Rhythmic Regulation of DNA Methylation Factors and Core-Clock Genes in Brain Structures Activated by Cocaine or Sucrose: Potential Role of Chromatin Remodeling
Abstract
:1. Introduction
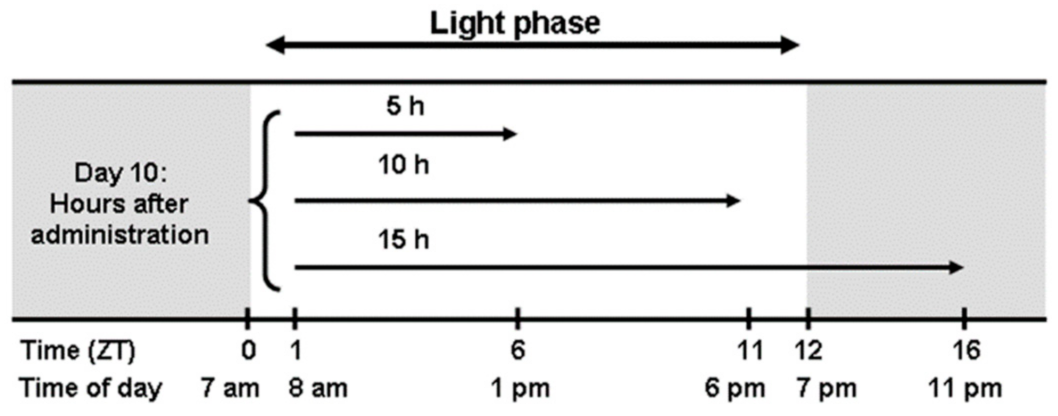
2. Materials and Methods
2.1. Animals
2.2. Brain Dissection and RNA Extraction
2.3. Reverse Transcription-Quantitative PCR Analysis
2.4. Global DNA Methylation Analysis
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
3.1. Chronic Daily Cocaine and Sucrose Administration Does Not Affect Rat Body Weight
3.2. Cocaine and Sucrose Induce Opposite Effects on Dnmt3a and Dnmt3b in the PFCx and the CPu in a Time Dependent Manner
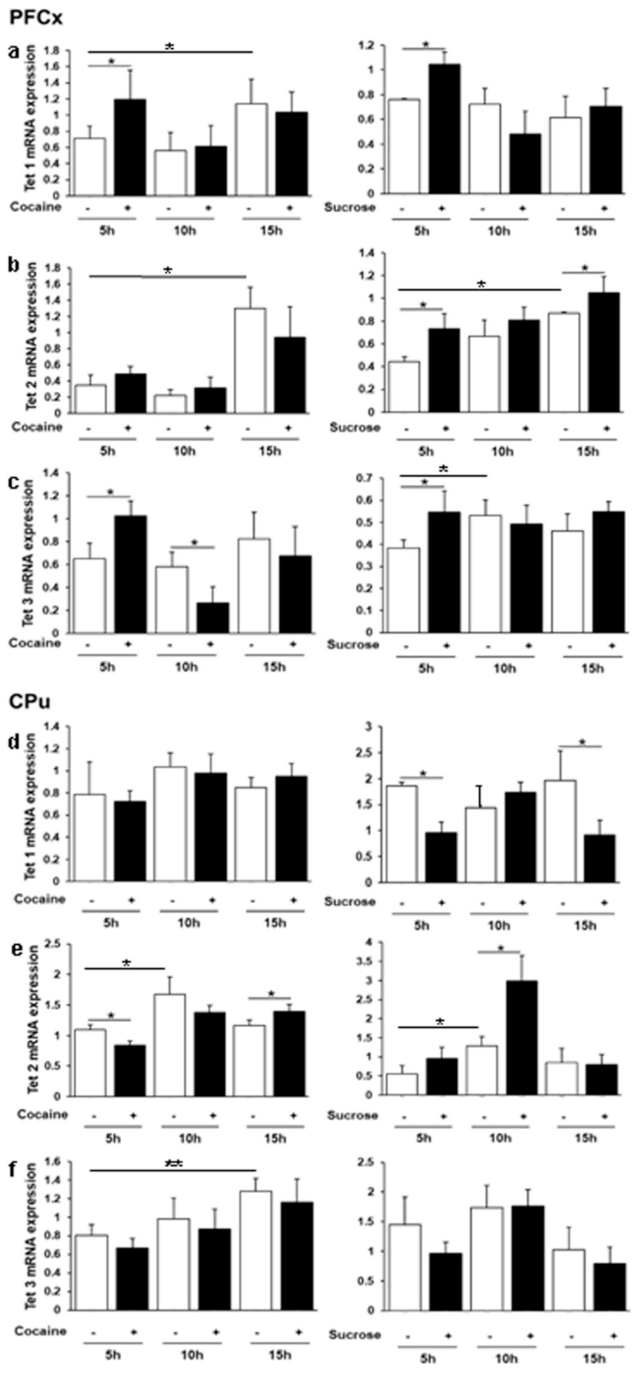
3.3. Daily Regulation of Tet 1, 2 and 3 Gene Expression by Cocaine and Sucrose
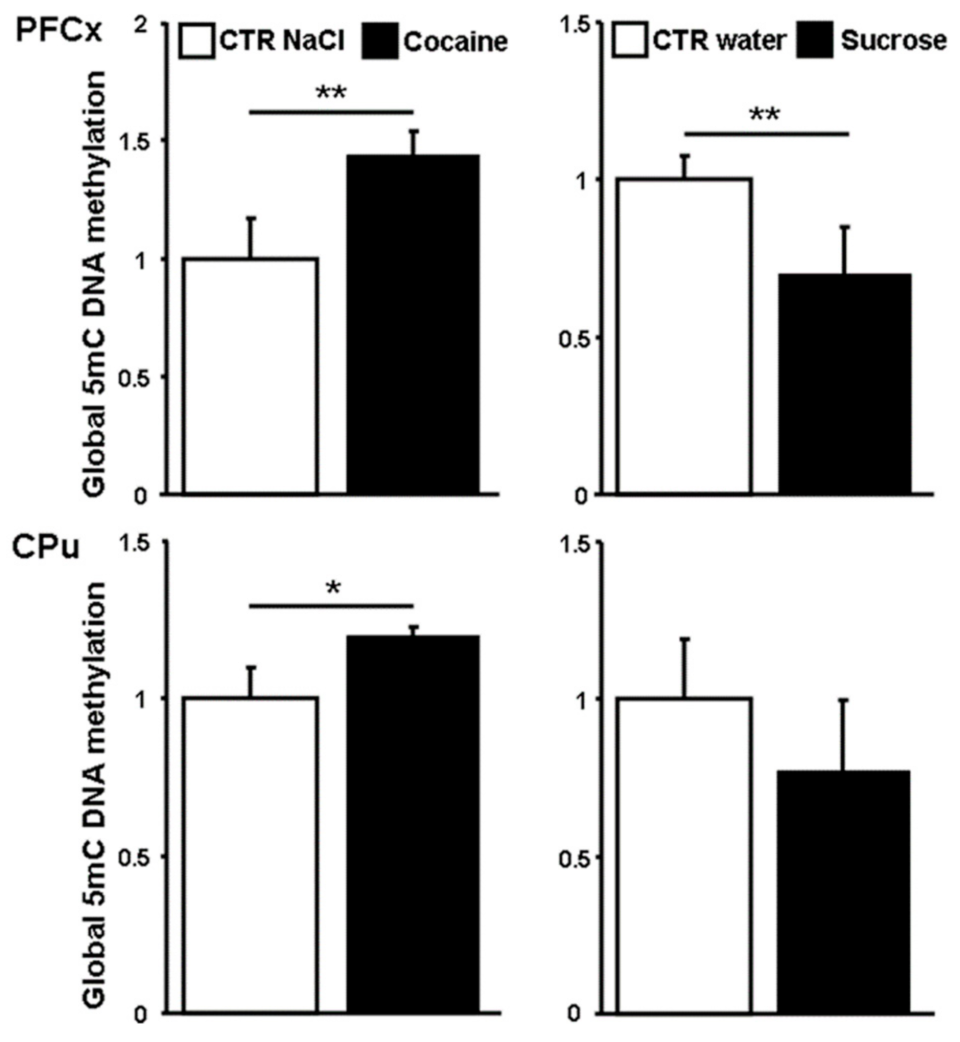
3.4. Global 5-methylcytosines Analysis
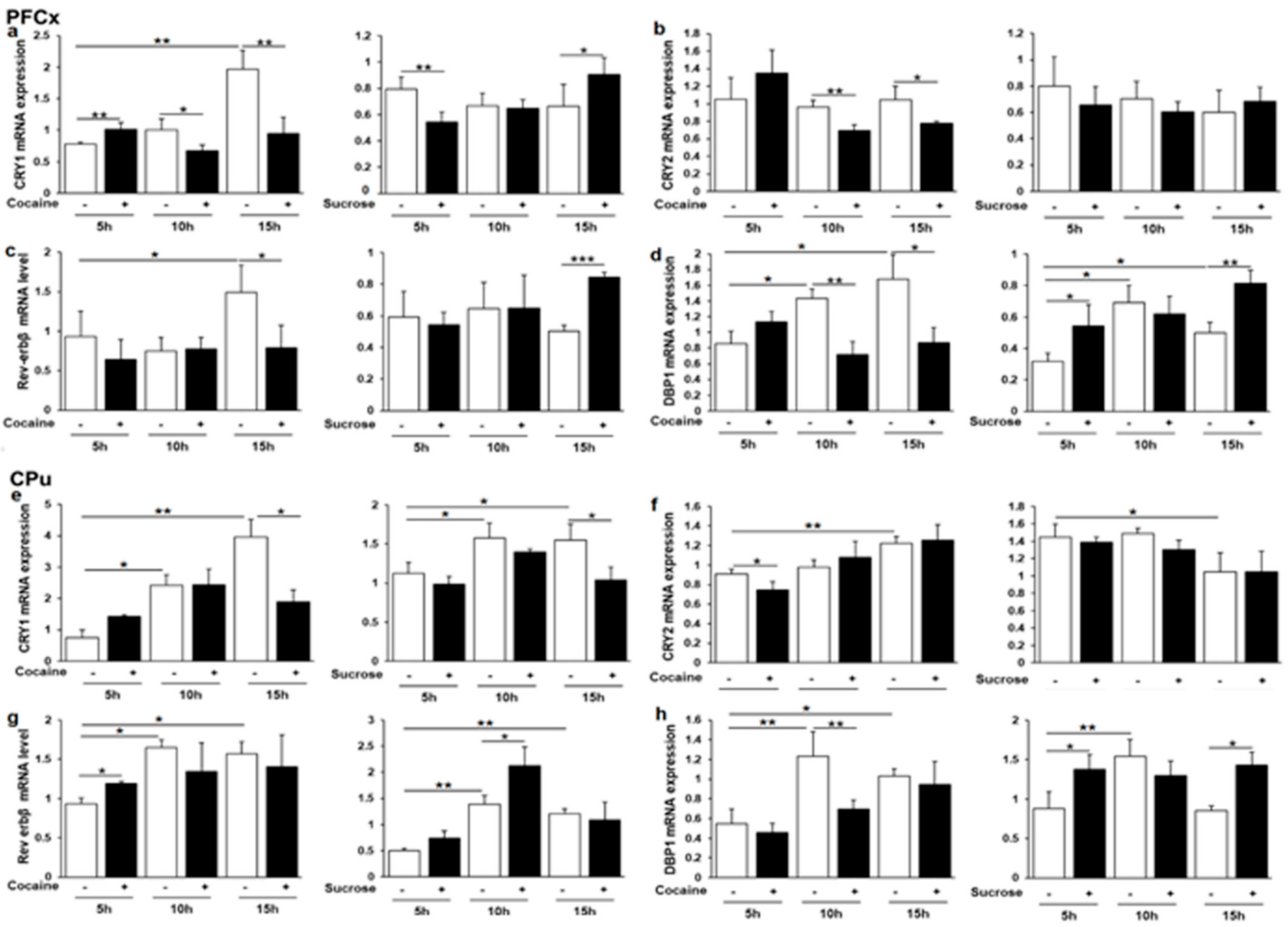
3.5. Effects of Cocaine and Sucrose on Clock and Clock-Controlled Gene Expression
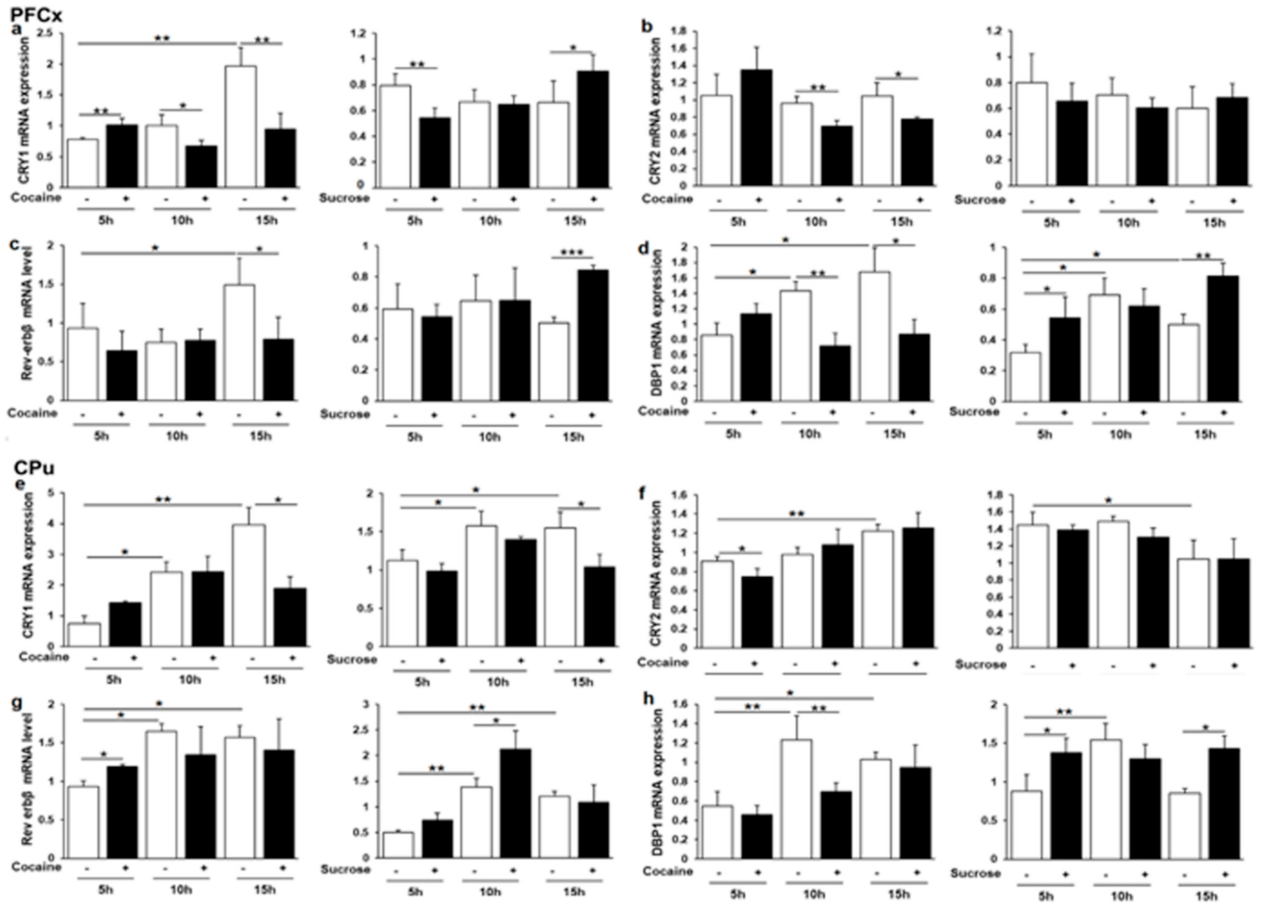
3.6. Cry1, Cry2, Rev-erbβ and Dbp1 Expression in Response to Cocaine and Sucrose
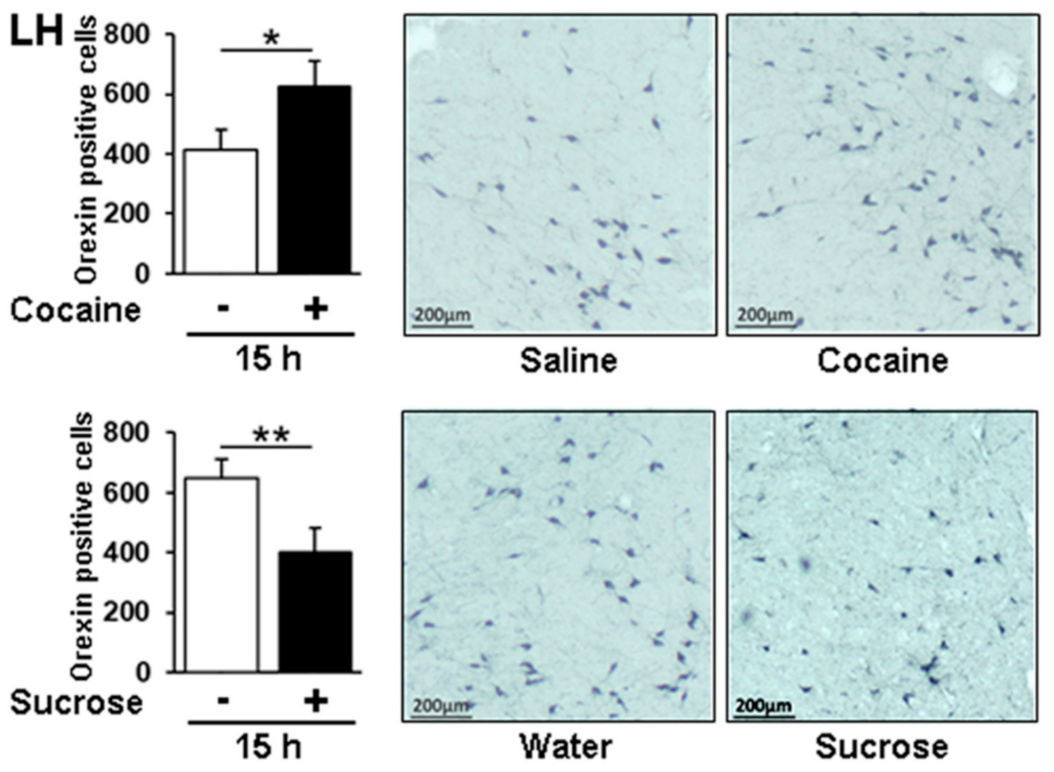
3.7. Neuropeptide Y and Orexin Regulation by Cocaine and Sucrose
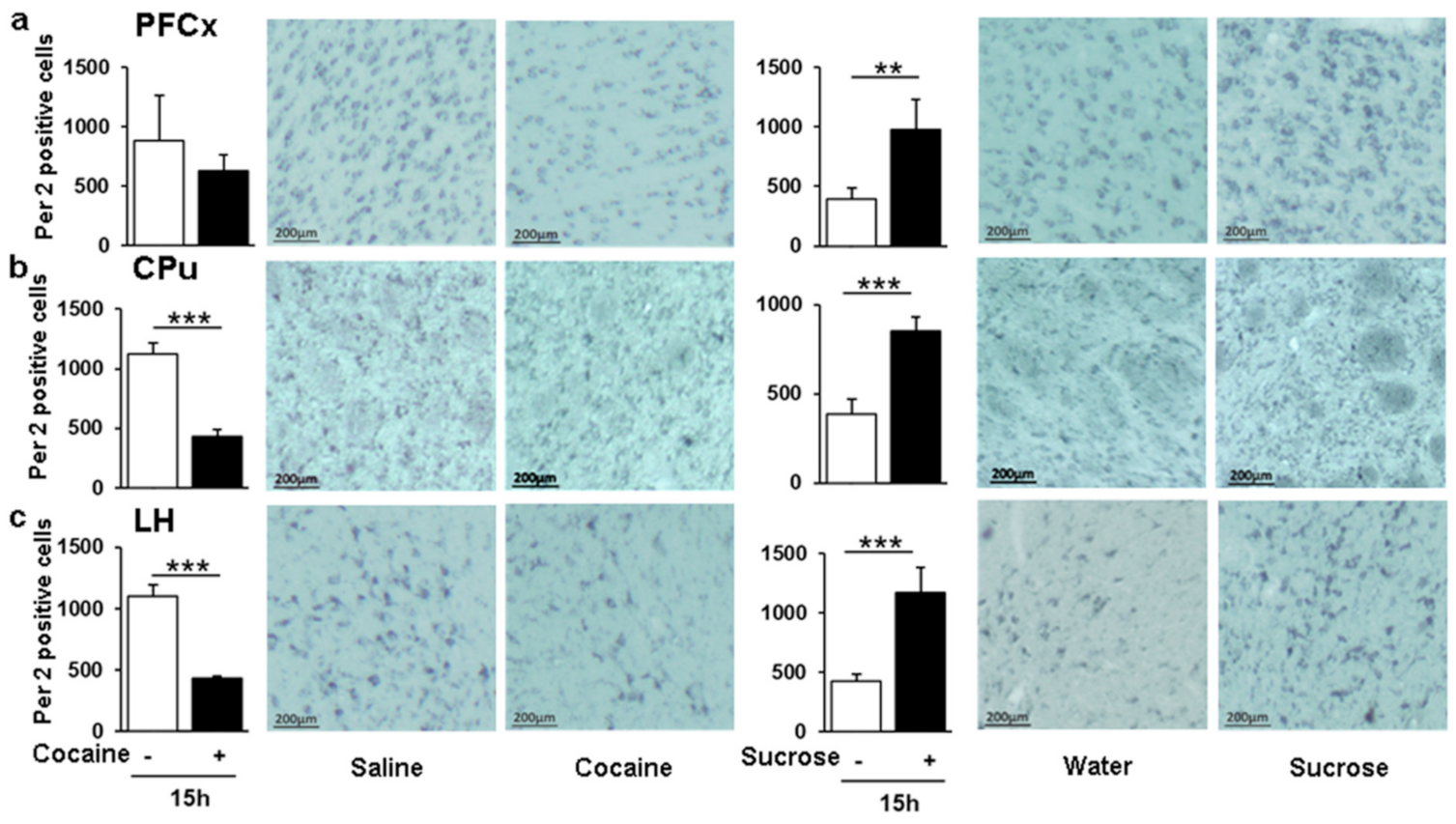
3.8. Opposite Pattern of Per2 Positive Cells in Response to Sucrose and Cocaine in the LH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Chefer, S.; Kurup, P.K.; Guillem, K.; Vaupel, D.B.; Ross, T.J.; Moore, A.; Yang, Y.; Peoples, L.L.; Stein, E.A. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. Neuroimage 2012, 62, 1857–1866. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Shabat-Simon, M.; Shalev, U.; Barnea-Ygael, N.; Cooper, A.; Zangen, A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J. Neurosci. 