Genome-Wide Association Studies of Conotruncal Heart Defects with Normally Related Great Vessels in the United States
Abstract
:1. Introduction
2. Materials and Methods
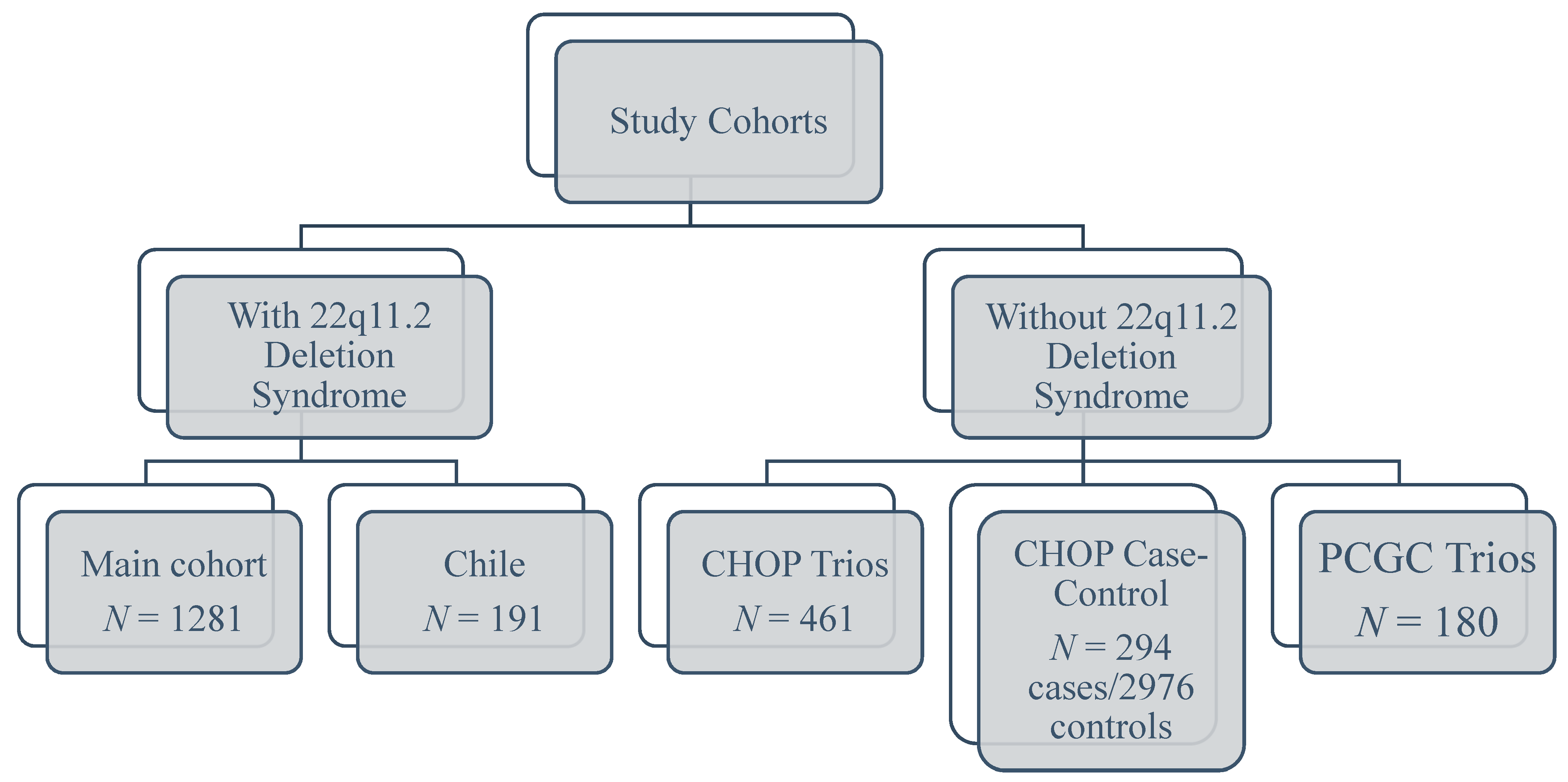
2.1. Study Subjects
2.1.1. Subjects without 22q11.2DS
2.1.2. Subjects with 22q11.2DS
2.2. Genotyping, Quality Control, and Prior Analyses
2.2.1. Subjects without 22q11.2DS
2.2.2. Subjects with 22q11.2DS
2.3. Statistical Methods
2.3.1. SNP-Level Analyses
2.3.2. Gene-Level Analyses
2.3.3. Candidate Gene Set Analyses
2.3.4. Interpretation
3. Results
3.1. SNP-Level
3.2. Gene-Level
3.3. Gene Sets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howell, H.B.; Zaccario, M.; Kazmi, S.H.; Desai, P.; Sklamberg, F.E.; Mally, P. Neurodevelopmental outcomes of children with congenital heart disease: A review. Curr. Probl. Pediatr. Adolesc. Health Care 2019, 49, 100685. [Google Scholar] [CrossRef]
- Shabana, N.A.; Shahid, S.U.; Irfan, U. Genetic Contribution to Congenital Heart Disease (CHD). Pediatr. Cardiol. 2020, 41, 12–23. [Google Scholar] [CrossRef]
- Kirby, R.S. The prevalence of selected major birth defects in the United States. Semin. Perinatol. 2017, 41, 338–344. [Google Scholar] [CrossRef]
- Lahiri, S.; Gil, W.; Daria, S.; Joshua, G.; Parul, J.; Redmond, B.; Elizabeth, W. Genetic abnormalities/syndromes significantly impact perioperative outcomes of conotruncal heart defects. Ann. Pediatr. Cardiol. 2020, 13, 38–45. [Google Scholar] [CrossRef] [PubMed]
- DeLea, M.; Espeche, L.D.; Bruque, C.D.; Bidondo, M.P.; Massara, L.S.; Oliveri, J.; Brun, P.; Cosentino, V.R.; Martinoli, C.; Tolaba, N.; et al. Genetic Imbalances in Argentinean Patients with Congenital Conotruncal Heart Defects. Genes 2018, 9, 454. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Diacou, A.; Johnston, H.R.; Musfee, F.I.; McDonald-McGinn, D.M.; McGinn, D.; Crowley, T.B.; Repetto, G.M.; Swillen, A.; Breckpot, J.; et al. Complete Sequence of the 22q11.2 Allele in 1,053 Subjects with 22q11.2 Deletion Syndrome Reveals Modifiers of Conotruncal Heart Defects. Am. J. Hum. Genet. 2020, 106, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Webber, D.M.; MacLeod, S.L.; Hobbs, C.A.; Li, M.; the National Birth Defects Prevention Study. Gene-by-gene interactions associated with the risk of conotruncal heart defects. Mol. Genet. Genom. Med. 2019, 8, e1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mlynarski, E.E.; Sheridan, M.B.; Xie, M.; Guo, T.; Racedo, S.E.; McDonald-McGinn, D.M.; Gai, X.; Chow, E.W.; Vorstman, J.; Swillen, A.; et al. Copy-Number Variation of the Glucose Transporter Gene SLC2A3 and Congenital Heart Defects in the 22q11.2 Deletion Syndrome. Am. J. Hum. Genet. 2015, 96, 753–764. [Google Scholar] [CrossRef] [Green Version]
