Biochemical and Molecular Effects Induced by Triacontanol in Acquired Tolerance of Rice to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Growth Conditions
2.2. Imposition of Treatments
2.3. Methods
2.3.1. Measurement of Number of Stomata
2.3.2. Chlorophyll Fluorescence Measurements
2.3.3. Measurement of Photosynthetic Pigments
2.3.4. Measurement of Total Soluble Sugars
2.3.5. Measurement of Free Amino Acids
2.3.6. Determination of Free Proline
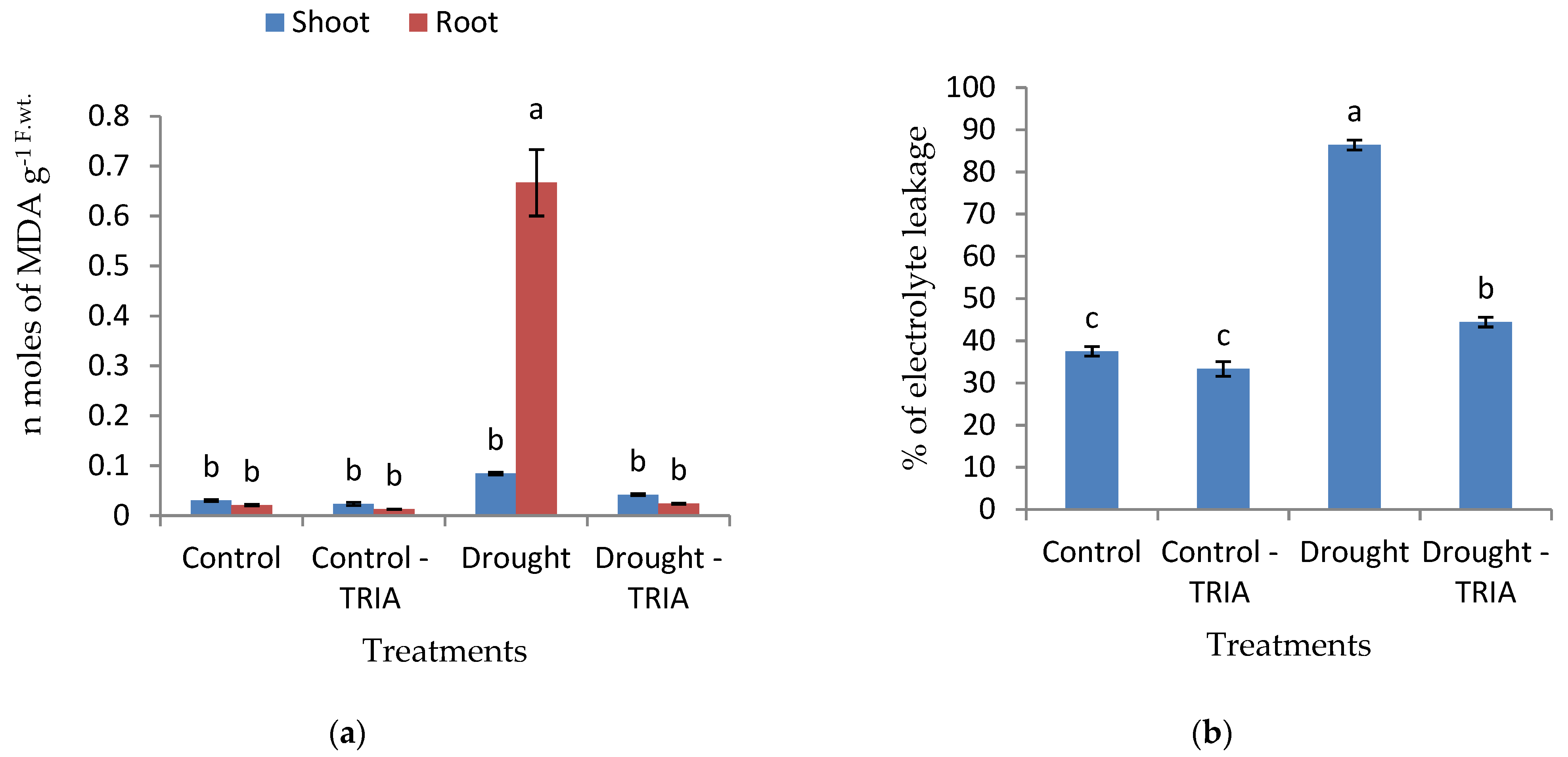
2.3.7. Determination of Electrolyte Leakage (EL)
2.3.8. Lipid Peroxidation
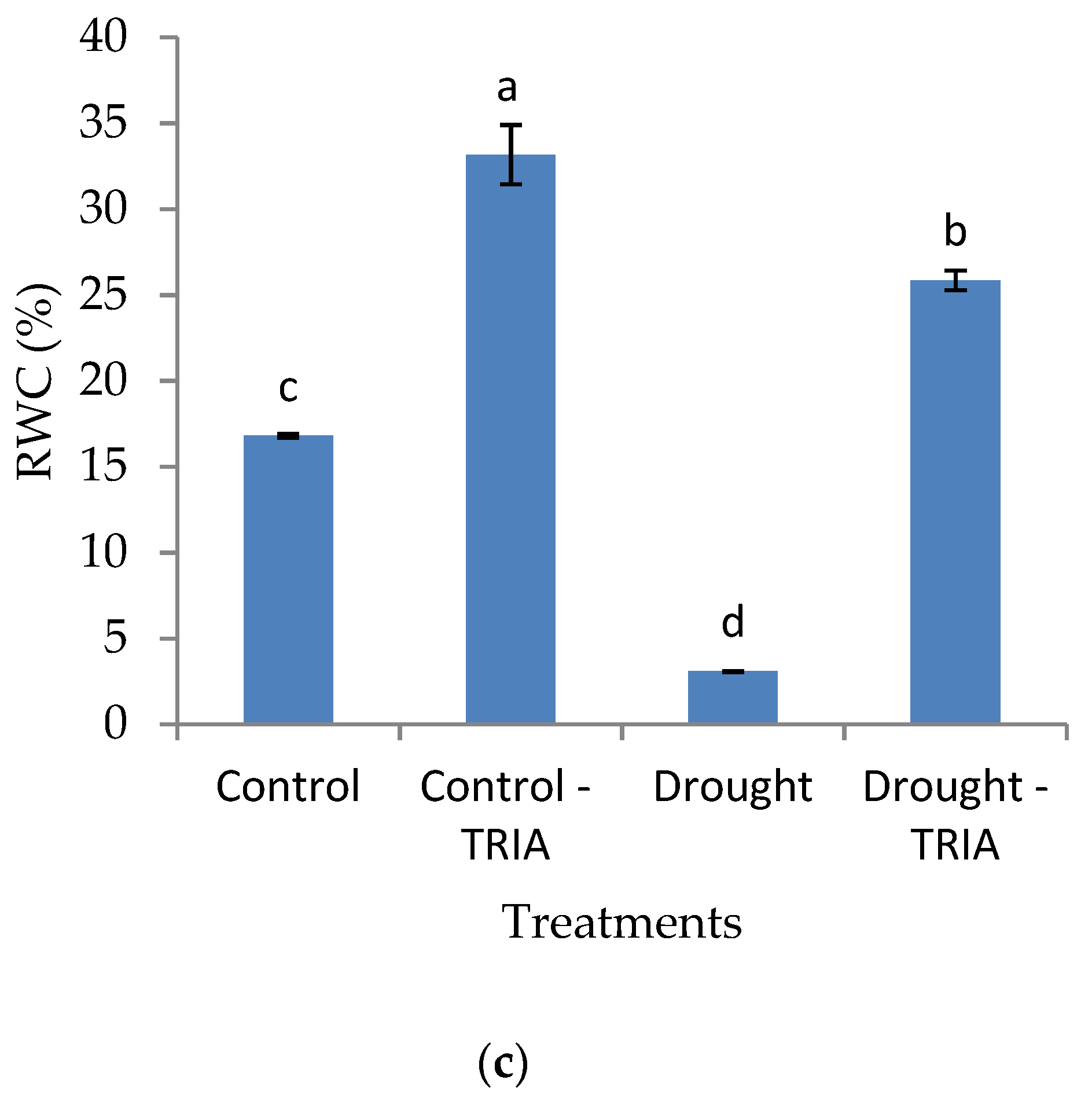
2.3.9. Relative Water Content (RWC)
2.3.10. Extraction, Separation, and Determination of Abscisic Acid (ABA)
2.3.11. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.4. Statistical Analysis
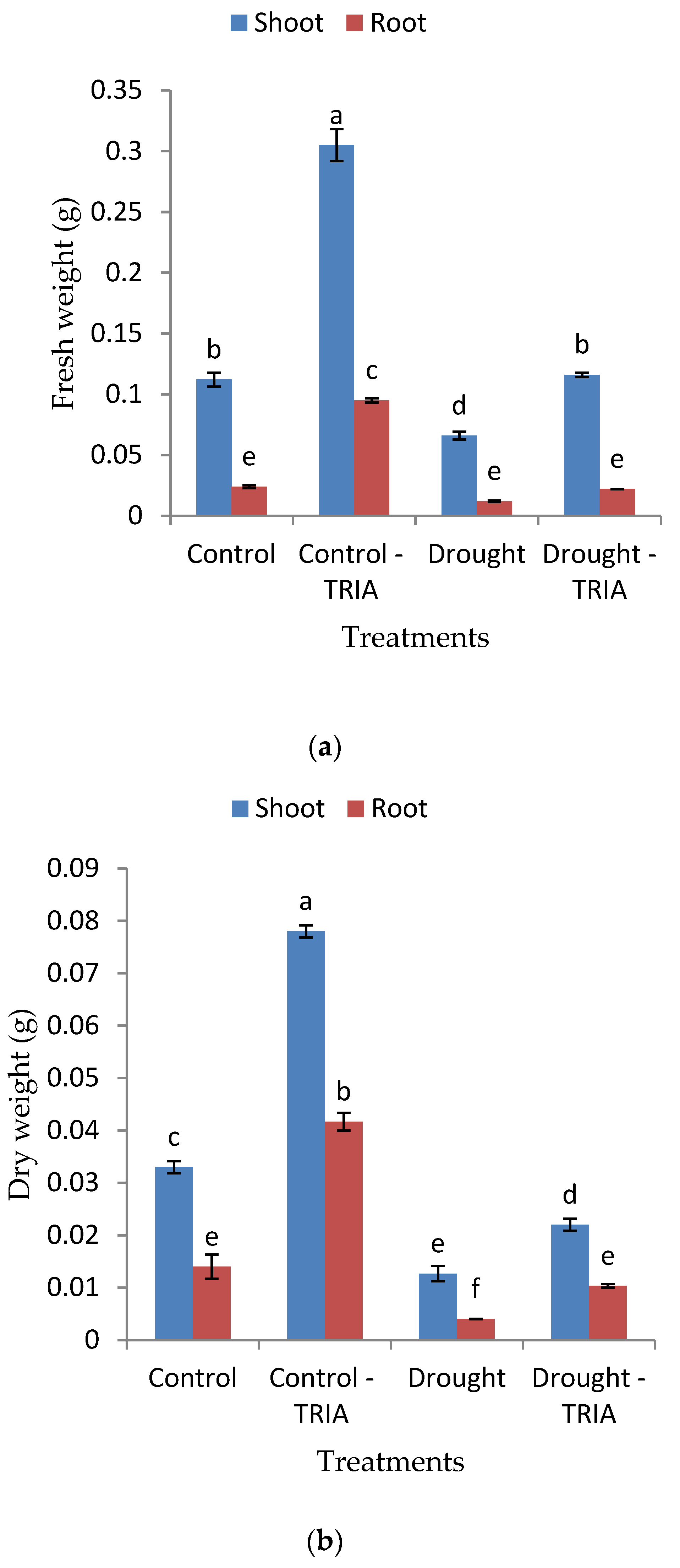
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| EL | electrolyte leakage |
| Fv/Fm | the maximal photochemical efficiency |
| ABA | Abscisic acid |
| AQPs | Aquaporins |
| PIP1,1, PIP1,2, PIP2,4 and PIP2,5 | Plasma membrane intrinsic proteins 1,1; 1,2; 2,4; and 2,5 genes |
| TRIA | triacontanol |
| RWC | relative water content |
References
- Kour, D.; Rana, K.L.; Yadav, A.N.; Sheikh, I.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Amelioration of drought stress in Foxtail millet (Setaria italica L.) by P-solubilizing drought-tolerant microbes with multifarious plant growth promoting attributes. Environ. Sustain. 2020, 3, 23–34. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef]
- Sharma, S.; Mujumdar, P. Increasing frequency and spatial extent of concurrent meteorological droughts and heat waves in India. Sci. Rep. 2017, 7, 15582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mckersie, B.D.; Ya’acov, Y.L. Oxidative stress. In Stress and Stress Coping in Cultivated Plants; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994; pp. 15–54. [Google Scholar]
- Bray, E.A. Molecular responses to water deficit. Plant Physiol. 1993, 103, 1035. [Google Scholar] [CrossRef] [Green Version]
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.; Li, H.; Li, Y. Physiological responses of seedling roots of the desert plant Reaumuria soongorica to drought stress. Acta Pratacult. Sin. 2015, 24, 72–80. [Google Scholar]
- Zeid, I.M.; Shedeed, Z.A. Response of alfalfa to putrescine treatment under drought stress. Biol. Plant. 2006, 50, 635–640. [Google Scholar] [CrossRef]
