Reelin Alleviates Mesenchymal Stem Cell Senescence and Reduces Pathological α-Synuclein Expression in an In Vitro Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cell Culture
2.3. Cell Viability and Senescence Analysis
2.4. RNA Preparation
2.5. RNA Sequencing Transcriptome Analysis
2.6. Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
2.7. Reverse Transcription and Quantitative Reverse Transcription Polymerase Chain Reaction
2.8. Western Blot Analysis
2.9. Preformed Fibril and Human Recombinant Reelin Treatment
2.10. Statistical Analysis
3. Results
3.1. Cell Viability Was Decreased in Parkinson’s Disease
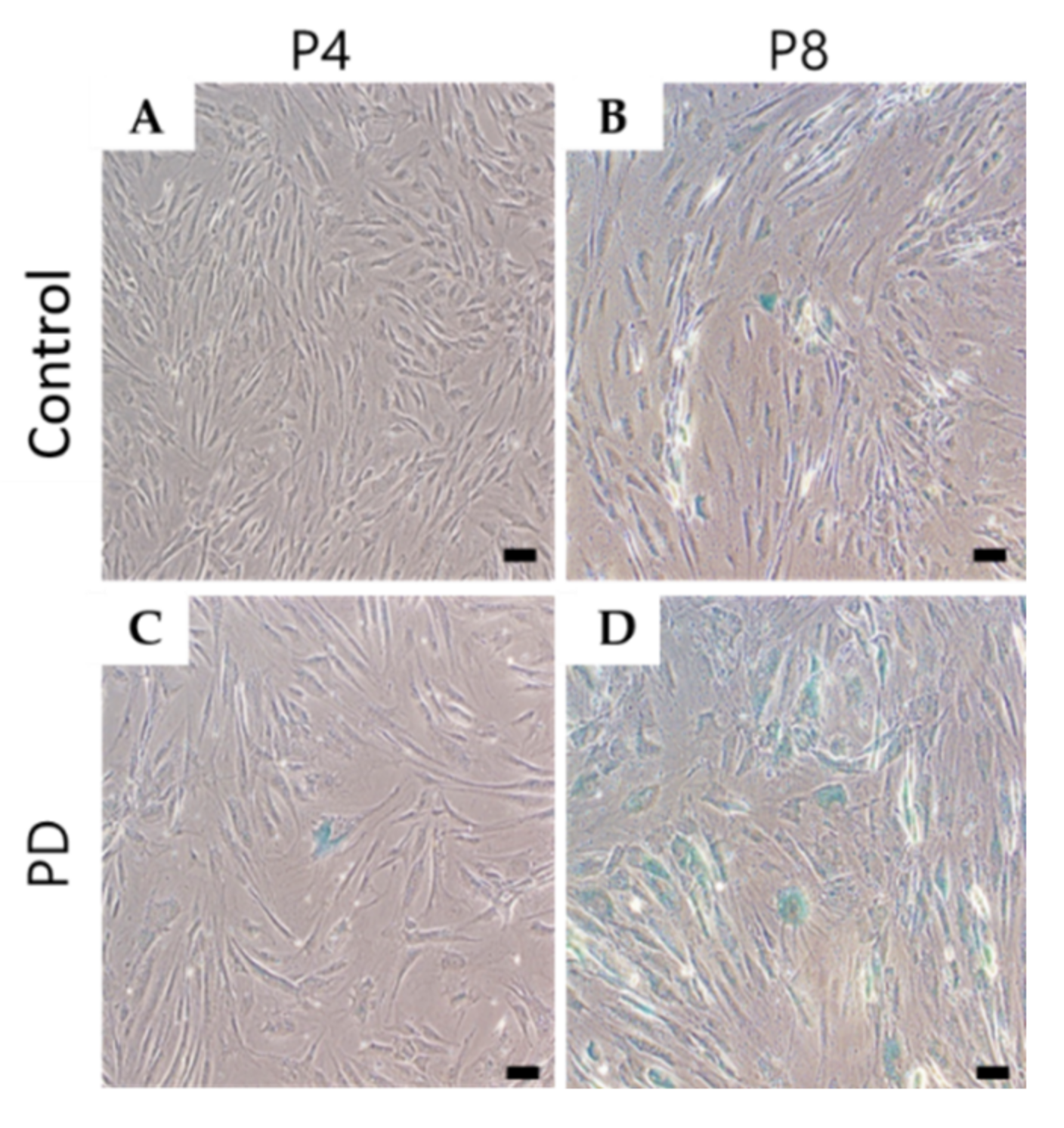
3.2. Cell Senescence Was Increased in Parkinson’s Disease
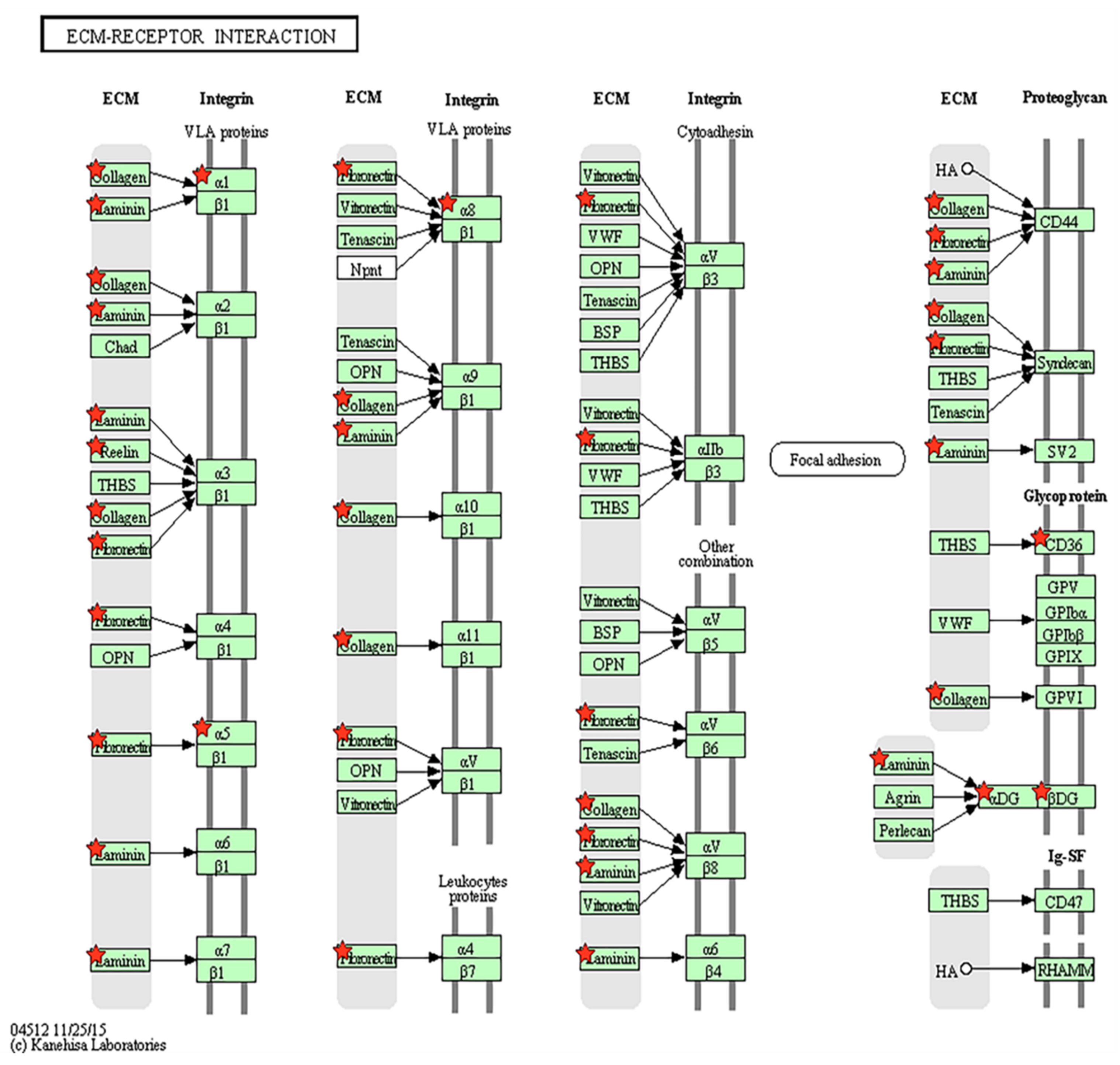
3.3. Extracellular Matrix-Receptor Interaction-Related Genes Were Down-Regulated in Parkinson’s Disease
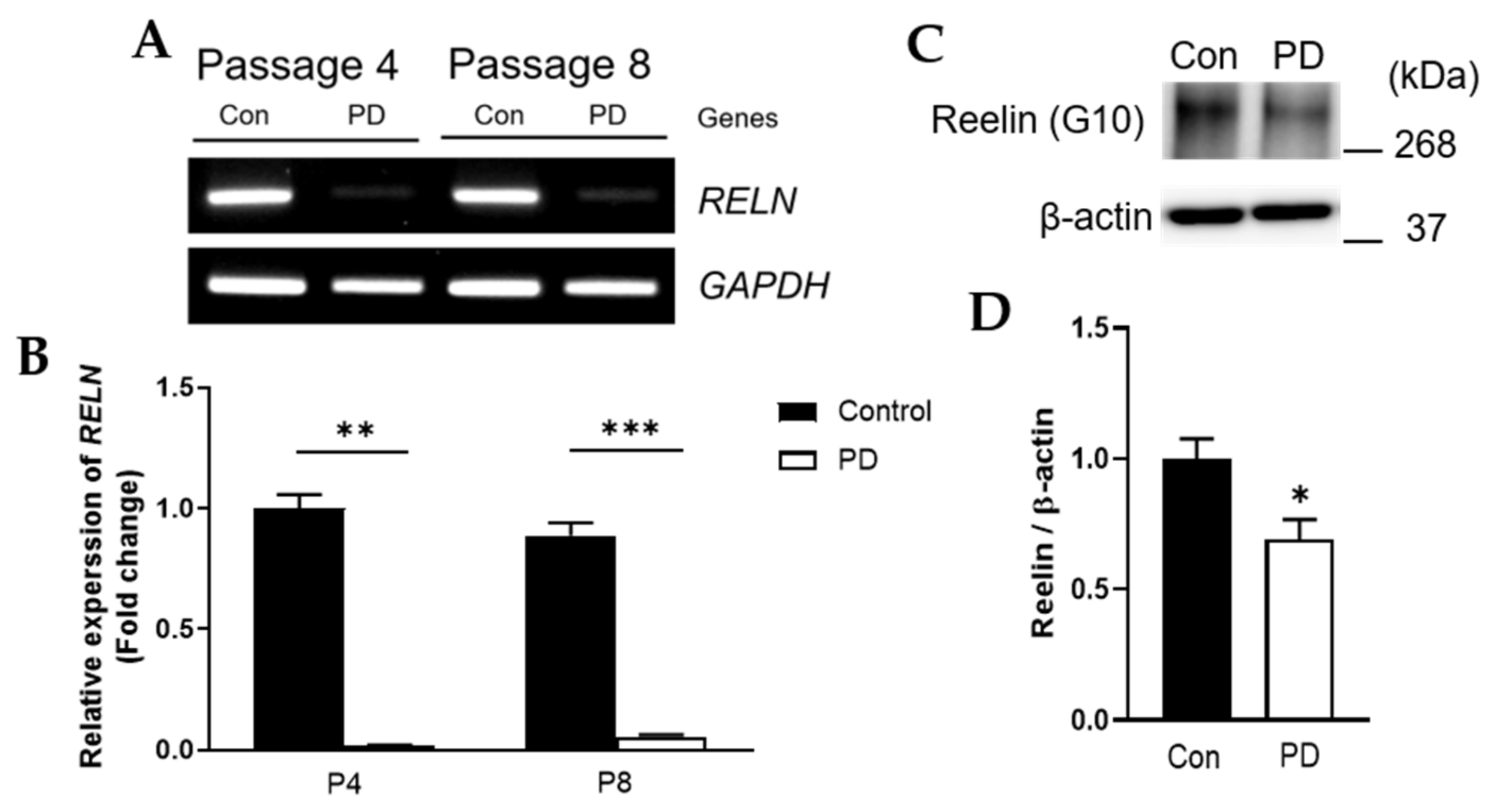
3.4. Both RELN mRNA and Reelin Protein Levels Were Down-Regulated in Parkinson’s Disease
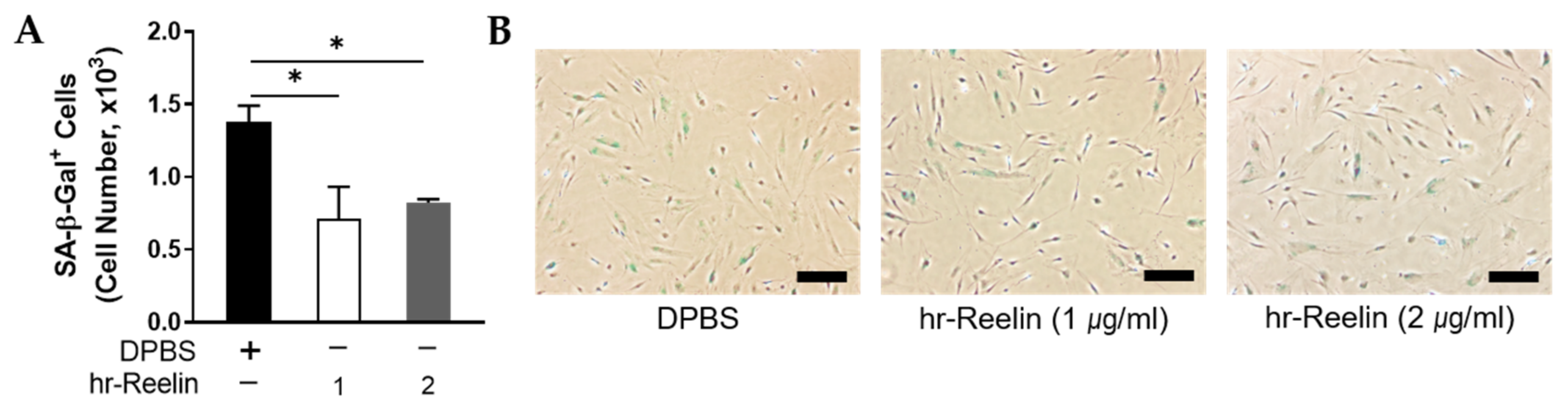
3.5. Recombinant Reelin Protein Regulates Cell Viability and Senescence
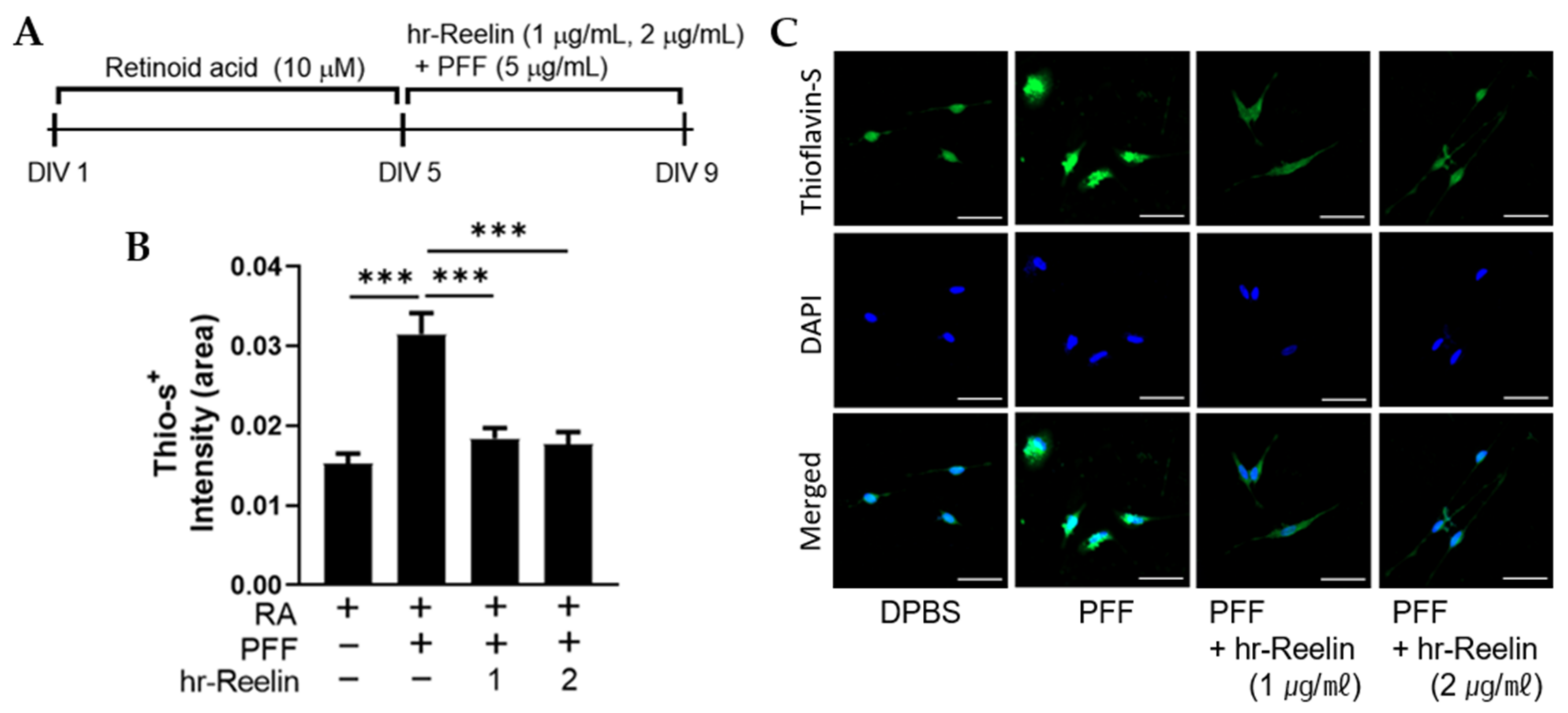
3.6. Recombinant Reelin Protein Attenuates α-Synuclein Aggragation in SH-SY5Y
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantuti-Castelvetri, I.; Keller-McGandy, C.; Bouzou, B.; Asteris, G.; Clark, T.W.; Frosch, M.P.; Standaert, D.G. Effects of gender on nigral gene expression and parkinson disease. Neurobiol. Dis. 2007, 26, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherzer, C.R.; Eklund, A.C.; Morse, L.J.; Liao, Z.; Locascio, J.J.; Fefer, D.; Schwarzschild, M.A.; Schlossmacher, M.G.; Hauser, M.A.; Vance, J.M. Molecular markers of early Parkinson’s disease based on gene expression in blood. Proc. Natl. Acad. Sci. USA 2007, 104, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstner, M.; Morris, C.M.; Heim, K.; Bender, A.; Mehta, D.; Jaros, E.; Klopstock, T.; Meitinger, T.; Turnbull, D.M.; Prokisch, H. Expression analysis of dopaminergic neurons in Parkinson’s disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathol. 