Association of GC Variants with Bone Mineral Density and Serum VDBP Concentrations in Mexican Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Analysis
2.3. BMD Measurements
2.4. Genotyping of GC Gene Polymorphisms
2.5. Other Measurements
2.6. Statistical Analysis
3. Results
3.1. Description of the Sample
3.2. Determinants of VDBP Levels
3.3. Association between GC Gene and BMD
3.4. Association between VDBP Levels and BMD
3.5. Association between Different Forms of VitD and BMD
3.6. Interactions between VDBP Levels and VitD Intake in Low-Hip BMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Cleve, H.; Constans, J. The mutants of the vitamin-D-binding protein: More than 120 variants of the GC/DBP system. Vox Sang. 1988, 54, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Kamboh, M.I.; Ferrell, R.E. Ethnic variation in vitamin D-binding protein (GC): A review of isoelectric focusing studies in human populations. Hum. Genet. 1986, 72, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, A.L.; Vestergaard, P.; Nexo, E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin. Chem. 2001, 47, 753–756. [Google Scholar] [CrossRef]
- Lauridsen, A.L.; Vestergaard, P.; Hermann, A.P.; Brot, C.; Heickendorff, L.; Mosekilde, L.; Nexo, E. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): A cross-sectional study on 595-Early postmenopausal women. Calcif. Tissue Int. 2005. [Google Scholar] [CrossRef]
- WHO. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Available online: http://www.who.int/chp/topics/Osteoporosis.pdf (accessed on 17 March 2018).
- Kanis, J.A.; Melton, L.J., III; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-Analysis. Lancet 2014. [Google Scholar] [CrossRef]
- Bhan, I. Vitamin D binding protein and bone health. Int. J. Endocrinol. 2014. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Aguilar, M.M.; Aparicio-Bautista, D.I.; Ramírez-Salazar, E.G.; Reyes-Grajeda, J.P.; De la Cruz-Montoya, A.H.; Antuna-Puente, B.; Hidalgo-Bravo, A.; Rivera-Paredez, B.; Ramírez-Palacios, P.; Quiterio, M.; et al. Serum proteomic analysis reveals vitamin d-binding protein (Vdbp) as a potential biomarker for low bone mineral density in mexican postmenopausal women. Nutrients 2019, 11, 2853. [Google Scholar] [CrossRef] [Green Version]
- Bolland, M.J.; Grey, A.B.; Ames, R.W.; Horne, A.M.; Mason, B.H.; Wattie, D.J.; Gamble, G.D.; Bouillon, R.; Reid, I.R. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin. Endocrinol. 2007, 67, 259–264. [Google Scholar] [CrossRef]