- Manickavelu, A.; Nadarajan, N.; Ganesh, S.K.; Gnanamalar, R.P.; Babu, R.C. Drought tolerance in rice: Morphological and molecular genetic consideration. Plant Growth Regul. 2006, 50, 121–138. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, S.E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Sci. Hortic. 2009, 122, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Jia, L.; Shi, W.; Liang, J.; Zhou, F.; Li, Q.; Zhang, J. Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol. 2013, 197, 139–150. [Google Scholar] [CrossRef]
- Puertolas, J.; Alcobendas, R.; Alarcón, J.J.; Dodd, I.C. Long-distance abscisic acid signalling under different vertical soil moisture gradients depends on bulk root water potential and average soil water content in the root zone. Plant Cell Environ. 2013, 36, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, J.; Conesa, M.R.; Ballester, C.; Dodd, I.C. Local root abscisic acid (ABA) accumulation depends on the spatial distribution of soil moisture in potato: Implications for ABA signalling under heterogeneous soil drying. J. Exp. Bot. 2015, 66, 2325–2334. [Google Scholar] [CrossRef] [Green Version]
- Khattab, H.I.; Emam, M.A.; Emam, M.M.; Helal, N.M.; Mohamed, M.R. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol. Plant. 2014, 58, 265–273. [Google Scholar] [CrossRef]
- Luu, D.T.; Maurel, C. Aquaporins in a challenging environment: Molecular gears for adjusting plant water status. Plant Cell Environ. 2005, 28, 85–96. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Ullah, M.A.; Sulaman, S.; Nawaz, M.; Zhiqiang, W.; Yanqin, M.; Guoqin, H. The role of potassium in plants under drought stress: Mini review. J. Basic Appl. Sci. 2017, 13, 268–271. [Google Scholar]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehlein, N.; Lovisolo, C.; Siefritz, F.; Kaldenhoff, R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 2003, 425, 734–737. [Google Scholar] [CrossRef]
- Hachez, C.; Moshelion, M.; Zelazny, E.; Cavez, D.; Chaumont, F. Localization and quantification of plasma membrane aquaporin expression in maize primary root: A clue to understanding their role as cellular plumbers. Plant Mol. Biol. 2006, 62, 305–323. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Khan, M.M.; Moinuddin, N.M. Augmentation of nutraceuticals, productivity and quality of ginger (Zingiber officinale Rosc.) through triacontanol application. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2012, 146, 106–113. [Google Scholar]
