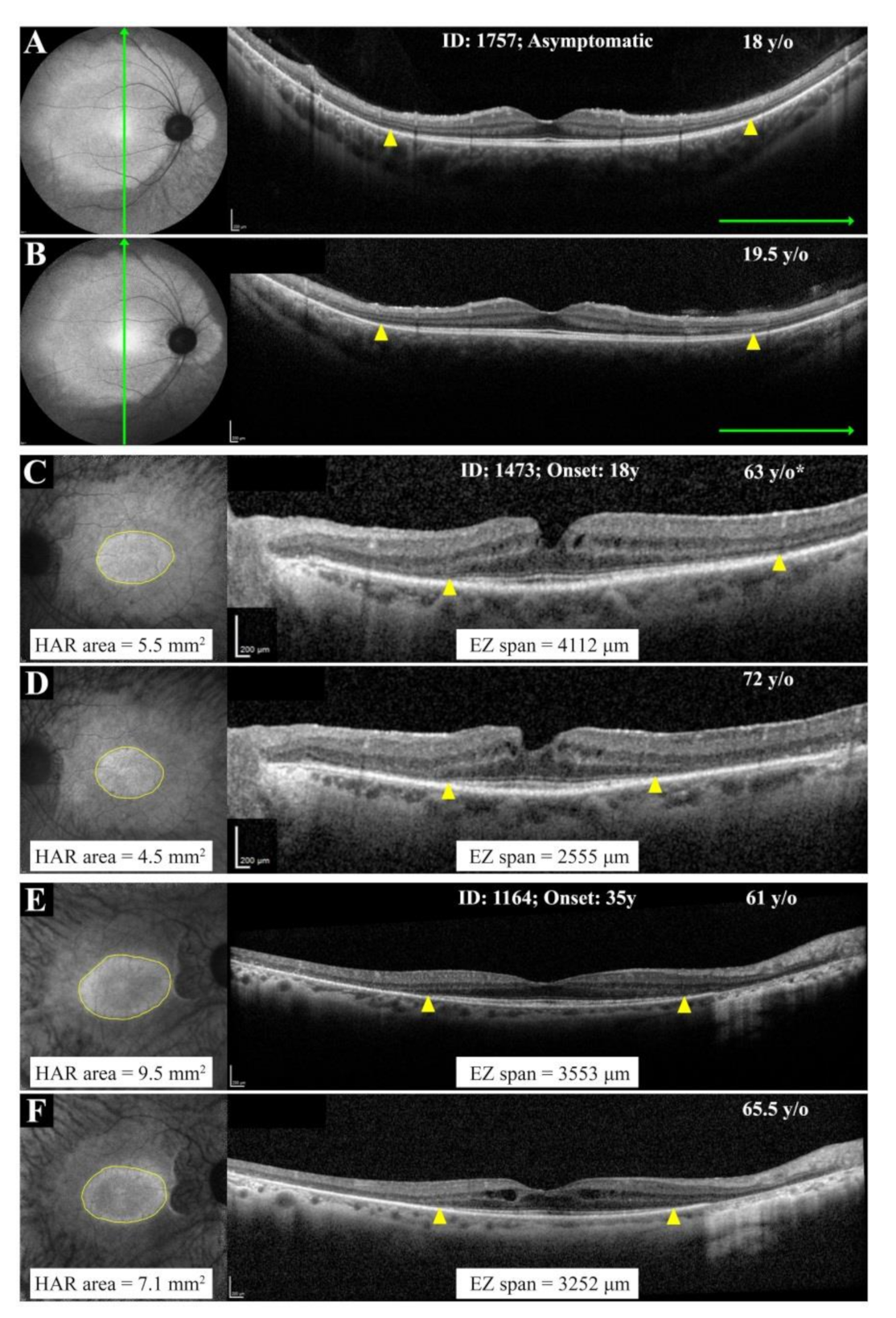
| AIRDR Pedigree ID | WARD Study ID | Age (y) * | Sex | Onset (y) ** | BCVA (ETDRS) *** | Lens § | Mutation | Phenotype | ||
|---|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | |||||||
| 0255 | 1332 | 29 | F | 20 | 68 (20/50) | 68 (20/50) | PSCC + | PSCC + | c.267del | B |
| 1506 | 34 | F | 25 | 84 (20/20) | 74 (20/32) | Clear | Clear | c.267del | C | |
| 1651 † | 41 | M | - | 92 (20/16) | 92 (20/16) | Clear | Clear | c.267del | D | |
| 1150 | 48 | F | 6 | 45 (20/125) | 62 (20/63) | IOL (44) | IOL (43) | c.267del | A | |
| 1477 | 61 | M | 49 | 50 (20/100) | 42 (20/160) | IOL (NA) | IOL (NA) | c.267del | B | |
| 1313 | 81 | F | 6 | CF | CF | IOL (56) | IOL (54) | c.267del | A | |
| 3200 | 1681 | 16 | F | 16 | 83 (20/25) | 85 (20/20) | Clear | Clear | c.772_773delins16 | B |
| 1757 | 18 | M | - | 84 (20/20) | 83 (20/25) | Clear | Clear | c.772_773delins16 | C | |
| 1816 † | 56 | M | - | NA | NA | Clear | Clear | c.772_773delins16 | D | |
| 0244 | 1473 | 63 | M | 18 | HM ‡ | 35 (20/200) | NSC + | NSC + | c.-9+1G>T | C |
| 2097 | 1708 | 70 | F | 3 | CF | CF | IOL (56) | IOL (56) | c.527+1G>T | A |
| 0213 | 1705 | 62 | F | 26 | 35 (20/200) | 64 (20/50) | IOL (56) | IOL (51) | Exon 2–3del | B |
| 0725 | 1164 | 61 | F | 35 | 70 (20/40) | 70 (20/40) | IOL (58) | IOL (58) | Exon 2–8del | C |
| 0155 | 1175 | 35 | M | 4 | 59 (20/63) | 64 (20/50) | PSCC + | PSCC + | Exon 9–14del | A |
| Mutation Class | Phenotype A | Phenotype B | Phenotype C | Phenotype D | Total |
|---|---|---|---|---|---|