- Denova-Gutierrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramirez-Palacios, P.; Rivera-Paredez, B.; Munoz-Aguirre, P.; Velazquez-Cruz, R.; Torres-Ibarra, L.; Meneses-Leon, J.; Mendez-Hernandez, P.; et al. Health workers cohort study: Methods and study design. Salud Publica Mex. 2016, 58, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Bhattoa, H.P.; Cavalier, E.; Phinney, K.; Sempos, C.T.; Ulmer, C.Z.; Vasikaran, S.D.; Vesper, H.; Heijboer, A.C. Recommendations on the measurement and the clinical use of vitamin D metabolites and vitamin D binding protein—A position paper from the IFCC Committee on bone metabolism. Clin. Chim. Acta 2021, 517, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Wilson, K.; Spears, R.; Shalhoub, V.; Sibley, P. Performance evaluation of four 25-hydroxyvitamin D assays to measure 25-hydroxyvitamin D2. Clin. Biochem. 2015, 48, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Beckman Coulter. SYNCRON System. Chemistry Information Sheet; Beckman Coulter: Brea, CA, USA, 2015. [Google Scholar]
- Carrillo-Vega, M.F.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Vitamin D deficiency in older adults and its associated factors: A cross-sectional analysis of the Mexican Health and Aging Study. Arch. Osteoporos. 2017. [Google Scholar] [CrossRef]
- Johnsen, M.S.; Grimnes, G.; Figenschau, Y.; Torjesen, P.A.; Almås, B.; Jorde, R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand. J. Clin. Lab. Investig. 2014, 74, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, J.; Constans, J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum. Genet. 1993, 92, 183–188. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef]
- Kawakami, M.; Blum, C.B.; Ramakrishnan, R.; Dell, R.B.; Goodman, D.S. Turnover of the plasma binding protein for vitamin D and its metabolites in normal human subjects. J. Clin. Endocrinol. Metab. 1981, 53, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D binding protein, total and free Vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Powe, C.E.; Ricciardi, C.; Berg, A.H.; Erdenesanaa, D.; Collerone, G.; Ankers, E.; Wenger, J.; Karumanchi, S.A.; Thadhani, R.; Bhan, I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 2011, 26, 1609–1616. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.-J.; Zhan, J.-K.; Tang, Z.-Y.; Huang, W.; Tan, P.; Gao, S.; Ma, C.-L.; Jian, Z.-J.; Liu, Y.-S. Vitamin D Binding Protein Affects the Correlation of 25(OH)D and Frailty in the Older Men. Int. J. Endocrinol. 2014, 2014, 543783. [Google Scholar] [CrossRef] [Green Version]