2011, 122, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Liao, Z.; Locascio, J.J.; Lesniak, K.A.; Roderick, S.S.; Watt, M.L.; Eklund, A.C.; Zhang-James, Y.; Kim, P.D.; Hauser, M.A.; et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci. Transl. Med. 2010, 2, 52ra73. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Moyeed, R.; Carroll, C.; Albani, D.; Li, X. Gene expression meta-analysis of Parkinson’s disease and its relationship with Alzheimer’s disease. Mol. Brain 2019, 12, 16. [Google Scholar] [CrossRef]
- Tsui, A.; Isacson, O. Functions of the nigrostriatal dopaminergic synapse and the use of neurotransplantation in Parkinson’s disease. J. Neurol. 2011, 258, 1393–1405. [Google Scholar] [CrossRef]
- Pantcheva, P.; Reyes, S.; Hoover, J.; Kaelber, S.; Borlongan, C.V. Treating non-motor symptoms of Parkinson’s disease with transplantation of stem cells. Expert Rev. Neurother. 2015, 15, 1231–1240. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal stem cell therapy in Parkinson’s disease animal models. Curr. Res. Transl. Med. 2017, 65, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yin, F.; Meng, H.Q.; Ling, L.; Hu-He, T.N.; Li, P.; Zhang, C.X.; Yu, S.; Duan, D.S.; Fan, H.X. Differentiation of mesenchymal stem cells into dopaminergic neuron-like cells in vitro. Biomed. Environ. Sci. 2005, 18, 36–42. [Google Scholar]