- Mlynarski, E.E.; Xie, M.; Taylor, D.; Sheridan, M.B.; Guo, T.; Racedo, S.E.; McDonald-McGinn, D.M.; Chow, E.W.C.; Vorstman, J.; Swillen, A.; et al. Rare copy number variants and congenital heart defects in the 22q11.2 deletion syndrome. Qual. Life Res. 2016, 135, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.M.; Taylor, D.M.; Zhang, Z.; McDonald-McGinn, D.M.; Zackai, E.H.; Stambolian, D.; Hakonarson, H.; Morrow, B.E.; Emanuel, B.S.; Goldmuntz, E. Copy number variations in individuals with conotruncal heart defects reveal some shared developmental pathways irrespective of 22q11.2 deletion status. Birth Defects Res. 2019, 111, 888–905. [Google Scholar] [CrossRef]
- Xie, H.M.; Werner, P.; Stambolian, D.; Bailey-Wilson, J.E.; Hakonarson, H.; White, P.S.; Taylor, D.M.; Goldmuntz, E. Rare copy number variants in patients with congenital conotruncal heart defects. Birth Defects Res. 2017, 109, 271–295. [Google Scholar] [CrossRef] [Green Version]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Agopian, A.J.; Goldmuntz, E.; Hakonarson, H.; Sewda, A.; Taylor, D.; Mitchell, L.E.; Pediatric Cardiac Genomics Consortium. Genome-Wide Association Studies and Meta-Analyses for Congenital Heart Defects. Circ. Cardiovasc. Genet. 2017, 10, e001449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewda, A.; Agopian, A.J.; Goldmuntz, E.; Hakonarson, H.; Morrow, B.E.; Musfee, F.; Taylor, D.; Mitchell, L.E.; Pediatric Cardiac Genomics Consortium. Gene-based analyses of the maternal genome implicate maternal effect genes as risk factors for conotruncal heart defects. PLoS ONE 2020, 15, e0234357. [Google Scholar] [CrossRef]
- Goldmuntz, E.; Clark, B.J.; Mitchell, L.E.; Jawad, A.F.; Cuneo, B.F.; Reed, L.; McDonald-McGinn, D.; Chien, P.; Feuer, J.; Zackai, E.H.; et al. Frequency of 22q11 deletions in patients with conotruncal defects. J. Am. Coll. Cardiol. 1998, 32, 492–498. [Google Scholar] [CrossRef] [Green Version]
- Peyvandi, S.; Lupo, P.J.; Garbarini, J.; Woyciechowski, S.; Edman, S.; Emanuel, B.S.; Mitchell, L.E.; Goldmuntz, E. 22q11.2 Deletions in Patients with Conotruncal Defects: Data from 1,610 Consecutive Cases. Pediatr. Cardiol. 2013, 34, 1687–1694. [Google Scholar] [CrossRef]
- Wilcox, A.; Weinberg, C.; Lie, R.T. Distinguishing the Effects of Maternal and Offspring Genes through Studies of “Case-Parent Triads”. Am. J. Epidemiol. 1998, 148, 893–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Repetto, G.M.; McGinn, D.M.M.; Chung, J.H.; Nomaru, H.; Campbell, C.L.; Blonska, A.; Bassett, A.S.; Chow, E.W.; Mlynarski, E.E.; et al. Genome-Wide Association Study to Find Modifiers for Tetralogy of Fallot in the 22q11.2 Deletion Syndrome Identifies Variants in the GPR98 Locus on 5q14.3. Circ. Cardiovasc. Genet. 2017, 10, e001690. [Google Scholar] [CrossRef] [Green Version]
- Sewda, A.; Agopian, A.J.; Goldmuntz, E.; Hakonarson, H.; Morrow, B.E.; Taylor, D.; Mitchell, L.E.; on behalf of the Pediatric Cardiac Genomics Consortium. Gene-based genome-wide association studies and meta-analyses of conotruncal heart defects. PLoS ONE 2019, 14, e0219926. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, H.F.; Unwin, J.; Jamison, D.L.; Cordell, H.J. Investigation of maternal effects, maternal-fetal interactions and parent-of-origin effects (imprinting), using mothers and their offspring. Genet. Epidemiol. 2011, 35, 19–45. [Google Scholar] [CrossRef] [Green Version]