- Karam, E.A.; Keramat, B. Foliar spray of triacontanol improves growth by alleviating oxidative damage in coriander under salinity. Indian J. Plant Physiol. 2017, 22, 120–124. [Google Scholar] [CrossRef]
- Naeem, M.; Khan, M.M.A.; Moinuddin. Triacontanol: A potent plant growth regulator in agriculture. J. Plant Interact. 2012, 7, 129–142. [Google Scholar] [CrossRef]
- Ahmad, H.F.S.; Hassan, H.M.; El-Shafey, A.S. Effect of cadmium on growth, flowering and fruiting of Zea mays L. and possible roles of triacontanol in alleviating cadmium toxicity. Egypt. J. Bot. 2013, 53, 23–44. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Zaid, A.; Mohammad, F. Role of triacontanol in counteracting the ill effects of salinity in plants: A review. J. Plant Growth Regul. 2021, 40, 1–10. [Google Scholar] [CrossRef]
- Borowski, E.; Blamowski, Z.K. The effect of triacontanol ‘TRIA’ and Asahi-SL on the development and metabolic activity of sweet basil (Ocimum basilicum L.) plants treated with chilling. Folia Hortic. 2009, 21, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Perveen, S.; Shahbaz, M.; Ashraf, M. Influence of foliar-applied triacontanol on growth, gas exchange characteristics, and chlorophyll fluorescence at different growth stages in wheat under saline conditions. Photosynthetica 2013, 51, 541–551. [Google Scholar] [CrossRef]
- Perveen, S.; Iqbal, M.; Nawaz, A.; Parveen, A.; Mahmood, S. Induction of drought tolerance in Zea mays L. by foliar application of triacontanol. Pak. J. Bot. 2016, 48, 907–915. [Google Scholar]
- Perveen, S.; Iqbal, M.; Parveen, A.; Akram, M.S.; Shahbaz, M.; Akber, S.; Mehboob, A. Exogenous triacontanol-mediated increase in phenolics, proline, activity of nitrate reductase, and shoot k+ confers salt tolerance in maize (Zea mays L.). Braz. J. Bot. 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Maresca, V.; Sorbo, S.; Keramat, B.; Basile, A. Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium-induced oxidative stress. Ecotoxicol. Environ. Saf. 2017, 144, 268–274. [Google Scholar]
