Association of TGF-β1 and IL-10 Gene Polymorphisms with Osteoporosis in a Study of Taiwanese Osteoporotic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. DNA Extraction and PCR
2.3. PCR-RFLP Analysis
2.4. Assessments of BMD
2.5. Statistical Analysis
3. Results
3.1. Analysis of SNPs within the TGF-β1 and IL-10 Genes
3.2. Characteristics of the Study Population
3.3. Multivariate Analysis of SNPs and BMD Correlation Using a Linear Regression Model
3.4. Association of Combined Polymorphisms with Lumbar Spine BMD in Osteoporotic Patients
3.5. Association of Combined SNPs with Femoral Neck BMD in Osteoporotic Patients
3.6. Association of Combined SNPs with Total Hip BMD in Osteoporotic Patients
3.7. Association of Combined SNPs with Body Height
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Koromani, F.; Trajanoska, K.; Rivadeneira, F.; Oei, L. Recent Advances in the Genetics of Fractures in Osteoporosis. Front. Endocrinol. 2019, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Young, D.; Hopper, J.L.; Nowson, C.A.; Green, R.M.; Sherwin, J.A.; Kaymakci, B.; Smid, M.; Guest, C.S.; Larkins, R.G.; Wark, J.D. Determinants of bone mass in 10- to 26-year-old females: A twin study. J. Bone Miner. Res. 2009, 10, 558–567. [Google Scholar] [CrossRef]
- Arden, N.K.; Spector, T.D. Genetic Influences on Muscle Strength, Lean Body Mass, and Bone Mineral Density: A Twin Study. J. Bone Miner. Res. 1997, 12, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006, 20, 2492–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Huang, Z.; Chen, G.; Zhou, K.; Zhang, Y.; Chen, J.; Xu, J.; Yin, X. Network and pathway-based analyses of genes associated with osteoporosis. Medicine 2020, 99, e19120. [Google Scholar] [CrossRef]
- Tural, S.; Alayli, G.; Kara, N.; Tander, B.; Bilgici, A.; Kuru, O. Association between osteoporosis and polymorphisms of the IL-10 and TGF-β genes in Turkish postmenopausal women. Hum. Immunol. 2013, 74, 1179–1183. [Google Scholar] [CrossRef]
- Yuan, J.; Tickner, J.; Mullin, B.H.; Zhao, J.; Zeng, Z.; Morahan, G.; Xu, J. Advanced genetic approaches in discovery and characterization of genes involved with osteoporosis in mouse and human. Front. Genet. 2019, 10, 288. [Google Scholar] [CrossRef]
- Janssens, K.; Ten Dijke, P.; Janssens, S.; Van Hul, W. Transforming growth factor-β1 to the bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef] [Green Version]
- Kasagi, S.; Chen, W. TGF-β1 on osteoimmunology and the bone component cells. Cell Biosci. 2013, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Langdahl, B.L.; Carstens, M.; Stenkjær, L.; Eriksen, E.F. Polymorphisms in the transforming growth factor β1 gene and osteoporosis. Bone 2003, 32, 297–310. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.; Xu, L.; Yang, M.; Yangl, H. The transforming growth factor-β1 (TGF-β1) gene polymorphisms (TGF-β1 T869C and TGF-β1 T29C) and susceptibility to postmenopausal osteoporosis: A meta-analysis. Medicine 2015, 94, e461. [Google Scholar] [CrossRef]