2007, 27, 14179–14189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, C.M.; Wightman, R.M.; Carelli, R.M. Dynamics of rapid dopamine release in the nucleus accumbens during goal-directed behaviors for cocaine versus natural rewards. Neuropharmacology 2014, 86, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pol Bodetto, S.; Romieu, P.; Sartori, M.; Tesone-Coelho, C.; Majchrzak, M.; Barbelivien, A.; Zwiller, J.; Anglard, P. Differential regulation of MeCP2 and PP1 in passive or voluntary administration of cocaine or food. Int. J. Neuropsychopharmacol. 2014, 17, 2031–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, L.; Sartori, M.; Pol Bodetto, S.; Romieu, P.; Kalsbeek, A.; Zwiller, J.; Anglard, P. Regulation of Brain DNA Methylation Factors and of the Orexinergic System by Cocaine and Food Self-Administration. Mol. Neurobiol. 2019, 56, 5315–5331. [Google Scholar] [CrossRef] [Green Version]
- Cassel, S.; Carouge, D.; Gensburger, C.; Anglard, P.; Burgun, C.; Dietrich, J.B.; Aunis, D.; Zwiller, J. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol. Pharmacol. 2006, 70, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Newton, S.S.; Duman, R.S. Chromatin remodeling: A novel mechanism of psychotropic drug action. Mol. Pharmacol. 2006, 70, 440–443. [Google Scholar] [CrossRef]
- Schenk, S.; Highgate, Q. Methylenedioxymethamphetamine (MDMA): Serotonergic and dopaminergic mechanisms related to its use and misuse. J. Neurochem. 2021, 157, 1714–1724. [Google Scholar] [CrossRef]
- Good, K.V.; Vincent, J.B.; Ausio, J. MeCP2: The Genetic Driver of Rett Syndrome Epigenetics. Front. Genet. 2021, 12, 620859. [Google Scholar] [CrossRef]