- Howey, R.; Cordell, H.J. PREMIM and EMIM: Tools for estimation of maternal, imprinting and interaction effects using multinomial modelling. BMC Bioinform. 2012, 13, 149. [Google Scholar] [CrossRef] [Green Version]
- Magi, R.; Morris, A.P. GWAMA: Software for genome-wide association meta-analysis. BMC Bioinform. 2010, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- De Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef] [PubMed]
- Norris, F.A.; Atkins, R.C.; Majerus, P.W. The cDNA cloning and characterization of inositol polyphosphate 4-phosphatase type II. Evidence for conserved alternative splicing in the 4-phosphatase family. J. Biol. Chem. 1997, 272, 23859–23864. [Google Scholar] [CrossRef] [Green Version]
- Gewinner, C.; Wang, Z.C.; Richardson, A.; Teruya-Feldstein, J.; Etemadmoghadam, D.; Bowtell, D.; Barretina, J.; Lin, W.M.; Rameh, L.; Salmena, L.; et al. Evidence that Inositol Polyphosphate 4-Phosphatase Type II is a Tumor Suppressor that Inhibits PI3K Signaling. Cancer Cell 2009, 16, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Chen, Y.; Chen, Q.; Wang, G.; Xu, X.; Peng, A.; Hao, J.; He, J.; Huang, L.; Dai, J. Clinical presentation and genetic profiles of Chinese patients with velocardiofacial syndrome in a large referral centre. J. Genet. 2019, 98, 42. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, T.; Yu, M.S.; McKeithan, W.L.; Spiering, S.; Carrette, F.; Huang, C.-T.; Bushway, P.J.; Tierney, M.; Albini, S.; Giacca, M.; et al. Id genes are essential for early heart formation. Genes Dev. 2017, 31, 1325–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, L.A.; Kirby, M.L. The role of secondary heart field in cardiac development. Dev. Biol. 2009, 336, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoak, I.W.; Byrd, N.; Abu-Issa, R.; Goddeeris, M.; Anderson, R.; Morris, J.; Yamamura, K.; Klingensmith, J.; Meyers, E. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev. Biol. 2005, 283, 357–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A.F.; Yu, K.P.; Brueckner, M.; Brailey, L.L.; Johnson, L.; McGrath, J.M.; Bale, A.E. Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development 2005, 132, 4407–4417. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, M.; Wirrig, E.; Phelps, A.; Wessels, A. Extracellular matrix and heart development. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 535–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene Name (Gene Symbol) | Chr 3 | Start 4 | Stop | Cohort with 22q.11.2 Deletion Syndrome | Cohort without a 22q.11.2 Deletion | All Cohorts Combined |
|---|---|---|---|---|---|---|
| Even-Skipped Homeobox 1 (EVX1) | 7 | 27281164 | 27288438 | 2.40 × 10−3 | 4.20 × 10−2 | 8.33 × 10−5 |