- Hughes, D.E.; Dai, A.; Tiffee, J.C.; Li, H.H.; Mundy, G.R.; Boyce, B.F. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat. Med. 1996, 2, 1132–1136. [Google Scholar] [CrossRef]
- Geiser, A.G.; Zeng, Q.Q.; Sato, M.; Helvering, L.M.; Hirano, T.; Turner, C.H. Decreased bone mass and bone elasticity in mice lacking the transforming growth factor-β1 gene. Bone 1998, 23, 87–93. [Google Scholar] [CrossRef]
- Cebinelli, G.C.M.; Trugilo, K.P.; Garcia, S.B.; De Oliveira, K.B. TGF-β1 functional polymorphisms: A review. Eur. Cytokine Netw. 2016, 27, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Stanilova, S.; Stanilov, N.; Julianov, A.; Manolova, I.; Miteva, L. Transforming growth factor-β1 gene promoter -509C/T polymorphism in association with expression affects colorectal cancer development and depends on gender. PLoS ONE 2018, 13, e0201775. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.J.; Heathcote, K.; Chiano, M.; Snieder, H.; Kemp, P.R.; Metcalfe, J.C.; Carter, N.D.; Spector, T.D. Genetic control of the circulating concentration of transforming growth factor type β1. Hum. Mol. Genet. 1999, 8, 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Miyauchi, A.; Goto, J.; Takagi, Y.; Okuizumi, H.; Kanematsu, M.; Hase, H.; Takai, H.; Harada, A.; Ileda, K. Association of a polymorphism of the transforming growth factor-β1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese women. J. Bone Miner. Res. 1998, 13, 1569–1576. [Google Scholar] [CrossRef]
- Yamada, Y.; Miyauchi, A.; Takagi, Y.; Tanaka, M.; Mizuno, M.; Harada, A. Association of the C-509-->T polymorphism, alone of in combination with the T869-->C polymorphism, of the transforming growth factor-β1 gene with bone mineral density and genetic susceptibility to osteoporosis in Japanese women. J. Mol. Med. 2001, 79, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Shachar, I.; Karin, N. The dual roles of inflammatory cytokines and chemokines in the regulation of autoimmune diseases and their clinical implications. J. Leukoc. Biol. 2013, 93, 51–61. [Google Scholar] [CrossRef]
- Bakiri, A.H.; Mingomataj, E.Ç. Novel insights on interleukin-10 functions: A manipulative tool for the deviation of immune response and disease outcome. EMJ Allergy Immunol. 2019, 4, 88–94. [Google Scholar]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yangc, W. Interleukin-10 Inhibits Bone Resorption: A Potential Therapeutic Strategy in Periodontitis and Other Bone Loss Diseases. BioMed. Res. Int. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Dresner-Pollak, R.; Gelb, N.; Rachmilewitz, D.; Karmeli, F.; Weinreb, M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology 2004, 127, 792–801. [Google Scholar] [CrossRef]