- LaPlant, Q.; Vialou, V.; Covington, H.E., 3rd; Dumitriu, D.; Feng, J.; Warren, B.L.; Maze, I.; Dietz, D.M.; Watts, E.L.; Iniguez, S.D.; et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci. 2010, 13, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Pol Bodetto, S.; Carouge, D.; Fonteneau, M.; Dietrich, J.B.; Zwiller, J.; Anglard, P. Cocaine represses protein phosphatase-1Cbeta through DNA methylation and Methyl-CpG Binding Protein-2 recruitment in adult rat brain. Neuropharmacology 2013, 73, 31–40. [Google Scholar] [CrossRef]
- Carouge, D.; Host, L.; Aunis, D.; Zwiller, J.; Anglard, P. CDKL5 is a brain MeCP2 target gene regulated by DNA methylation. Neurobiol. Dis. 2010, 38, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Anier, K.; Malinovskaja, K.; Aonurm-Helm, A.; Zharkovsky, A.; Kalda, A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology 2010, 35, 2450–2461. [Google Scholar] [CrossRef] [Green Version]
- Ploense, K.L.; Li, X.; Baker-Andresen, D.; Carr, A.E.; Woodward, N.; Bagley, J.; Szumlinski, K.K.; Bredy, T.W.; Kippin, T.E. Prolonged-access to cocaine induces distinct Homer2 DNA methylation, hydroxymethylation, and transcriptional profiles in the dorsomedial prefrontal cortex of Male Sprague-Dawley rats. Neuropharmacology 2018, 143, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Levy, K.A.; Brodnik, Z.D.; Shaw, J.K.; Perrey, D.A.; Zhang, Y.; Espana, R.A. Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology 2017, 234, 2761–2776. [Google Scholar] [CrossRef] [PubMed]
- Sadri-Vakili, G. Cocaine triggers epigenetic alterations in the corticostriatal circuit. Brain Res. 2015, 1628, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadet, J.L. Epigenetics of Stress, Addiction, and Resilience: Therapeutic Implications. Mol. Neurobiol. 2016, 53, 545–560. [Google Scholar] [CrossRef] [Green Version]