- Oleröd, G.; Hultén, L.M.; Hammarsten, O.; Klingberg, E. The variation in free 25-hydroxy vitamin D and vitamin D-binding protein with season and vitamin D status. Endocr. Connect. 2017, 6, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Taes, Y.E.C.; Goemaere, S.; Huang, G.; Van Pottelbergh, I.; De Bacquer, D.; Verhasselt, B.; Van den Broeke, C.; Delanghe, J.R.; Kaufman, J.-M. Vitamin D binding protein, bone status and body composition in community-dwelling elderly men. Bone 2006, 38, 701–707. [Google Scholar] [CrossRef]
- Pop, L.C.; Shapses, S.A.; Chang, B.; Sun, W.; Wang, X. Vitamin d-binding protein in healthy pre- and postmenopausal women: Relationship with estradiol concentrations. Endocr. Pract. 2015, 21, 936–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Wiepjes, C.M.; van Schoor, N.M.; Heijboer, A.C.; de Jongh, R.T.; den Heijer, M.; Lips, P. Changes of Vitamin D-Binding Protein, and Total, Bioavailable, and Free 25-Hydroxyvitamin D in Transgender People. J. Clin. Endocrinol. Metab. 2019, 104, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Rapado, A.; Hawkins, F.; Sobrinho, L.; Díaz-Curiel, M.; Galvao-Telles, A.; Arver, S.; Melo Gomes, J.; Mazer, N.; Garcia e Costa, J.; Horcajada, C.; et al. Bone mineral density and androgen levels in elderly males. Calcif. Tissue Int. 1999, 65, 417–421. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; Van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Murad, R.; Mahboob, T.; Rehman, R.; Baig, R. Comparison of serum levels of vitamin d and vitamin d-binding protein in normal, osteopenic and osteoporotic postmenopausal women. Pak. J. Med. Sci. 2019, 35, 543–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef]
- Lauridsen, A.L.; Vestergaard, P.; Hermann, A.P.; Moller, H.J.; Mosekilde, L.; Nexo, E. Female premenopausal fracture risk is associated with Gc phenotype. J. Bone Miner. Res. 2004, 19, 875–881. [Google Scholar] [CrossRef]
- Fang, Y.; Van Meurs, J.B.J.; Arp, P.; Van Leeuwen, J.P.T.; Hofman, A.; Pols, H.A.P.; Uitterlinden, A.G. Vitamin D binding protein genotype and osteoporosis. Calcif. Tissue Int. 2009, 85, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.C.; Zhu, Y.; Gong, C.; Liang, X.; Zhou, X.; Xu, Y.; Lyu, D.; Mo, J.; Xu, J.; Song, J.; et al. The GC2 haplotype of the vitamin D binding protein is a risk factor for a low plasma 25-hydroxyvitamin D concentration in a Han Chinese population 11 Medical and Health Sciences 1103 Clinical Sciences. Nutr. Metab. 