- Holgersen, K.; Dobie, R.; Farquharson, C.; Van't Hof, R.; Ahmed, S.F.; Hansen, A.K.; Holm, T.L. Piroxicam treatment augments bone abnormalities in interleukin-10 knockout mice. Inflamm. Bowel Dis. 2015, 21, 257–266. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, W.C.; Hsu, C.M.; Tsai, F.J.; Tsai, C.H. Tumor necrosis factor α, CYP 17, urokinase, and interleukin 10 gene polymorphisms in postmenopausal women: Correlation to bone mineral density and susceptibility to osteoporosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 73–78. [Google Scholar] [CrossRef]
- Tu, M.Y.; Chen, H.L.; Tung, Y.T.; Kao, C.C.; Hu, F.C.; Chen, C.M. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Chen, W.C.; Hsu, C.D.; Tsai, F.J.; Tsai, C.H. Relation of vitamin D receptor FokI start codon polymorphism to bone mineral density and occurrence of osteoporosis in postmenopausal women in Taiwan. Acta Obstet. Gynecol. Scand. 2002, 81, 93–98. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, I.C.; Huang, C.S.; Hu, F.C.; Kuo, W.H.; Kuo, K.; Wang, C.C.; Wu, P.F.; Chang, D.Y.; Wang, M.Y.; et al. TP53 Mutational Analysis Enhances the Prognostic Accuracy of IHC4 and PAM50 Assays. Sci. Rep. 2015, 5, 17879. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.Y.; Huang, J.W.; Tsai, W.C.; Peng, Y.S.; Chen, H.Y.; Yang, J.Y.; Hsu, S.-P.; Pai, M.-F.; Ko, M.-J.; Hung, K.-Y.; et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: A prospective cohort study. PLoS ONE 2018, 13, e0203474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yee, T.W.; Wild, C.J. Vector Generalized Additive Models. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 481–493. [Google Scholar] [CrossRef]
- Tzakas, P.; Wong, B.Y.; Logan, A.G.; Rubin, L.A.; Cole, D.E. Transforming growth factor β-1 (TGFB1) and peak bone mass: Association between intragenic polymorphisms and quantitative ultrasound of the heel. BMC Musculoskelet. Disord. 2005, 6. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Ng, C.T.; Song, Y.Q. Genetic disorders associated with osteoporosis. In Advances in Osteoporosis; IntechOpen: London, UK, 2015; pp. 19–36. [Google Scholar]
- Al Anouti, F.; Taha, Z.; Shamim, S.; Khalaf, K.; Al Kaabi, L.; Alsafar, H.S. An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone Rep. 2019, 11, 100216. [Google Scholar] [CrossRef]
- Li, J.; Ayoub, A.; Xiu, Y.; Yin, X.; Sanders, J.O.; Mesfin, A.; Xing, L.; Yao, Z.; Boyce, B.F. TGFβ-induced degradation of TRAF3 in mesenchymal progenitor cells causes age-related osteoporosis. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Loots, G.G.; Keller, H.; Leupin, O.; Murugesh, D.; Collette, N.M.; Genetos, D.C. TGF-β regulates sclerostin expression via the ECR5 enhancer. Bone 2012, 50, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Langdahl, B.L.; Uitterlinden, A.G.; Ralston, S.H.; Trikalinos, T.A.; Balcells, S.; Brandi, M.L.; Scollen, S.; Lips, P.; Lorenc, R.S.; Obermayer-Pietsch, B.; et al. Large-scale analysis of association between polymorphisms in the transforming growth factor β1 gene (TGFB1) and osteoporosis: The GENOMOS study. Bone 2008, 42, 969–981. [Google Scholar] [CrossRef]