- Vaillancourt, K.; Ernst, C.; Mash, D.; Turecki, G. DNA Methylation Dynamics and Cocaine in the Brain: Progress and Prospects. Genes 2017, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anglard, P.; Zwiller, J. Cocaine and Epigenetics: An Overview. Book Chapter in the Neuroscience of Cocaine: Mechanisms and Treatment; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; pp. 79–88. [Google Scholar]
- Campbell, R.R.; Wood, M.A. How the epigenome integrates information and reshapes the synapse. Nat. Rev. Neurosci. 2019, 20, 133–147. [Google Scholar] [CrossRef]
- Coulson, R.L.; LaSalle, J.M. Epigenetics of Circadian Rhythms in Imprinted Neurodevelopmental Disorders. Prog. Mol. Biol. Transl. Sci. 2018, 157, 67–92. [Google Scholar]
- Kadayifci, F.Z.; Zheng, S.; Pan, Y.X. Molecular Mechanisms Underlying the Link between Diet and DNA Methylation. Int. J. Mol. Sci. 2018, 19, 4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Hajkova, P.; Ecker, J.R. Dynamic DNA methylation: In the right place at the right time. Science 2018, 361, 1336–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, U. The circadian clock, reward, and memory. Front. Mol. Neurosci 2011, 4, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, H.T.; Sergeeva, A.; Stark, G.; Sorg, B.A. Circadian discrimination of reward: Evidence for simultaneous yet separable food- and drug-entrained rhythms in the rat. Chronobiol. Int. 2012, 29, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Abarca, C.; Albrecht, U.; Spanagel, R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc. Natl. Acad. Sci. USA 2002, 99, 9026–9030. [Google Scholar] [CrossRef] [Green Version]
- Stowie, A.C.; Amicarelli, M.J.; Prosser, R.A.; Glass, J.D. Chronic cocaine causes long-term alterations in circadian period and photic entrainment in the mouse. Neuroscience 2015, 284, 171–179. [Google Scholar] [CrossRef]
