The Effect of Exogenous Nitrate on LCO Signalling, Cytokinin Accumulation, and Nodule Initiation in Medicago truncatula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Testing the Effects of Varying Nitrate Concentration on Nodulation
2.3. Application of Nod Factors and Rhizobia
2.4. RNA Isolation, cDNA Synthesis, and Quantitative RT-PCR
2.5. Cytokinin Extraction
2.6. Detection and Quantification of Cytokinins via Liquid Chromatography–Tandem Mass Spectrometry
2.7. ACC Extraction, Detection, and Quantification via Liquid Chromatography–Tandem Mass Spectrometry
2.8. Statistical Analysis
3. Results
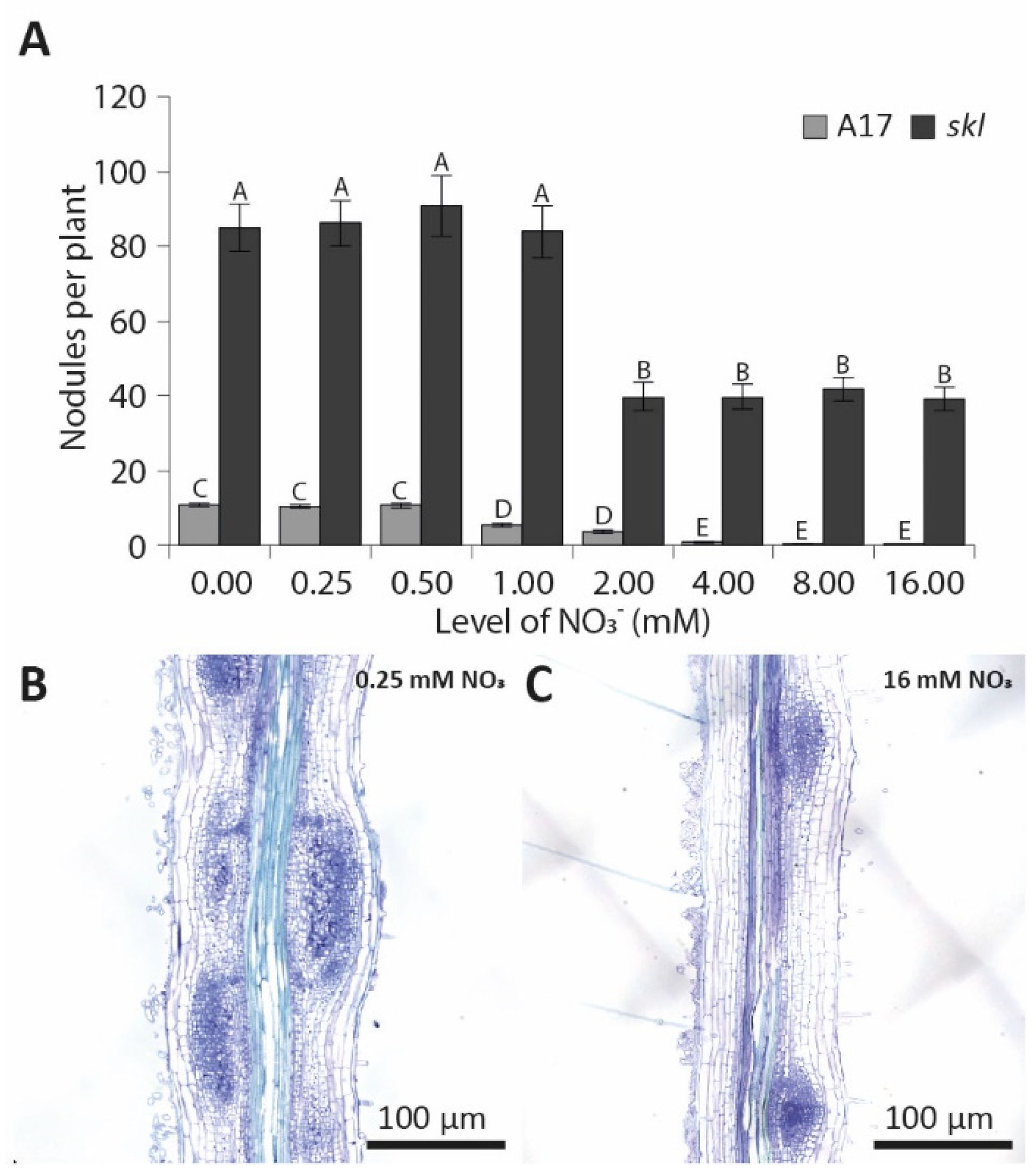
3.1. Elevated Nitrate Concentrations Inhibit Nodulation in a Synchronized In Vitro Assay
3.2. Root Hair Deformation Is Not Affected by Exogenous Nitrate
3.3. Pericycle and Cortical Cell Divisions Are Blocked by High Nitrate
3.4. Nitrate Inhibition of Pericycle and Cortical Cell Divisions Is Mediated By Ethylene
4. Discussion
4.1. Nitrate Interferes with Both Nodule Initiation and Nitrogen-Fixation Rates
4.2. Nitrate Interferes with Pericycle and Cortical Cell Divisions in an Ethylene-Dependent Manner
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant. Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guha, S.; Sarkar, M.; Ganguly, P.; Uddin, M.R.; Mandal, S.; DasGupta, M. Segregation of nod -containing and nod -deficient bradyrhizobia as endosymbionts of Arachis hypogaea and as endophytes of Oryza sativa in intercropped fields of Bengal Basin, India. Environ. Microbiol. 2016, 18, 2575–2590. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Moulin, L.; Vallenet, D.; Barbe, V.; Cytryn, E.; Avarre, J.-C.; Jaubert, M.; Simon, D.; Cartieaux, F.; Prin, Y.; et al. Legumes symbioses: Absence of Nod genes in photosynthetic Bradyrhizobia. Science 2007, 316, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Limpens, E.; van Zeijl, A.; Geurts, R. Lipochitooligosaccharides modulate plant host immunity to enable endosymbioses. Annu. Rev. Phytopathol. 2015, 53, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Van Zeijl, A.; den Camp, R.H.M.O.; Deinum, E.E.; Charnikhova, T.; Franssen, H.; den Camp, H.J.M.O.; Bouwmeester, H.; Kohlen, W.; Bisseling, T.; Geurts, R. Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol. Plant 2015, 8, 1213–1226. [Google Scholar] [CrossRef] [Green Version]
- Larrainzar, E.; Riely, B.; Kim, S.C.; Carrasquilla-Garcia, N.; Yu, H.-J.; Hwang, H.; Oh, M.; Kim, G.B.; Surendrarao, A.; Chasman, D.; et al. Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between Nod factor and ethylene signals. Plant Physiol. 2015, 169, 233–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breakspear, A.; Liu, C.; Roy, S.; Stacey, N.; Rogers, C.; Trick, M.; Morieri, G.; Mysore, K.S.; Wen, J.; Oldroyd, G.E.D.; et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 2014, 26, 4680–4701. [Google Scholar] [CrossRef] [Green Version]