- Yamada, Y.; Miyauchi, A.; Takagi, Y.; Nakauchi, K.; Miki, N.; Mizuno, M.; Harada, A. Association of a polymorphism of the transforming growth factor β-1 gene with prevalent vertebral fractures in Japanese women. Am. J. Med. 2000, 109, 244–247. [Google Scholar] [CrossRef]
- Hinke, V.; Seck, T.; Clanget, C.; Scheidt-Nave, C.; Ziegler, R.; Pfeilschifter, J. Association of transforming growth factor-β1 T29→C gene polymorphism with bone mineral density (BMD), changes in BMD, and serum concentrations of TGF-β 1 in a population-based sample of postmenopausal German women. Calcif. Tissue Int. 2001, 69, 315–320. [Google Scholar] [CrossRef]
- Ziv, E.; Kahn, A.; Cauley, J.; Morin, P.; Saiz, R.; Browner, W. No association between the TGF-β 1 Leu10Pro polymorphism and osteoporosis among white women in the United States. Am. J. Med. 2003, 114, 227–231. [Google Scholar] [CrossRef]
- Utennam, D.; Tungtrongchitr, A.; Phonrat, B.; Tungtrongchitr, R.; Preutthipan, S. Association of T869C gene polymorphism of transforming growth factor-β1 with low protein levels and anthropometric indices in osteopenia/osteoporosis postmenopausal Thai women. Genet. Mol. Res. 2012, 11, 87–99. [Google Scholar] [CrossRef]
- Xue, C.; Nie, W.; Xu, J.; Dai, B.; Mei, C.L. C509T and T869C polymorphisms of transforming growth factor β1 and the risk of IgA nephropathy: A meta-analysis. Chin. Med. J. 2013, 126, 4562–4567. [Google Scholar]
- Yim, H.E.; Bae, I.S.; Yoo, K.H.; Hong, Y.S.; Lee, J.W. Genetic control of VEGF and TGF-β 1gene polymorphisms in childhood urinary tract infection and vesicoureteral reflux. Pediatr. Res. 2007, 62, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Park, B.L.; Han, I.K.; Lee, H.S.; Kim, L.H.; Kim, S.J.; Shin, J.S.; Kim, S.-Y.; Shin, H.D. Association of Interleukin 10 Haplotype with Low Bone Mineral Density in Korean Postmenopausal Women. J. Biochem. Mol. Biol. 2004, 37, 691–699. [Google Scholar] [CrossRef]
- Kotrych, D.; Dziedziejko, V.; Safranow, K.; Sroczynski, T.; Staniszewska, M.; Juzyszyn, Z.; Pawlik, A. TNF-α and IL10 gene polymorphisms in women with postmenopausal osteoporosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 199, 92–95. [Google Scholar] [CrossRef]
- Tseng, L.H.; Lin, M.T.; Shau, W.Y.; Lin, W.C.; Chang, F.Y.; Chien, K.L.; Hansen, J.A.; Chen, D.-S.; Chen, D.S. Correlation of interleukin-10 gene haplotype with hepatocellular carcinoma in Taiwan. Tissue Antigens 2006, 67, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, W.; Zhao, Y.; Chen, Y.; Qin, L.; Zhang, H.; Zhang, S. IL-10 and IL-10RB gene polymorphisms are correlated with hepatitis B-related hepatocellular carcinoma in the Chinese Han population. Transl. Cancer Res. 2017, 6, 432–440. [Google Scholar] [CrossRef]