- Prosser, R.A.; Stowie, A.; Amicarelli, M.; Nackenoff, A.G.; Blakely, R.D.; Glass, J.D. Cocaine modulates mammalian circadian clock timing by decreasing serotonin transport in the SCN. Neuroscience 2014, 275, 184–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosser, R.A.; Glass, J.D. Neurological aspects of cocaine and the suprachiasmatic circadian clock. In Book Chapter in the Neuroscience of Cocaine: Mechanisms and Treatment; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; pp. 163–172. [Google Scholar] [CrossRef]
- Joska, T.M.; Zaman, R.; Belden, W.J. Regulated DNA methylation and the circadian clock: Implications in cancer. Biology 2014, 3, 560–577. [Google Scholar] [CrossRef] [Green Version]
- Azzi, A.; Evans, J.A.; Leise, T.; Myung, J.; Takumi, T.; Davidson, A.J.; Brown, S.A. Network Dynamics Mediate Circadian Clock Plasticity. Neuron 2017, 93, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Zhao, M.; Li, M.; Song, T.; Zhang, M.; Quan, L.; Li, S.; Sun, Z.S. Reversal of cocaine-conditioned place preference through methyl supplementation in mice: Altering global DNA methylation in the prefrontal cortex. PLoS ONE 2012, 7, e33435. [Google Scholar] [CrossRef]
- Wright, K.N.; Hollis, F.; Duclot, F.; Dossat, A.M.; Strong, C.E.; Francis, T.C.; Mercer, R.; Feng, J.; Dietz, D.M.; Lobo, M.K.; et al. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J. Neurosci. 2015, 35, 8948–8958. [Google Scholar] [CrossRef] [PubMed]
- Anier, K.; Zharkovsky, A.; Kalda, A. S-adenosylmethionine modifies cocaine-induced DNA methylation and increases locomotor sensitization in mice. Int. J. Neuropsychopharmacol. 2013, 16, 2053–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonteneau, M.; Filliol, D.; Anglard, P.; Befort, K.; Romieu, P.; Zwiller, J. Inhibition of DNA methyltransferases regulates cocaine self-administration by rats: A genome-wide DNA methylation study. Genes Brain Behav. 