- Schiessl, K.; Lilley, J.L.S.; Lee, T.; Tamvakis, I.; Kohlen, W.; Bailey, P.C.; Thomas, A.; Luptak, J.; Ramakrishnan, K.; Carpenter, M.D.; et al. Nodule Inception recruits the lateral root developmental program for symbiotic nodule organogenesis in Medicago truncatula. Curr. Biol. 2019, 29, 3657–3668.e5. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rizzo, S.; Crespi, M.; Frugier, F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 2006, 18, 2680–2693. [Google Scholar] [CrossRef] [Green Version]
- Plet, J.; Wasson, A.; Ariel, F.; Le Signor, C.; Baker, D.; Mathesius, U.; Crespi, M.; Frugier, F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011, 65, 622–633. [Google Scholar] [CrossRef]
- Murray, J.D.; Karas, B.J.; Sato, S.; Tabata, S.; Amyot, L.; Szczyglowski, K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 2007, 315, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Vernié, T.; Kim, J.; Frances, L.; Ding, Y.; Sun, J.; Guan, D.; Niebel, A.; Gifford, M.L.; de Carvalho-Niebel, F.; Oldroyd, G.E.D. The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 2015, 27, 3410–3424. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Rutten, L.; Limpens, E.; van der Molen, T.; van Velzen, R.; Chen, R.; Chen, Y.; Geurts, R.; Kohlen, W.; Kulikova, O.; et al. A remote cis-regulatory region is required for NIN expression in the pericycle to initiate nodule primordium formation in Medicago truncatula. Plant Cell 2019, 31, 68–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, A.C.; Auriac, M.C.; Truchet, G. Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 1999, 126, 3617–3628. [Google Scholar] [CrossRef]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate map of Medicago truncatula root nodules. Development 2014, 141, 3517–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geurts, R.; Fedorova, E.; Bisseling, T. Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr. Opin. Plant Biol. 2005, 8, 346–352. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef] [Green Version]
- Streeter, J.G. Nitrate inhibition of legume nodule growth and activity: I. long term studies with a continuous supply of nitrate. Plant Physiol. 1985, 77, 321–324. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. CRC. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Carroll, B.J.; Gresshoff, P.M. Nitrate inhibition of nodulation and nitrogen fixation in white clover. Z. Für Pflanzenphysiol. 1983, 110, 77–88. [Google Scholar] [CrossRef]
- Heidstra, R.; Geurts, R.; Franssen, H.; Spaink, H.P.; van Kammen, A.; Bisseling, T. Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 1994, 105, 787–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbulova, A.; Rogato, A.; D’Apuzzo, E.; Omrane, S.; Chiurazzi, M. Differential effects of combined N sources on early steps of the Nod factor-dependent transduction pathway in Lotus japonicus. Mol. Plant Microbe Interact. 2007, 20, 994–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligero, F.; Caba, J.M.; Lluch, C.; Olivares, J. Nitrate inhibition of nodulation can be overcome by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 1991, 97, 1221–1225. [Google Scholar] [CrossRef] [Green Version]