- Yin, Q.; Xu, N.; Xu, D.; Dong, M.; Shi, X.; Wang, Y.; Hao, Z.; Zhu, S.; Zhao, D.; Jin, H.; et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Alicka, M.; Kornicka-Garbowska, K.; Kucharczyk, K.; Kępska, M.; Röcken, M.; Marycz, K. Age-dependent impairment of adipose-derived stem cells isolated from horses. Stem Cell Res. Ther. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fričová, D.; Korchak, J.A.; Zubair, A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 2020, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheung, H.H. Stem Cell-Based Therapies for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 8060. [Google Scholar] [CrossRef]
- Adams, B.; Nunes, J.M.; Page, M.J.; Roberts, T.; Carr, J.; Nell, T.A.; Kell, D.B.; Pretorius, E. Parkinson’s Disease: A Systemic Inflammatory Disease Accompanied by Bacterial Inflammagens. Front. Aging Neurosci. 2019, 11, 210. [Google Scholar] [CrossRef] [Green Version]
- Levy, O.A.; Malagelada, C.; Greene, L.A. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis 2009, 14, 478–500. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Wiley, C.A. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009, 19, 573–585. [Google Scholar] [CrossRef]
- Berezin, V.; Walmod, P.S.; Filippov, M.; Dityatev, A. Targeting of ECM molecules and their metabolizing enzymes and receptors for the treatment of CNS diseases. Prog. Brain Res. 2014, 214, 353–388. [Google Scholar] [CrossRef]
- Raghunathan, R.; Hogan, J.D.; Labadorf, A.; Myers, R.H.; Zaia, J. A glycomics and proteomics study of aging and Parkinson’s disease in human brain. Sci. Rep. 2020, 10, 12804. [Google Scholar] [CrossRef]
- Byeon, J.S.; Jeong, J.Y.; Kim, M.J.; Lee, S.M.; Nam, W.H.; Myung, S.J.; Kim, J.G.; Yang, S.K.; Kim, J.H.; Suh, D.J. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int. J. Cancer 2010, 127, 2758–2767. [Google Scholar] [CrossRef]
- Ai, B.; Gao, Y.; Zhang, X.; Tao, J.; Kang, M.; Huang, H. Comparative transcriptome resources of eleven Primulina species, a group of ‘stone plants’ from a biodiversity hot spot. Mol. Ecol. Resour. 2015, 15, 619–632. [Google Scholar] [CrossRef]