- Cavedon, V.; Milanese, C.; Laginestra, F.G.; Giuriato, G.; Pedrinolla, A.; Ruzzante, F.; Schena, F.; Venturelli, M. Bone and skeletal muscle changes in oldest-old women: The role of physical inactivity. Aging Clin. Exp. Res. 2019, 32, 207–214. [Google Scholar] [CrossRef]
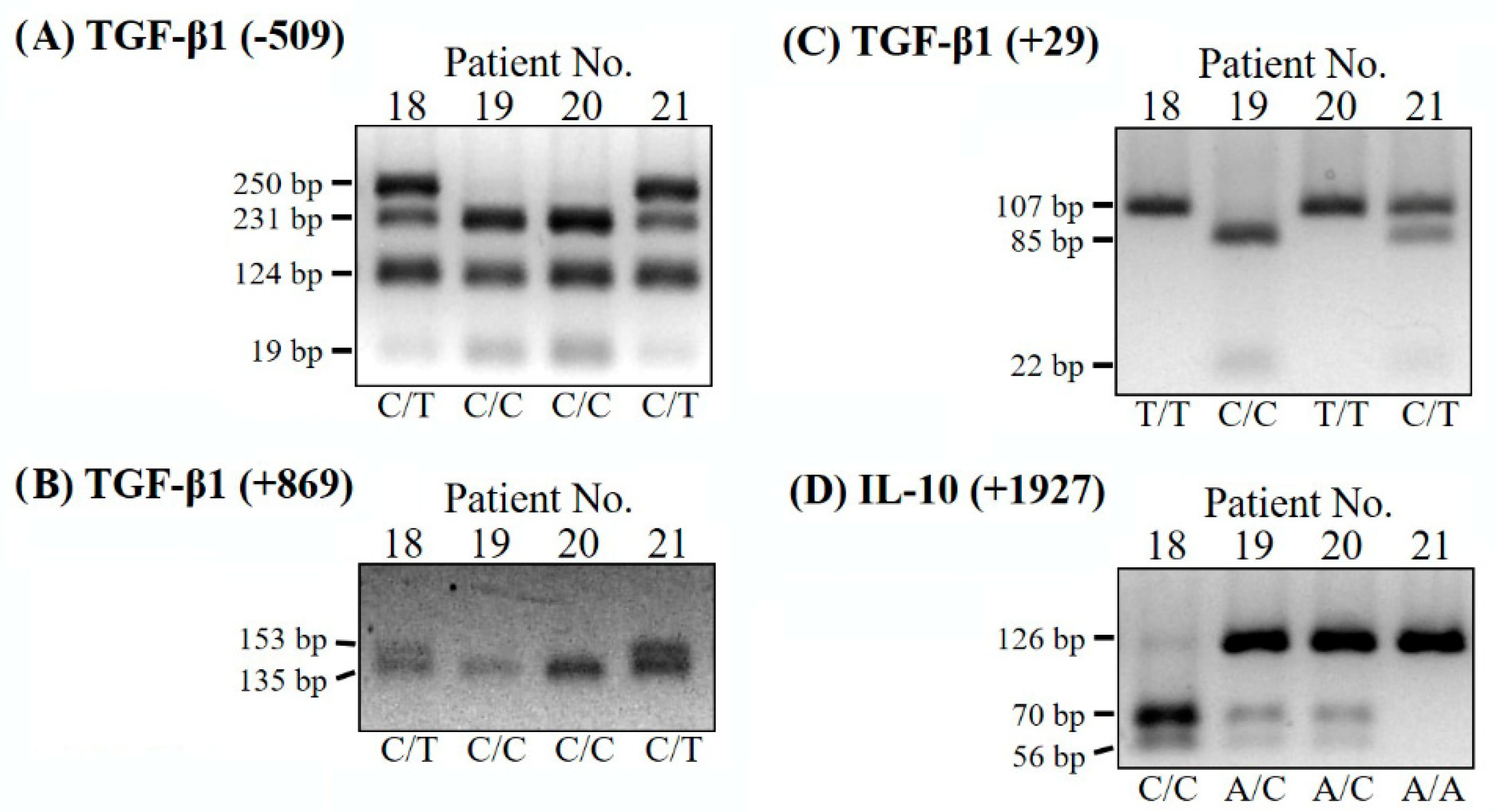
| SNPs | Oligonucleotide PRIMERS (F: Forward Primer; R: Reverse Primer) | Restriction Enzyme (PCR Size) | PCR-RFLP Fragments (bp) | Genotype |
|---|---|---|---|---|
| TGF-β1 SNP (−509 C/T) | F: 5′-CCAGCTAAGGCATGGCACCG-3′ R: 5′-GCGGTGTGGGTCACCAGAGA-3′ | Dde I (380 bp) | 231, 124, 19, 6 250, 231,124, 19, 6 250, 124, 6 | C/C C/T T/T |
| TGF-β1 SNP (+29 C/T) | F: 5′-ACCACACCAGCCCTGTTCGCGC-3′ R: 5′-AGCCACAGCAGCGGTAGCAGGA-3′ | BsrB I (107 bp) | 107 107, 85, 22 85, 22 | T/T C/T C/C |
| TGF-β1 SNP (+869 C/T) | F: 5′-TCCGGGCTGCGGCTGCAGC-3′ R: 5′-CAGGATCTGGCCGCGGATGG-3′ | Pvu II (153 bp) | 153 153, 135, 18 135, 18 | T/T C/T C/C |
| IL-10 SNP (+1927 A/C) | F: 5′-GCTTCTGCTTTCCCTTCAAAAT-3′ R: 5′-AAATCAAAAGGGGAGTTTTAAA-3′ | Aci I (126 bp) | 126 126, 70, 56 70, 56 | A/A A/C C/C |
| Parameter 1 | Patients with Osteoporosis/Osteopenia with Fractures (n = 88) | Controls (n = 129) | p-Value 2 |
|---|---|---|---|
| Sample Size (n) | 88/217 (40.6%) | 129/217 (59.4%) | |
| Gender | 0.534 | ||
| Male | 21/57 (36.8%) | 36/57 (63.2%) | |
| Female | 67/160 (41.9%) | 93/160 (58.1%) | |
| Age (years old) | 65.69 ± 13.12 | 57.71 ± 10.44 | <0.001 |
| Height (cm) | 154.72 ± 9.27 | 160.69 ± 7.25 | <0.001 |
| Weight (kg) | 58.53 ± 10.41 | 62.62 ± 9.81 | 0.008 |
| BMI (kg/m2) | 24.48 ± 4.18 | 24.26 ± 3.50 | 0.786 |
| Ca Intake (mg/day) | 697.20 ± 234.11 | 822.16 ± 271.25 | 0.001 |
| Vegetarian | 0.064 | ||
| Yes | 6/8 (75.0%) | 2/8 (25.0%) | |
| No | 82/209 (39.2%) | 127/209 (60.8%) | |
| TGF-β1 SNP (−509) | 0.838 | ||
| C/C | 13/35 (37.1%) | 22/35 (62.9%) | |
| C/T | 52/121 (43.0%) | 69/121 (57.0%) | |
| T/T | 23/61 (37.7%) | 38/61(62.3%) | |
| IL-10 SNP (+1927) | 0.439 | ||
| A/A | 22/64 (34.4%) | 42/64 (65.6%) | |
| A/C | 43/98 (43.9%) | 55/98 (56.1%) | |
| C/C | 23/55 (41.8%) | 32/55 (58.2%) | |
| Lumbar Spine T-Score | −2.01 ± 1.14 | −0.29 ± 1.10 | <0.001 |
| Femoral Neck T-Score | −2.19 ± 0.85 | −0.61 ± 0.91 | <0.001 |
| Total Hip T-Score | −1.66 ± 1.03 | 0.03 ± 0.90 | <0.001 |
| Covariate | Estimate | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Y1 = Spinal BMD T-Score 1 | ||||
| Intercept | −10.5181 | 1.8483 | −5.691 | <0001 |
| Height (cm) | 0.0577 | 0.0116 | 4.970 | <0001 |
| 33 < Age ≤ 55 (years old) | 0.6541 | 0.1928 | 3.393 | 0.0009 |
| Ca Intake > 800 (mg/day) | 0.3764 | 0.1869 | 2.014 | 0.0458 |
| TGF-β1 SNP (−509 T/T) × IL-10 SNP (+1927 A/A + A/C) | 0.4242 | 0.2374 | 1.787 | 0.0759 |
| Female × TGF-β1 SNP (−509 C/C) × IL-10 SNP (+1927 C/C) | 1.0417 | 0.6944 | 1.500 | 0.1356 |
| Covariate | Estimate | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Y2 = Femoral Neck BMD T-Score 1 | ||||