2017, 16, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmeichel, B.E.; Matzeu, A.; Koebel, P.; Vendruscolo, L.F.; Sidhu, H.; Shahryari, R.; Kieffer, B.L.; Koob, G.F.; Martin-Fardon, R.; Contet, C. Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats. Neuropsychopharmacology 2018, 43, 2373–2382. [Google Scholar] [CrossRef] [Green Version]
- Boutrel, B.; Steiner, N.; Halfon, O. The hypocretins and the reward function: What have we learned so far? Front. Behav. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Gentile, T.A.; Simmons, S.J.; Barker, D.J.; Shaw, J.K.; Espana, R.A.; Muschamp, J.W. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict. Biol. 2017, 23, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Wiskerke, J.; James, M.H.; Aston-Jones, G. The orexin-1 receptor antagonist SB-334867 reduces motivation, but not inhibitory control, in a rat stop signal task. Brain Res. 2019, 1731, 146222. [Google Scholar] [CrossRef]
- Rotter, A.; Bayerlein, K.; Hansbauer, M.; Weiland, J.; Sperling, W.; Kornhuber, J.; Biermann, T. Orexin A expression and promoter methylation in patients with cannabis dependence in comparison to nicotine-dependent cigarette smokers and nonsmokers. Neuropsychobiology 2012, 66, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Hirosawa, M.; Tabei, Y.; Arai, D.; Tanaka, S.; Murakami, N.; Yagi, S.; Shiota, K. Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. J. Biol. Chem. 2013, 288, 17099–17110. [Google Scholar] [CrossRef] [Green Version]
- Dehan, P.; Canon, C.; Trooskens, G.; Rehli, M.; Munaut, C.; Van Criekinge, W.; Delvenne, P. Expression of type 2 orexin receptor in human endometrium and its epigenetic silencing in endometrial cancer. J. Clin. Endocrinol. Metab. 2013, 98, 1549–1557. [Google Scholar] [CrossRef] [Green Version]