- El-Shehaby, O.A. The effect of water stress-induced by polyethylene glycol on stomata and photosynthesis in Lupinus termis plants. Egypt. J. Physiol. Sci. 1994, 18, 381–392. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis, Advances in Photosynthesis and Respiration Series; George, C., Papageorgiou, C., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen zur synchronisierbarkeit einzelner pigmentmangel-mutanten von Chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Fairbairn, N.J. A modified anthrone reagent. Chem. Ind. 1953, 4, 285–313. [Google Scholar]
- Muting, D.; Kaiser, E. Spectrophotometric method of determining of amino-N in biological material by means of the ninhydrin reaction. Hoppe-Seylers Z. Physiol. Chem. 1963, 323, 276–332. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free prolin for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Valentovič, P.; Luxová, M.; Kolarovič, L.; Gašparíková, O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in maize cultivars. Plant Soil Environ. 2006, 52, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konopka-Postupolska, D.; et al. Downregulation of CBP80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef]
- Wasfi, W.S.; Orrin, E.S. Identification of plant hormones from cotton ovules. Plant Physiol. 1975, 55, 550–554. [Google Scholar]
- Tenea, G.N.; Bota, A.P.; Raposo, F.C.; Maquet, A. Reference genes for gene expression studies in wheat flag leaves grown under different farming conditions. BMC Res. Notes 2011, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hortic. 2018, 30, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Delfine, S.; Tognetti, R.; Loreto, F.; Alvino, A. Physiological and growth responses to water stress in field-grown bell pepper (Capsicum annuum L.). J. Hortic. Sci. Biotechnol. 2002, 77, 697–704. [Google Scholar] [CrossRef]
- Kabiri, R.; Nasibi, F.; Farahbakhsh, H. Effect of exogenous salicylic acid on some physiological parameters and alleviation of drought stress in Nigella sativa plant under hydroponic culture. Plant Prot. Sci. 2014, 50, 43–45. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.E.; Huang, C.; Deng, X.; Zhou, S.; Chen, L.; Li, Y.I.; Wang, C.; Ma, Z.; Yuan, Q.; Wang, Y.A.; et al. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 2013, 36, 1449–1464. [Google Scholar] [CrossRef]