- Won, Y.H.; Lee, M.Y.; Choi, Y.C.; Ha, Y.; Kim, H.; Kim, D.Y.; Kim, M.S.; Yu, J.H.; Seo, J.H.; Kim, M.; et al. Elucidation of Relevant Neuroinflammation Mechanisms Using Gene Expression Profiling in Patients with Amyotrophic Lateral Sclerosis. PLoS ONE 2016, 11, e0165290. [Google Scholar] [CrossRef]
- Lin, J.Q.; Zhao, X.X.; Zhi, Q.Q.; Zhao, M.; He, Z.M. Transcriptomic profiling of Aspergillus flavus in response to 5-azacytidine. Fungal Genet. Biol. 2013, 56, 78–86. [Google Scholar] [CrossRef]
- Lee, E.J.; Malik, A.; Pokharel, S.; Ahmad, S.; Mir, B.A.; Cho, K.H.; Kim, J.; Kong, J.C.; Lee, D.M.; Chung, K.Y.; et al. Identification of genes differentially expressed in myogenin knock-down bovine muscle satellite cells during differentiation through RNA sequencing analysis. PLoS ONE 2014, 9, e92447. [Google Scholar] [CrossRef]
- Jiao, X.; Sherman, B.T.; Huang da, W.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. DAVID-WS: A stateful web service to facilitate gene/protein list analysis. Bioinformatics 2012, 28, 1805–1806. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.Y.; Yu, J.H.; Kim, J.Y.; Seo, J.H.; Park, E.S.; Kim, C.H.; Kim, H.; Cho, S.R. Alteration of synaptic activity-regulating genes underlying functional improvement by long-term exposure to an enriched environment in the adult brain. Neurorehabil. Neural Repair 2013, 27, 561–574. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Losada, N.; de la Rosa, J.; Larriva, M.; Wendelbo, R.; Aguirre, J.A.; Castresana, J.S.; Ballaz, S.J. Overexpression of α -synuclein promotes both cell proliferation and cell toxicity in human SH-SY5Y neuroblastoma cells. J. Adv. Res. 2020, 23, 37–45. [Google Scholar] [CrossRef]