- Ligero, F.; Lluch, C.; Olivares, J. Evolution of ethylene from roots and nodulation rate of Alfalfa (Medicago sativa L.) plants inoculated with Rhizobium meliloti as affected by the presence of nitrate. J. Plant Physiol. 1987, 129, 461–467. [Google Scholar] [CrossRef]
- Penmetsa, R.V. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 1997, 275, 527–530. [Google Scholar] [CrossRef]
- Penmetsa, R.V.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.-C.; Nam, Y.W.; Engstrom, E.; Xu, K.; Sckisel, G.; Pereira, M.; et al. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008, 55, 580–595. [Google Scholar] [CrossRef]
- Kende, H. Ethylene biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 283–307. [Google Scholar] [CrossRef]
- Herrbach, V.; Chirinos, X.; Rengel, D.; Agbevenou, K.; Vincent, R.; Pateyron, S.; Huguet, S.; Balzergue, S.; Pasha, A.; Provart, N.; et al. Nod factors potentiate auxin signaling for transcriptional regulation and lateral root formation in Medicago truncatula. J. Exp. Bot. 2017, 68, 569–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, D.; Liu, H.; Kelly, S.; Kawaharada, Y.; Mun, T.; Andersen, S.U.; Desbrosses, G.; Stougaard, J. Dynamics of ethylene production in response to compatible Nod factor. Plant Physiol. 2018, 176, 1764–1772. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.; Chang, C. Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 2015, 169, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Miyata, K.; Kawaguchi, M.; Nakagawa, T. Two distinct EIN2 genes cooperatively regulate ethylene signaling in Lotus japonicus. Plant Cell Physiol. 2013, 54, 1469–1477. [Google Scholar] [CrossRef] [Green Version]
- Prayitno, J. Root and nodulation phenotypes of the ethylene-insensitive sickle mutant of Medicago truncatula. HAYATI J. Biosci. 2010, 17, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Gresshoff, P.M.; Lohar, D.; Chan, P.-K.; Biswas, B.; Jiang, Q.; Reid, D.; Ferguson, B.; Stacey, G. Genetic analysis of ethylene regulation of legume nodulation. Plant Signal. Behav. 2009, 4, 818–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortier, V.; Holsters, M.; Goormachtig, S. Never too many? How legumes control nodule numbers. Plant. Cell Environ. 2012, 35, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Fåhraeus, G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. Microbiology 1957, 16, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Spaink, H.P.; Sheeley, D.M.; van Brussel, A.A.N.; Glushka, J.; York, W.S.; Tak, T.; Geiger, O.; Kennedy, E.P.; Reinhold, V.N.; Lugtenberg, B.J.J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 1991, 354, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kohlen, W.; Charnikhova, T.; Liu, Q.; Bours, R.; Domagalska, M.A.; Beguerie, S.; Verstappen, F.; Leyser, O.; Bouwmeester, H.; Ruyter-Spira, C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host arabidopsis. Plant Physiol. 2011, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155. [Google Scholar] [CrossRef] [Green Version]