- Shahbaz, M.; Noreen, N.; Perveen, S. Triacontanol modulates photosynthesis and osmoprotectants in canola (Brassica napus L.) under saline stress. J. Plant Interact. 2013, 8, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Zambrano, E.; Juárez-Yáñez, T.E.; Tapia-Maruri, D.; Camacho-Díaz, B.H.; Jiménez-Aparicio, A.R.; Martínez-Ayala, A.L. Effects of triacontanol and light on stomatal and photochemical responses in Solanum lycopersicum L. J. Plant Growth Regul. 2020, 1–13. [Google Scholar] [CrossRef]
- Khan, M.M.; Bhardwaj, G.; Naeem, M.; Mohammad, F.; Singh, M.; Nasir, S.; Idrees, M. Response of tomato (Solanum lycopersicum L.) to application of potassium and triacontanol. XI Int. Symp. Process. Tomato 2008, 823, 199–208. [Google Scholar] [CrossRef]
- Pandey, N.; Upadhyay, S.K.; Tripathi, R.S. Effect of plant growth regulators and fertiuty levels on growth and yield of transplanted rice. Indian J. Agric. Res. 2001, 35, 205–207. [Google Scholar]
- Moorthy, P.; Kathiresan, K. Physiological responses of mangrove seedling to triacontanol. Biol. Plant. 1993, 35, 577. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Sharma, S. Effect of triacontanol on photosynthesis, alkaloid content and growth in opium poppy (Papaver somniferum L.). Plant Growth Regul. 1990, 9, 65–71. [Google Scholar] [CrossRef]
- Kumaravelu, G.; Livingstone, V.D.; Ramanujam, M.P. Triacontanol-induced changes in the growth, photosynthetic pigments, cell metabolites, flowering and yield of green gram. Biol. Plant. 2000, 43, 287–290. [Google Scholar] [CrossRef]
- Naeem, M.; Khan, M.M.; Siddiqui, M.H. Triacontanol stimulates nitrogen-fixation, enzyme activities, photosynthesis, crop productivity and quality of hyacinth bean (Lablab purpureus L.). Sci. Hortic. 2009, 121, 389–396. [Google Scholar] [CrossRef]
- Borowski, E.; Blamowski, Z.K.; Michałek, W. Effects of Tomatex/Triacontanol/on chlorophyll fluorescence and tomato (Lycopersicon esculentum Mill.) yields. Acta Physiol. Plant 2000, 22, 271–274. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, H.; Chen, R.; Zhu, L.; He, G. Biochemical and photochemical changes in response to triacontanol in rice (Oryza sativa L.). Plant Growth Regul. 2003, 40, 249–256. [Google Scholar] [CrossRef]
- Muthuchelian, K.; Velayutham, M.; Nedunchezhian, N. Ameliorating effect of triacontanol on acidic mist-treated Erythrina variegata seedlings: Changes in growth and photosynthetic activities. Plant Sci. 2003, 165, 1253–1259. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, H.; Chen, R.; Zhu, L.; Du, B.; Weng, Q.; He, G. Isolation and characterization of triacontanol-regulated genes in rice (Oryza sativa L.): Possible role of triacontanol as a plant growth stimulator. Plant Cell Physiol. 2002, 43, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Trewavas, A.J.; Gilory, S. Signal transduction in plant cells. Trends Genet. 1991, 7, 356–361. [Google Scholar] [CrossRef]