- Tan, F.C.; Hutchison, E.R.; Eitan, E.; Mattson, M.P. Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 2014, 15, 643–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Bree, R.T.; Stenson-Cox, C.; Grealy, M.; Byrnes, L.; Gorman, A.M.; Samali, A. Cellular longevity: Role of apoptosis and replicative senescence. Biogerontology 2002, 3, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Li, T.; Ding, Y.; Sun, L.; Tu, T.; Zhu, W.; Hu, J.; Sun, X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 2016, 13, 5207–5215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Shao, X.Y.; Qi, G.J.; Chen, Q.; Bu, L.L.; Chen, L.J.; Shi, J.; Ming, J.; Tian, B. Cdk5-Dependent Activation of Neuronal Inflammasomes in Parkinson’s Disease. Mov. Disord. 2016, 31, 366–376. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, D.; Jha, N.K.; Jha, S.K.; Ambasta, R.K.; Kumar, P. Re-expression of cell cycle markers in aged neurons and muscles: Whether cells should divide or die? Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 324–336. [Google Scholar] [CrossRef]
- Sah, E.; Krishnamurthy, S.; Ahmidouch, M.Y.; Gillispie, G.J.; Milligan, C.; Orr, M.E. The Cellular Senescence Stress Response in Post-Mitotic Brain Cells: Cell Survival at the Expense of Tissue Degeneration. Life 2021, 11, 229. [Google Scholar] [CrossRef]
- Levi, N.; Papismadov, N.; Solomonov, I.; Sagi, I.; Krizhanovsky, V. The ECM path of senescence in aging: Components and modifiers. FEBS J. 2020, 287, 2636–2646. [Google Scholar] [CrossRef]
- Truong, N.C.; Bui, K.H.; Van Pham, P. Characterization of Senescence of Human Adipose-Derived Stem Cells After Long-Term Expansion. Adv. Exp. Med. Biol. 2019, 1084, 109–128. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef]
- In, C. Genetics Home Reference, Medlineplus. 2013. Available online: https://medlineplus.gov/genetics/gene/reln/ (accessed on 13 August 2020).