2019, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Ezura, Y.; Nakajima, T.; Kajita, M.; Ishida, R.; Inoue, S.; Yoshida, H.; Suzuki, T.; Shiraki, M.; Hosoi, T.; Orimo, H.; et al. Association of molecular variants, haplotypes, and linkage disequilibrium within the human vitamin D-binding protein (DBP) gene with postmenopausal bone mineral density. J. bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2003, 18, 1642–1649. [Google Scholar] [CrossRef]
- Abidin, N.Z.; Mitra, S.R. Total vs. Bioavailable: Determining a Better 25(OH)D Index in Association with Bone Density and Muscle Mass in Postmenopausal Women. Metabolites 2020, 11, 23. [Google Scholar] [CrossRef]
- Jemielita, T.O.; Leonard, M.B.; Baker, J.; Sayed, S.; Zemel, B.S.; Shults, J.; Herskovitz, R.; Denburg, M.R. Association of 25-hydroxyvitamin D with areal and volumetric measures of bone mineral density and parathyroid hormone: Impact of vitamin D-binding protein and its assays. Osteoporos. Int. 2016, 27, 617–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, J.G. Plasma vitamin D-binding protein (Gc-globulin): Multiple tasks. J. Steroid Biochem. Mol. Biol. 1995, 53, 579–582. [Google Scholar] [CrossRef]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E.C. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]


| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| VDBP Levels | VDBP Levels | |||||||
| Characteristics | Low c (106.8–250.8) * n = 430 | Medium (250.9–303.9) * n = 430 | High (304.2–449.7) * n = 429 | p Value ** | Low c (114.6–239.3) * n = 188 | Medium (239.9–287.9) * n = 188 | High (288.0–423.9) * n = 188 | p Value ** |
| Age (years) a | 55.5 (20) | 53.0 (21) | 53.0 (19) | <0.001 | 48.0 (22.5) | 44.5 (19) | 47.0 (20.5) | 0.162 |
| Leisure time physical activity (min/day) a | 9.6 (28.6) | 9.6 (28.6) | 9.6 (26.8) | 0.493 | 12.9 (43.9) | 19.1 (42.6) | 14.2 (28.6) | 0.433 |
| Active (≥30 min/day), % | 32.1 | 30.5 | 31.5 | 0.850 | 38.8 | 45.7 | 41.5 | 0.593 |
| Smoking status | ||||||||
| Current, % | 10.2 | 7.2 | 8.1 | 0.286 | 21.7 | 22.3 | 19.8 | 0.650 |
| Past, % | 22.9 | 22.5 | 25.7 | 0.175 | 38.6 | 36.2 | 44.5 | 0.246 |
| BMI (kg/m2) a | 26.8 (5.9) | 26.7 (6.0) | 26.9 (6.4) | 0.493 | 26.5 (4.8) | 26.6 (4.3) | 26.4 (5.7) | 0.433 |
| Overweight, % | 48.9 | 51.1 | 45.2 | 0.277 | 41.6 | 38.5 | 39.6 | 0.693 |
| Obesity, % | 20.7 | 17.6 | 18.1 | 0.335 | 25.8 | 25.9 | 27.0 | 0.792 |