- Bours, R.; van Zanten, M.; Pierik, R.; Bouwmeester, H.; van der Krol, A. Antiphase light and temperature cycles affect PHYTOCHROME B-controlled ethylene sensitivity and biosynthesis, limiting leaf movement and growth of Arabidopsis. Plant Physiol. 2013, 163, 882–895. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation of Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Goodlass, G.; Smith, K.A. Effects of ethylene on root extension and nodulation of pea (Pisum sativum L.) and white clover (Trifolium repens L.). Plant Soil 1979, 51, 387–395. [Google Scholar] [CrossRef]
- Lee, K.H.; LaRue, T.A. Ethylene as a possible Mediator of light- and nitrate-induced inhibition of nodulation of Pisum sativum L. cv. Sparkle. Plant Physiol. 1992, 100, 1334–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidstra, R.; Yang, W.C.; Yalcin, Y.; Peck, S.; Emons, A.M.; van Kammen, A.; Bisseling, T. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 1997, 124, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Buttery, B.R. Ethyl-methane sulphonate (EMS) induced nodulation mutants of common bean (Phaseolus vulgaris L.) lacking effective nodules. Plant Soil 1992, 139, 295–298. [Google Scholar] [CrossRef]
- Duc, G.; Messager, A. Mutagenesis of pea (Pisum sativum L.) and the isolation of mutants for nodulation and nitrogen fixation. Plant Sci. 1989, 60, 207–213. [Google Scholar] [CrossRef]
- Jiang, Y.; MacLean, D.E.; Perry, G.E.; Marsolais, F.; Hill, B.; Pauls, K.P. Evaluation of beneficial and inhibitory effects of nitrate on nodulation and nitrogen fixation in common bean (Phaseolus vulgaris). Legum. Sci. 2020, 2, e45. [Google Scholar] [CrossRef]
- Becana, M.; Aparicio-Tejo, P.M.; Sanchez-Diaz, M. Nitrate and nitrite reduction by alfalfa root nodules: Accumulation of nitrite in Rhizobium melioti bacteroids and senescence of nodules. Physiol. Plant. 1985, 64, 353–358. [Google Scholar] [CrossRef]
- Swaraj, K.; Laura, J.S.; Bishnoi, N.R. Nitrate induced nodule senescence and changes in activities of enzymes scavenging H2O2 in Clusterbean (Cyamopsis tetragonaloba Taub.). J. Plant Physiol. 1993, 141, 202–205. [Google Scholar] [CrossRef]
- Schuller, K.A.; Minchin, F.R.; Gresshoff, P.M. Nitrogenase activity and oxygen diffusion in nodules of Soyabean cv. Bragg and a supernodulating mutant: Effects of nitrate. J. Exp. Bot. 1988, 39, 865–877. [Google Scholar] [CrossRef]
- Liese, R.; Schulze, J.; Cabeza, R.A. Nitrate application or P deficiency induce a decline in Medicago truncatula N2-fixation by similar changes in the nodule transcriptome. Sci. Rep. 2017, 7, 46264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Noorden, G.; Verbeek, R.; Dinh, Q.; Jin, J.; Green, A.; Ng, J.; Mathesius, U. Molecular signals controlling the inhibition of nodulation by nitrate in Medicago truncatula. Int. J. Mol. Sci. 2016, 17, 1060. [Google Scholar] [CrossRef] [Green Version]