- Lambert de Rouvroit, C.; Goffinet, A.M. The reeler mouse as a model of brain development. Adv. Anat. Embryol Cell Biol. 1998, 150, 1–106. [Google Scholar]
- D’Arcangelo, G. Reelin mouse mutants as models of cortical development disorders. Epilepsy Behav. 2006, 8, 81–90. [Google Scholar] [CrossRef]
- Krueger, D.D.; Howell, J.L.; Hebert, B.F.; Olausson, P.; Taylor, J.R.; Nairn, A.C. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology 2006, 189, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Renfro, A.; Quattrocchi, C.C.; Sheldon, M.; D’Arcangelo, G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 2004, 41, 71–84. [Google Scholar] [CrossRef] [Green Version]
- D’Arcangelo, G. Apoer2: A reelin receptor to remember. Neuron 2005, 47, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Weeber, E.J.; Beffert, U.; Jones, C.; Christian, J.M.; Förster, E.; Sweatt, J.D.; Herz, J. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 2002, 277, 39944–39952. [Google Scholar] [CrossRef] [Green Version]
- Herz, J.; Beffert, U. Apolipoprotein E receptors: Linking brain development and Alzheimer’s disease. Nat. Rev. Neurosci. 2000, 1, 51. [Google Scholar] [CrossRef]
- Herz, J.; Chen, Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 2006, 7, 850. [Google Scholar] [CrossRef]
- Baloyannis, S.J.; Costa, V.; Mauroudis, I.; Psaroulis, D.; Manolides, S.L.; Manolides, L.S. Dendritic and spinal pathology in the acoustic cortex in Alzheimer’s disease: Morphological and morphometric estimation by Golgi technique and electron microscopy. Acta Oto-Laryngol. 2007, 127, 351–354. [Google Scholar] [CrossRef]