| Body fat proportion a | 45.3 (8.6) | 45.0 (8.4) | 44.9 (8.1) | 0.322 | 31.5 (6.9) | 31.4 (6.7) | 31.2 (8.3) | 0.116 |
| Femoral neck BMD b, g/cm2 | 0.906 (0.156) | 0.931 (0.139) | 0.947 (0.135) | <0.001 | 1.012 (0.152) | 1.042 (0.141) | 1.035 (0.169) | 0.464 |
| Femur Low BMD, % | 47.2 | 43.0 | 36.6 | 0.002 | 29.4 | 22.6 | 29.6 | 0.966 |
| Hip BMD b, g/cm2 | 0.948 (0.163) | 0.967 (0.135) | 0.985 (0.129) | <0.001 | 1.064 (0.140) | 1.085 (0.132) | 1.083 (0.158) | 0.550 |
| Hip Low BMD, % | 34.7 | 27.0 | 22.1 | <0.001 | 17.0 | 16.0 | 20.2 | 0.425 |
| Lumbar spine BMD b, g/cm2 | 1.053 (0.174) | 1.064 (0.161) | 1.071 (0.155) | 0.243 | 1.147 (0.165) | 1.151 (0.1444) | 1.162 (0.162) | 1.000 |
| Lumbar spine Low BMD, % | 54.8 | 53.9 | 51.5 | 0.333 | 48.4 | 41.5 | 40.1 | 0.106 |
| Total 25-hydroxivitamin D (ng/mL)a | 20.6 (7.4) | 21.2 (8.3) | 20.8 (8.4) | 0.198 | 22.3 (8.5) | 22.1 (8.7) | 21.8 (8.7) | 0.460 |
| Free 25-hydroxivitamin D (pg/mL) a | 7.5 (2.5) | 5.8 (2.2) | 4.5 (2.1) | <0.001 | 7.5 (3.0) | 6.3 (2.3) | 5.1 (2.5) | <0.001 |
| Bioavailable 25-hydroxivitamin D (ng/mL) a | 2.6 (1.1) | 2.2 (0.9) | 1.7 (0.8) | <0.001 | 2.9 (1.3) | 2.4 (1.0) | 1.9 (0.9) | <0.001 |
| Free 25-hydroxivitamin D-SNP adjusted (pg/mL) a | 7.3 (4.3) | 6.6 (3.9) | 5.3 (3.5) | <0.001 | 8.1 | 7.3 | 5.9 | <0.001 |
| Bioavailable 25-hydroxivitamin D-SNP adjusted (ng/mL) a | 2.7 (1.6) | 2.5 (1.5) | 1.9 (1.3) | <0.001 | 3.1 | 2.8 | 2.3 | <0.001 |
| Vitamin D intake (UI/day) a | 150.3 (166.0) | 147.4 (166.6) | 133.1 (166.6) | 0.092 | 141.7 | 155.3 | 140.5 | 0.335 |
| Albumin (g/dL) a | 4.2 (0.4) | 4.2 (0.4) | 4.2 (0.4) | 1.000 | 4.3 (0.4) | 4.3 (0.4) | 4.3 (0.5) | 1.000 |
| Alcohol (g/day) a | 0.43 (1.8) | 0.58 (1.8) | 0.79 (1.5) | 0.011 | 2.2 | 3.1 | 2.7 | 0.051 |
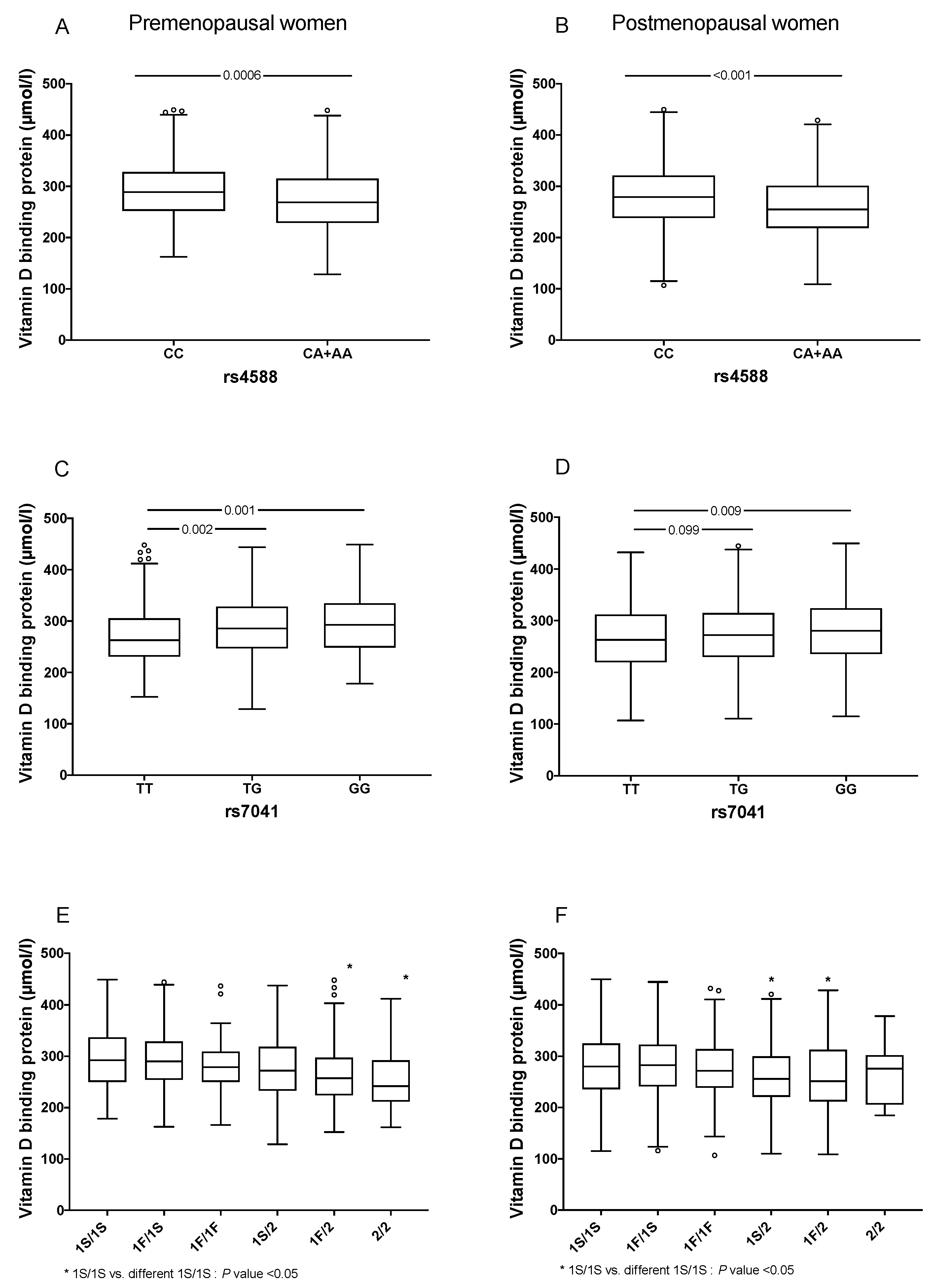
| rs4588 | ||||||||
| C, n (%) | 621 (72.9) | 676 (80.0) | 706 (84.0) | <0.001 | 279 (75.4) | 298 (81.9) | 310 (83.3) | 0.009 |
| A, n (%) | 231 (27.0) | 168 (20.0) | 134 (16.0) | <0.001 | 91 (24.6) | 66 (18.1) | 62 (16.6) | 0.007 |
| CC, n (%) | 214 (50.2) | 266 (63.0) | 293 (69.8) | <0.001 | 101 (54.6) | 120 (65.9) | 128 (68.8) | 0.005 |
| CA+AA, n (%) | 212 (49.8) | 156 (37.0) | 127 (30.2) | <0.001 | 84 (45.4) | 62 (34.1) | 58 (31.2) | 0.015 |
| rs7041 | ||||||||
| T, n (%) | 475 (55.8) | 455 (53.9) | 395 (46.9) | 0.0003 | 214 (57.8) | 186 (50.8) | 178 (47.9) | 0.003 |
| G, n (%) | 377 (44.3) | 389 (46.1) | 447 (53.1) | 0.0003 | 156 (42.2) | 180 (49.2) | 194 (52.2) | 0.024 |
| TT, n (%) | 138 (32.4) | 124 (29.4) | 93 (22.1) | <0.001 | 63 (34.1) | 44 (24.1) | 37 (19.9) | 0.002 |