- Waqas, M.; Shahzad, R.; Khan, A.L.; Asaf, S.; Kim, Y.H.; Kang, S.M.; Bilal, S.; Hamayun, M.; Lee, I.J. Salvaging effect of triacontanol on plant growth, thermotolerance, macro-nutrient content, amino acid concentration and modulation of defense hormonal levels under heat stress. Plant Physiol. Biochem. 2016, 99, 118–125. [Google Scholar] [CrossRef]
- Sanadhya, D.; Kathuria, E.; Malik, C.P. Effect of drought stress and its interaction with two phytohormones on Vigna radiata seed germination and seedling growth. LS Int. J. Life Sci. 2012, 1, 201–207. [Google Scholar] [CrossRef]
- Suman, K.; Kondamudi, R.; Rao, Y.V.; Kiran, T.V.; Swamy, K.N.; Rao, P.R.; Subramanyam, D.; Voleti, S.R. Effect of triacontanol on seed germination, seedling growth and antioxidant enzyme in rice under poly ethylene glycol induced drought stress. Agric. J. 2013, 60, 132–137. [Google Scholar]
- Franks, P.J. Passive and active stomatal control: Either or both? New Phytol. 2013, 198, 325–327. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Walton, T.J. The biochemistry of plant cuticular lipids. Prog. Chem. Fats Other Lipids 1973, 13, 119–175. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates Inc. Publishers: Sunderland, MA, USA, 2006. [Google Scholar]
- Dodd, I.C. Hormonal interactions and stomatal responses. J. Plant Growth Regul. 2003, 22, 32–46. [Google Scholar] [CrossRef]
- Thakur, A.; Thakur, P.S.; Singh, R.P. Influence of paclobutrazol and triacontanol on growth and water relations in olive varieties under water stress. Indian J. Plant. Physiol. 1998, 3, 116–120. [Google Scholar]
- Rahdari, P.; Hosseini, S.M.; Tavakoli, S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plants Res. 2012, 6, 1539–1547. [Google Scholar]
- Demiral, T.; Türkan, I. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? Plant Physiol. 2004, 161, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.N.; Gaur, J.P. Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.B.; Sadique, S.; Hassan, S.; Sania, R.I.; Rashid, B.; Mohamed, B.B.; Husnain, T. Physio-biochemical and molecular responses in transgenic cotton under drought stress. J. Agric. Sci. 2017, 23, 157–166. [Google Scholar]