- Baloyannis, S.J. Morphological and morphometric alterations of Cajal-Retzius cells in early cases of Alzheimer’s disease: A Golgi and electron microscope study. Int. J. Neurosci. 2005, 115, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.S.; Curran, T. Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 2001, 24, 1005–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.; Jeong, H.; Kwon, J.; Heo, H.Y.; Kwon, J.J.; Yun, H.J.; Kim, C.H.; Han, B.S.; Tong, Y.; Shen, J.; et al. LRRK2 regulates synaptic vesicle endocytosis. Exp. Cell Res. 2008, 314, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.A.; Ingrassia, A.; de Menezes, R.X.; van Kesteren, R.E.; Rozemuller, A.J.; Heutink, P.; van de Berg, W.D. Evidence for Immune Response, Axonal Dysfunction and Reduced Endocytosis in the Substantia Nigra in Early Stage Parkinson’s Disease. PLoS ONE 2015, 10, e0128651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaton, G.R.; Landeck, N.; Mamais, A.; Nalls, M.A.; Nixon-Abell, J.; Kumaran, R.; Beilina, A.; Pellegrini, L.; Li, Y.; Harvey, K.; et al. Sequential screening nominates the Parkinson’s disease associated kinase LRRK2 as a regulator of Clathrin-mediated endocytosis. Neurobiol. Dis. 2020, 141, 104948. [Google Scholar] [CrossRef]
- Kim, K.; Wi, S.; Seo, J.H.; Pyo, S.; Cho, S.R. Reduced Interaction of Aggregated α-Synuclein and VAMP2 by Environmental Enrichment Alleviates Hyperactivity and Anxiety in a Model of Parkinson’s Disease. Genes 2021, 12, 392. [Google Scholar] [CrossRef]
- Duda, J.E.; Lee, V.M.; Trojanowski, J.Q. Neuropathology of synuclein aggregates. J. Neurosci. Res. 2000, 61, 121–127. [Google Scholar] [CrossRef]
- Luk, K.C.; Song, C.; O’Brien, P.; Stieber, A.; Branch, J.R.; Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M. Exogenous α -synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20051–20056. [Google Scholar] [CrossRef] [Green Version]
- Marques, O.; Outeiro, T.F. α -synuclein: From secretion to dysfunction and death. Cell Death Dis. 2012, 3, e350. [Google Scholar] [CrossRef]