| TG, n (%) | 199 (46.7) | 207 (49.1) | 209 (49.6) | 0.415 | 88 (47.6) | 97 (53.3) | 104 (55.9) | 0.110 |
| GG, n (%) | 89 (20.9) | 91 (21.6) | 119 (28.3) | 0.011 | 34 (18.4) | 41 (22.5) | 45 (24.2) | 0.173 |
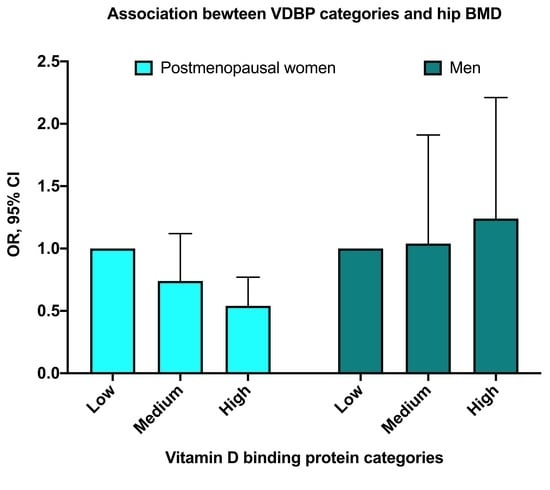
| Premenopausal Women, n = 513 | Postmenopausal Women, n = 776 | Men, n = 564 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VDBP Tertiles | VDBP Tertiles | VDBP Tertiles | |||||||||||||
| Low (128.6–255.9) * | Medium (256.0–308.9) * | High (309.0–449.0) * | Low (106.8–250.8) * | Medium (250.9–303.9) * | High (304.2–449.7) * | Low a (114.6–239.3) * | Medium a (239.9–287.9) * | High a (288.0–423.9) * | |||||||
| Model | OR (95% CI) | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | OR (95% CI) | OR (95% CI) | p Value | |
| Crude | Ref. | 1.06 (0.55, 2.03) | 0.868 | 1.48 (0.80, 2.74) | 0.215 | Ref. | 0.67 (0.47, 0.96) | 0.027 | 0.42 (0.29, 0.61) | <0.001 | Ref. | 0.926 (0.54, 1.60) | 0.781 | 1.25 (0.73, 2.08) | 0.427 |
| Adjusted 1 | Ref. | 1.06 (0.53, 2.15) | 0.860 | 1.41 (0.72, 2.79) | 0.318 | Ref. | 0.76 (0.51, 1.14) | 0.109 | 0.51 (0.34, 0.78) | 0.002 | Ref. | 1.05 (0.57, 1.91) | 0.884 | 1.28 (0.72, 2.28) | 0.402 |
| Adjusted 2 | Ref. | 1.14 (0.55, 2.34) | 0.742 | 1.43 (0.71, 2.87) | 0.319 | Ref. | 0.74 (0.49, 1.12) | 0.153 | 0.54 (0.33, 0.77) | 0.002 | Ref. | 1.04 (0.57, 1.91) | 0.892 | 1.24 (0.69, 2.21) | 0.473 |
| Normal Vitamin D, n = 1077 | Deficiency Vitamin D, n = 775 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VDBP Tertiles | VDBP Tertiles | |||||||||
| Low (106.8–247.5) * | Medium (247.6–298.6) * | High (298.7–448.0) * | Low (114.6–242.9) * | Medium (243.0–301.2) * | High (301.9–449.7) * | |||||
| Model | OR (95% CI) | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Crude | Ref. | 0.67 (0.48, 0.95) | 0.024 | 0.82 (0.58, 1.14) | 0.233 | Ref. | 0.79 (0.54, 1.16) | 0.237 | 0.57 (0.38, 0.85) | 0.006 |
| Adjusted 1 | Ref. | 0.79 (0.53, 1.17) | 0.234 | 0.94 (0.64, 1.40) | 0.770 | Ref. | 0.87 (0.55, 1.38) | 0.552 | 0.61 (0.38, 0.98) | 0.042 |
| Adjusted 2 | Ref. | 0.79 (0.53, 1.18) | 0.256 | 0.95 (0.64, 1.41) | 0.792 | Ref. | 0.86 (0.54, 1.38) | 0.536 | 0.60 (0.37, 0.97) | 0.038 |
| All Participants, n = 1893 | All Women, n = 1289 | All Men, n = 564 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip BMD (g/cm2) | Femoral Neck * (g/cm2) | Spine Lumbar * (g/cm2) | Hip BMD (g/cm2) | Femoral Neck * (g/cm2) | Spine Lumbar * (g/cm2) | Hip BMD (g/cm2) | Femoral Neck * (g/cm2) | Spine Lumbar * (g/cm2) | ||||||||||