- Tahkokorpi, M.; Taulavuori, K.; Laine, K.; Taulavuori, E. After-effects of drought-related winter stress in previous and current year stems of Vaccinium myrtillus L. Environ. Exp. Bot. 2007, 61, 85–93. [Google Scholar] [CrossRef]
- Sandhya, V.S.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Silva, E.N.; Vieira, S.A.; Ribeiro, R.V.; Ponte, L.F.; Ferreira-Silva, S.L.; Silveira, J.A. Contrasting physiological responses of Jatropha curcas plants to single and combined stresses of salinity and heat. J. Plant Growth Regul. 2013, 32, 159–169. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Lane, B.G. Cellular desiccation and hydration: Developmentally regulated proteins, and the maturation and germination of seed embryos. FASEB J. 1991, 5, 2893–2901. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Blake, T.J. New plant growth regulators protect photosynthesis and enhance growth under drought of jack pine seedlings. J. Plant Growth Regul. 1999, 18, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Khanam, D.; Mohammad, F. Plant growth regulators ameliorate the ill effect of salt stress through improved growth, photosynthesis, antioxidant system, yield and quality attributes in Mentha piperita L. Acta Physiol. Plant 2018, 40, 1–3. [Google Scholar] [CrossRef]
- Martre, P.; Morillon, R.; Barrieu, F.; North, G.B.; Nobel, P.S.; Chrispeels, M.J. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 2002, 130, 2101–2110. [Google Scholar] [CrossRef] [Green Version]
- Siefritz, F.; Tyree, M.T.; Lovisolo, C.; Schubert, A.; Kaldenhoff, R. PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell 2002, 14, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Aharon, R.; Shahak, Y.; Wininger, S.; Bendov, R.; Kapulnik, Y.; Galili, G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 2003, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.L.; Yu, X.; Ye, Q.; Ding, X.S.; Kitagawa, Y.; Kwak, S.S.; Su, W.A.; Tang, Z.C. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol. 2004, 45, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Kaldenhoff, R.; Ribas-Carbo, M.I.; Sans, J.F.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ. 2008, 31, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Parent, B.; Hachez, C.; Redondo, E.; Simonneau, T.; Chaumont, F.; Tardieu, F. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol. 2009, 149, 2000–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef]