| Intercept | −10.7755 | 1.9377 | −5.561 | <0.0001 |
| 30 < Age ≤ 68 (years old) | 0.4654 | 0.2055 | 2.265 | 0.0248 |
| Height (cm) | 0.0551 | 0.0119 | 4.642 | <0.0001 |
| Ca Intake ≥ 855 (mg/day) | 0.3726 | 0.1499 | 2.485 | 0.0140 |
| Female × Age ≥ 55 (years old) | −0.5283 | 0.1929 | −2.739 | 0.0068 |
| Height (cm) × Female | 0.0049 | 0.0016 | 3.093 | 0.0023 |
| Female × TGF-β1 SNP (−509 C/C) × IL-10 SNP (+1927 C/C) | 1.1666 | 0.5850 | 1.994 | 0.0478 |
| Female × TGF-β1 SNP (−509 T/T) × IL-10 SNP (+1927 C/C + A/C) | 0.4220 | 0.2191 | 1.926 | 0.0559 |
| Covariate | Estimate | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Y3 = Total Hip BMD T-Score 1 | ||||
| Intercept | −6.1780 | 2.2805 | −2.709 | 0.0075 |
| Height (cm) | 0.0331 | 0.0137 | 2.422 | 0.0165 |
| Ca Intake > 875 (mg) | 0.4561 | 0.1532 | 2.976 | 0.0034 |
| Height (cm) × Female | 0.0229 | 0.0034 | 6.658 | <0.0001 |
| Female × Age (years) | −0.0560 | 0.0102 | −5.509 | <0.0001 |
| TGF-β1 SNP (−509 T/T) × IL-10 SNP (+1927 A/A) | −1.4692 | 0.4704 | −3.123 | 0.0021 |
| Covariate | Estimate | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Y4 = Height (cm) 1 | ||||
| Intercept | 187.3222 | 2.5461 | 73.573 | <0.0001 |
| Female × TGF-β1 SNP (−509 T/C) × IL-10 SNP (+1927 A/C) | −11.4040 | 1.0245 | −11.132 | <0.0001 |
| Age × TGF-β1 SNP (−509 T/C) × IL-10 SNP (+1927 A/C) | −0.3503 | 0.0381 | −9.208 | <0.0001 |
| TGF-β1 SNP (−509 C/T) | 1.8464 | 0.8926 | 2.069 | 0.0402 |
| Female × TGF-β1 SNP (−509 C/C + C/T) × IL-10 SNP (+1927 C/C) | −4.3845 | 1.4081 | −3.114 | 0.0022 |
| Male × TGF-β1 SNP (−509 T/T) × IL-10 SNP (+1927 A/C) | 7.8140 | 2.7417 | 2.850 | 0.0049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, M.-Y.; Han, K.-Y.; Lan, Y.-W.; Chang, K.-Y.; Lai, C.-W.; Staniczek, T.; Lai, C.-Y.; Chong, K.-Y.; Chen, C.-M. Association of TGF-β1 and IL-10 Gene Polymorphisms with Osteoporosis in a Study of Taiwanese Osteoporotic Patients. Genes 2021, 12, 930. https://doi.org/10.3390/genes12060930
Tu M-Y, Han K-Y, Lan Y-W, Chang K-Y, Lai C-W, Staniczek T, Lai C-Y, Chong K-Y, Chen C-M. Association of TGF-β1 and IL-10 Gene Polymorphisms with Osteoporosis in a Study of Taiwanese Osteoporotic Patients. Genes. 2021; 12(6):930. https://doi.org/10.3390/genes12060930
Chicago/Turabian StyleTu, Min-Yu, Kuei-Yang Han, Ying-Wei Lan, Ku-Yi Chang, Cheng-Wei Lai, Theresa Staniczek, Chung-Yu Lai, Kowit-Yu Chong, and Chuan-Mu Chen. 2021. "Association of TGF-β1 and IL-10 Gene Polymorphisms with Osteoporosis in a Study of Taiwanese Osteoporotic Patients" Genes 12, no. 6: 930. https://doi.org/10.3390/genes12060930
APA StyleTu, M. -Y., Han, K. -Y., Lan, Y. -W., Chang, K. -Y., Lai, C. -W., Staniczek, T., Lai, C. -Y., Chong, K. -Y., & Chen, C. -M. (2021). Association of TGF-β1 and IL-10 Gene Polymorphisms with Osteoporosis in a Study of Taiwanese Osteoporotic Patients. Genes, 12(6), 930. https://doi.org/10.3390/genes12060930