| Model | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value |
| Crude | 0.04 (0.008, 0.07) | 0.012 | 0.05 (0.02, 0.08) | 0.001 | 0.03 (−0.006, 0.06) | 0.108 | 0.06 (0.02, 0.09) | 0.001 | 0.07 (0.03, 0.10) | <0.001 | 0.04 (0.001, 0.08) | 0.043 | 0.04 (−0.01, 0.10) | 0.138 | 0.05 (−0.01, 0.11) | 0.078 | 0.04 (−0.02, 0.10) | 0.217 |
| Adjusted 1 | 0.03 (0.003, 0.05) | 0.030 | 0.03 (0.005, 0.05) | 0.016 | 0.02 (−0.01, 0.05) | 0.235 | 0.01 (−0.02, 0.04) | 0.388 | 0.01 (−0.01, 0.04) | 0.260 | −0.002 (−0.04, 0.03) | 0.884 | 0.06 (0.01, 0.11) | 0.029 | 0.06 (0.01, 0.11) | 0.016 | 0.05 (−0.01, 0.11) | 0.081 |
| Adjusted 2 | 0.03 (0.002, 0.05) | 0.031 | 0.03 (0.005, 0.05) | 0.016 | 0.02 (−0.01, 0.05) | 0.214 | 0.01 (−0.02, 0.04) | 0.415 | 0.02 (−0.01, 0.04) | 0.259 | −0.002 (−0.03, 0.03) | 0.922 | 0.06 (0.007, 0.11) | 0.025 | 0.06 (0.01, 0.11) | 0.014 | 0.05 (−0.01, 0.11) | 0.078 |
| Women (n = 1277) | ||||||
|---|---|---|---|---|---|---|
| VDBP Levels | ||||||
| Low a (106.8–250.8) | Medium (250.9–303.9) | High (304.2–449.7) | ||||
| Ref. | OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Vitamin D intake | Low b (1.5–85.9) * | 1.0 | 1.55 (0.74–3.27) | 0.247 | 1.50 (0.73–3.07) | 0.274 |
| Medium (86.1–144.5) * | 1.0 | 1.08 (0.53–2.18) | 0.833 | 0.83 (0.40–1.74) | 0.623 | |
| High (144.7–253.1) * | 1.0 | 0.37 (0.19–0.74) | 0.004 | 0.39 (0.19–0.79) | 0.009 | |
| Very high (254.8–1275.6) * | 1.0 | 0.80 (0.39–1.62) | 0.538 | 0.44 (0.21–0.94) | 0.035 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Paredez, B.; Hidalgo-Bravo, A.; León-Reyes, G.; Antuna-Puente, B.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Association of GC Variants with Bone Mineral Density and Serum VDBP Concentrations in Mexican Population. Genes 2021, 12, 1176. https://doi.org/10.3390/genes12081176
Rivera-Paredez B, Hidalgo-Bravo A, León-Reyes G, Antuna-Puente B, Flores YN, Salmerón J, Velázquez-Cruz R. Association of GC Variants with Bone Mineral Density and Serum VDBP Concentrations in Mexican Population. Genes. 2021; 12(8):1176. https://doi.org/10.3390/genes12081176
Chicago/Turabian StyleRivera-Paredez, Berenice, Alberto Hidalgo-Bravo, Guadalupe León-Reyes, Bárbara Antuna-Puente, Yvonne N. Flores, Jorge Salmerón, and Rafael Velázquez-Cruz. 2021. "Association of GC Variants with Bone Mineral Density and Serum VDBP Concentrations in Mexican Population" Genes 12, no. 8: 1176. https://doi.org/10.3390/genes12081176