| Small deletion or deletion/insertion | 2 | 3 | 2 | 2 | 9 |
| Splice site mutations | 1 | 0 | 1 | 0 | 2 |
| Large deletions | 1 | 1 | 1 | 0 | 3 |
| Total | 4 | 4 | 4 | 2 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshandel, D.; Thompson, J.A.; Heath Jeffery, R.C.; Zhang, D.; Lamey, T.M.; McLaren, T.L.; De Roach, J.N.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Clinical Evidence for the Importance of the Wild-Type PRPF31 Allele in the Phenotypic Expression of RP11. Genes 2021, 12, 915. https://doi.org/10.3390/genes12060915
Roshandel D, Thompson JA, Heath Jeffery RC, Zhang D, Lamey TM, McLaren TL, De Roach JN, McLenachan S, Mackey DA, Chen FK. Clinical Evidence for the Importance of the Wild-Type PRPF31 Allele in the Phenotypic Expression of RP11. Genes. 2021; 12(6):915. https://doi.org/10.3390/genes12060915
Chicago/Turabian StyleRoshandel, Danial, Jennifer A. Thompson, Rachael C. Heath Jeffery, Dan Zhang, Tina M. Lamey, Terri L. McLaren, John N. De Roach, Samuel McLenachan, David A. Mackey, and Fred K. Chen. 2021. "Clinical Evidence for the Importance of the Wild-Type PRPF31 Allele in the Phenotypic Expression of RP11" Genes 12, no. 6: 915. https://doi.org/10.3390/genes12060915
APA StyleRoshandel, D., Thompson, J. A., Heath Jeffery, R. C., Zhang, D., Lamey, T. M., McLaren, T. L., De Roach, J. N., McLenachan, S., Mackey, D. A., & Chen, F. K. (2021). Clinical Evidence for the Importance of the Wild-Type PRPF31 Allele in the Phenotypic Expression of RP11. Genes, 12(6), 915. https://doi.org/10.3390/genes12060915