- Zhu, C.; Schraut, D.; Hartung, W.; Schäffner, A.R. Differential responses of maize MIP genes to salt stress and ABA. J. Exp. Bot. 2005, 56, 2971–2981. [Google Scholar] [CrossRef]
- Lian, H.L.; Yu, X.; Lane, D.; Sun, W.N.; Tang, Z.C.; Su, W.A. Upland rice and lowland rice exhibited different PIP expression under water deficit and ABA treatment. Cell Res. 2006, 16, 651–660. [Google Scholar] [CrossRef] [Green Version]




| pH | Electrical Conductivity (EC) (dS m−1) | Clay (%) | Silt (%) | Sand (%) | Texture |
|---|---|---|---|---|---|
| 8.2 | 2.8 | 53.3 | 31.4 | 15.3 | Clayey |
| Primer Name | Primer Sequence 5′-3′ | Gene Accession Number |
|---|---|---|
| PIP1,1 F′ | TGCGCAGCCGACGACATG | AK061769 |
| PIP1,1 R | CATACAGTGACTGAGTACTGGATTAC | |
| PIP1,2 F | CTGTCAAGATGCCAATCCAGAG | AK098849 |
| PIP1,2 R | GAACCGAACTCCAATAGGAGGA | |
| PIP2,4 F | GAGCTCGTCTGGTGATATCC | AK072632 |
| PIP2,4 R | CATGAAGACAACAGAGGGACAG | |
| PIP2,5 F | GCTTAAGCCGCAATCAAATGTGC | AK107700 |
| PIP2,5 R | CGATCGAACAATGTCACACTTGC | |
| OsActin F | CTGGGTTCGCCGGAGATGAT | XM_015774830.2 |
| OsActin R | TGAGATCACGCCCAGCAAGG |
| Treatments | Chl (a) | Chl (b) | Carotenoid | Fv/Fm |
|---|---|---|---|---|
| Control | 0.59 ± 0.0058 b | 0.26 ± 0.013 c | 0.39 ± 0.00 b | 0.78 ± 0.012 c |
| Control-TRIA | 0.79 ± 0.023 a | 0.31 ± 0.0135 b | 2.4 ± 0.205 a | 0.93 ± 0.09 a |
| Drought | 0.29 ± 0.012 d | 0.25 ± 0.015 c | 0.24 ± 0.012 b | 0.04 ± 00.00 d |
| Drought-TRIA | 0.52 ± 0.015 c | 0.39 ± 0.006 a | 0.5 ± 0.006 b | 0.7 ± 0.006 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, B.M.; Abdulmajeed, A.M.; Hassan, H. Biochemical and Molecular Effects Induced by Triacontanol in Acquired Tolerance of Rice to Drought Stress. Genes 2021, 12, 1119. https://doi.org/10.3390/genes12081119
Alharbi BM, Abdulmajeed AM, Hassan H. Biochemical and Molecular Effects Induced by Triacontanol in Acquired Tolerance of Rice to Drought Stress. Genes. 2021; 12(8):1119. https://doi.org/10.3390/genes12081119
Chicago/Turabian StyleAlharbi, Basmah M., Awatif Mahfouz Abdulmajeed, and Heba Hassan. 2021. "Biochemical and Molecular Effects Induced by Triacontanol in Acquired Tolerance of Rice to Drought Stress" Genes 12, no. 8: 1119. https://doi.org/10.3390/genes12081119