- Xiao, T.T. Root and Nodule: Lateral Organ Development in N2-Fixing Plants; Wageningen University: Wageningen, The Netherland, 2015. [Google Scholar]
- Caba, J.M.; Recalde, L.; Ligero, F. Nitrate-induced ethylene biosynthesis and the control of nodulation in alfalfa. Plant Cell Environ. 1998, 21, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Reid, D.E.; Ferguson, B.J.; Hayashi, S.; Lin, Y.H.; Gresshoff, P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011, 108, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.H.; Lin, Y.-H.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, D.; Frugoli, J. The regulation of nodule number in legumes is a balance of three signal transduction pathways. Int. J. Mol. Sci. 2021, 22, 1117. [Google Scholar] [CrossRef]
- Nishimura, R.; Hayashi, M.M.; Wu, G.-J.J.; Kouchi, H.; Imaizumi-Anraku, H.; Murakami, Y.; Kawasaki, S.; Akao, S.; Ohmori, M.; Nagasawa, M.; et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature 2002, 420, 426–429. [Google Scholar] [CrossRef]
- Schnabel, E.; Journet, E.-P.; de Carvalho-Niebel, F.; Duc, G.; Frugoli, J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol. Biol. 2005, 58, 809–822. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Ferguson, B.J. Molecular signals in nodulation control. Int. J. Mol. Sci. 2017, 18, 125. [Google Scholar] [CrossRef] [Green Version]
- Laffont, C.; Ivanovici, A.; Gautrat, P.; Brault, M.; Djordjevic, M.A.; Frugier, F. The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat. Commun. 2020, 11, 3167. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gühl, K.; Holmer, R.; Xiao, T.T.; Shen, D.; Wardhani, T.A.K.; Geurts, R.; van Zeijl, A.; Kohlen, W. The Effect of Exogenous Nitrate on LCO Signalling, Cytokinin Accumulation, and Nodule Initiation in Medicago truncatula. Genes 2021, 12, 988. https://doi.org/10.3390/genes12070988
Gühl K, Holmer R, Xiao TT, Shen D, Wardhani TAK, Geurts R, van Zeijl A, Kohlen W. The Effect of Exogenous Nitrate on LCO Signalling, Cytokinin Accumulation, and Nodule Initiation in Medicago truncatula. Genes. 2021; 12(7):988. https://doi.org/10.3390/genes12070988
Chicago/Turabian StyleGühl, Kerstin, Rens Holmer, Ting Ting Xiao, Defeng Shen, Titis A. K. Wardhani, René Geurts, Arjan van Zeijl, and Wouter Kohlen. 2021. "The Effect of Exogenous Nitrate on LCO Signalling, Cytokinin Accumulation, and Nodule Initiation in Medicago truncatula" Genes 12, no. 7: 988. https://doi.org/10.3390/genes12070988
APA StyleGühl, K., Holmer, R., Xiao, T. T., Shen, D., Wardhani, T. A. K., Geurts, R., van Zeijl, A., & Kohlen, W. (2021). The Effect of Exogenous Nitrate on LCO Signalling, Cytokinin Accumulation, and Nodule Initiation in Medicago truncatula. Genes, 12(7), 988. https://doi.org/10.3390/genes12070988