| Inositol Polyphosphate-4-Phosphatase Type II B (INPP4B) | 4 | 142948181 | 143768604 | 1.60 × 10−2 | 4.40 × 10−2 | 5.00 × 10−4 |
| Solute Carrier Family 35 Member G5 (SLC35G5) | 8 | 11187495 | 11190695 | 4.30 × 10−2 | 4.60 × 10−3 | 5.00 × 10−4 |
| Rho GTPase Activating Protein 26 (ARHGAP26) | 5 | 142148881 | 142609572 | 4.60 × 10−2 | 1.70 × 10−2 | 6.00 × 10−4 |
| Defensin Beta 134 (DEFB134) | 8 | 11850489 | 11854760 | 1.10 × 10−2 | 4.70 × 10−2 | 6.00 × 10−4 |
| Calsequestrin 2 (CASQ2) | 1 | 116241624 | 116312426 | 1.90 × 10−2 | 4.70 × 10−2 | 6.50 × 10−4 |
| Patched 1 (PTCH1) | 9 | 98204264 | 98280247 | 3.10 × 10−2 | 1.10 × 10−2 | 7.00 × 10−4 |
| Stabilin 2 (STAB2) | 12 | 103980069 | 104161502 | 2.50 × 10−2 | 1.12 × 10−2 | 8.50 × 10−4 |
| Cohort with 22q.11.2 Deletion Syndrome | Cohort without 22q.11.2 Deletion Syndrome | All Cohorts Combined | ||
|---|---|---|---|---|
| Gene Set | Number of Genes | p-Value | p-Value | p-Value |
| All gene sets combined | 250 | 0.274 | 0.333 | 0.016 |
| Gene Silencing by RNA | 6 | 0.453 | 0.007 | 0.007 |
| Integrin signaling pathway | 29 | 0.067 | 0.417 | 0.288 |
| TGF-beta signaling pathway | 22 | 0.358 | 0.148 | 0.085 |
| ECM-receptor interaction | 16 | 0.006 | 0.075 | 0.050 |
| Regulation of mitotic cell cycle | 10 | 0.840 | 0.736 | 0.191 |
| G alpha (q) signaling events | 26 | 0.036 | 0.649 | 0.230 |
| Basal transcription factors | 6 | 0.044 | 0.670 | 0.134 |
| Cardiac conduction | 20 | 0.266 | 0.773 | 0.253 |
| Mitochondrial translation | 4 | 0.923 | 0.187 | 0.448 |
| Mitotic Prometaphase | 34 | 0.757 | 0.352 | 0.399 |
| Processing of Capped Intron-Containing Pre-mRNA | 27 | 0.889 | 0.376 | 0.409 |
| MAPK signaling pathway | 40 | 0.915 | 0.648 | 0.516 |
| Neddylation | 6 | 0.774 | 0.877 | 0.794 |
| Chromatin organization | 4 | 0.327 | 0.987 | 0.983 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwafemi, O.O.; Musfee, F.I.; Mitchell, L.E.; Goldmuntz, E.; Xie, H.M.; Hakonarson, H.; Morrow, B.E.; Guo, T.; Taylor, D.M.; McDonald-McGinn, D.M.; et al. Genome-Wide Association Studies of Conotruncal Heart Defects with Normally Related Great Vessels in the United States. Genes 2021, 12, 1030. https://doi.org/10.3390/genes12071030
Oluwafemi OO, Musfee FI, Mitchell LE, Goldmuntz E, Xie HM, Hakonarson H, Morrow BE, Guo T, Taylor DM, McDonald-McGinn DM, et al. Genome-Wide Association Studies of Conotruncal Heart Defects with Normally Related Great Vessels in the United States. Genes. 2021; 12(7):1030. https://doi.org/10.3390/genes12071030
Chicago/Turabian StyleOluwafemi, Omobola O., Fadi I. Musfee, Laura E. Mitchell, Elizabeth Goldmuntz, Hongbo M. Xie, Hakon Hakonarson, Bernice E. Morrow, Tingwei Guo, Deanne M. Taylor, Donna M. McDonald-McGinn, and et al. 2021. "Genome-Wide Association Studies of Conotruncal Heart Defects with Normally Related Great Vessels in the United States" Genes 12, no. 7: 1030. https://doi.org/10.3390/genes12071030