| KEGG Pathway | Count | % | p-Value | Genes |
|---|---|---|---|---|
| ECM-receptor interaction | 10 | 0.120 | 0.007 | CD36, ITGA5, LAMA5, ITGA8, COL6A3, COL6A2, ITGA1, DAG1, RELN, COL5A3 |
| P53 signaling pathway | 8 | 0.096 | 0.021 | STEAP3, ZMAT3, CD82, BAX, SHISA5, MDM2, CCNG2, GADD45A |
| Arrhythmogenic right ventricular cardiomyopathy | 8 | 0.096 | 0.037 | ITGA5, ITGA8, ITGA1, DAG1, GJA1, CACNB3, TCF7L2, TCF7L1 |
| Focal adhesion | 15 | 0.181 | 0.038 | CAV1, TLN1, ITGA1, COL5A3, CAPN2, MYL9, DOCK1, LAMA5, ITGA5, ITGA8, COL6A3, COL6A2, PDGFRB, RELN, ZYX |
| Endocytosis | 14 | 0.168 | 0.041 | GIT1, ARFGAP1, CHMP2A, PLD1, ERBB3, RUFY1, ASAP3, EPS15, FAM125A, AP2A1, VPS24, VPS4A, MDM2, VPS28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Park, J.; Kim, K.; Kim, M.-G.; Cho, S.-R. Reelin Alleviates Mesenchymal Stem Cell Senescence and Reduces Pathological α-Synuclein Expression in an In Vitro Model of Parkinson’s Disease. Genes 2021, 12, 1066. https://doi.org/10.3390/genes12071066
Cho E, Park J, Kim K, Kim M-G, Cho S-R. Reelin Alleviates Mesenchymal Stem Cell Senescence and Reduces Pathological α-Synuclein Expression in an In Vitro Model of Parkinson’s Disease. Genes. 2021; 12(7):1066. https://doi.org/10.3390/genes12071066
Chicago/Turabian StyleCho, Eunju, Joonsang Park, Kyungri Kim, Min-Gi Kim, and Sung-Rae Cho. 2021. "Reelin Alleviates Mesenchymal Stem Cell Senescence and Reduces Pathological α-Synuclein Expression in an In Vitro Model of Parkinson’s Disease" Genes 12, no. 7: 1066. https://doi.org/10.3390/genes12071066
APA StyleCho, E., Park, J., Kim, K., Kim, M.-G., & Cho, S.-R. (2021). Reelin Alleviates Mesenchymal Stem Cell Senescence and Reduces Pathological α-Synuclein Expression in an In Vitro Model of Parkinson’s Disease. Genes, 12(7), 1066. https://doi.org/10.3390/genes12071066