- Boersma, G.J.; Liang, N.C.; Lee, R.S.; Albertz, J.D.; Kastelein, A.; Moody, L.A.; Aryal, S.; Moran, T.H.; Tamashiro, K.L. Failure to upregulate Agrp and Orexin in response to activity based anorexia in weight loss vulnerable rats characterized by passive stress coping and prenatal stress experience. Psychoneuroendocrinology 2016, 67, 171–181. [Google Scholar] [CrossRef] [Green Version]
- James, M.H.; Stopper, C.M.; Zimmer, B.A.; Koll, N.E.; Bowrey, H.E.; Aston-Jones, G. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol. Psychiatry 2018, 85, 925–935. [Google Scholar] [CrossRef]
- Anglard, P.; Tory, K.; Brauch, H.; Weiss, G.H.; Latif, F.; Merino, M.J.; Lerman, M.I.; Zbar, B.; Linehan, W.M. Molecular analysis of genetic changes in the origin and development of renal cell carcinoma. Cancer Res. 1991, 51, 1071–1077. [Google Scholar]
- Nugent, B.M.; Wright, C.L.; Shetty, A.C.; Hodes, G.E.; Lenz, K.M.; Mahurkar, A.; Russo, S.J.; Devine, S.E.; McCarthy, M.M. Brain feminization requires active repression of masculinization via DNA methylation. Nat. Neurosci. 2015, 18, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Feillet, C.A.; Bainier, C.; Mateo, M.; Blancas-Velazquez, A.; Salaberry, N.L.; Ripperger, J.A.; Albrecht, U.; Mendoza, J. Rev-erbalpha modulates the hypothalamic orexinergic system to influence pleasurable feeding behaviour in mice. Addict. Biol. 2017, 22, 411–422. [Google Scholar] [CrossRef]
- LeSauter, J.; Lambert, C.M.; Robotham, M.R.; Model, Z.; Silver, R.; Weaver, D.R. Antibodies for assessing circadian clock proteins in the rodent suprachiasmatic nucleus. PLoS ONE 2012, 7, e35938. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.A.; Suen, T.C.; Callif, B.L.; Mitchell, A.S.; Castanon-Cervantes, O.; Baker, K.M.; Kloehn, I.; Baba, K.; Teubner, B.J.; Ehlen, J.C.; et al. Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC Biol. 2015, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozek, C.; Krolewski, R.C.; Buchanan, S.M.; Rubin, L.L. Growth Differentiation Factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Sci. Rep. 2018, 8, 17293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajewski, P.A.; Eagle, A.L.; Williams, E.S.; Manning, C.E.; Lynch, H.; McCornack, C.; Maze, I.; Heller, E.A.; Robison, A.J. Epigenetic Regulation of Hippocampal Fosb Expression Controls Behavioral Responses to Cocaine. J. Neurosci. 2019, 39, 8305–8314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.X.; Serlie, M.J.; Ackermans, M.T.; Foppen, E.; Buijs, R.M.; Sauerwein, H.P.; Fliers, E.; Kalsbeek, A. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes 2009, 58, 1998–2005. [Google Scholar] [CrossRef] [Green Version]
- Oak, M.H.; El Bedoui, J.; Anglard, P.; Schini-Kerth, V.B. Red wine polyphenolic compounds strongly inhibit pro-matrix metalloproteinase-2 expression and its activation in response to thrombin via direct inhibition of membrane type 1-matrix metalloproteinase in vascular smooth muscle cells. Circulation 2004, 110, 1861–1867. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, M.; Serre, F.; Cantin, L.; Ahmed, S.H. Intense sweetness surpasses cocaine reward. PLoS ONE 2007, 2, e698. [Google Scholar] [CrossRef] [Green Version]
- Vanhille, N.; Belin-Rauscent, A.; Mar, A.C.; Ducret, E.; Belin, D. High locomotor reactivity to novelty is associated with an increased propensity to choose saccharin over cocaine: New insights into the vulnerability to addiction. Neuropsychopharmacology 2015, 40, 577–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [Green Version]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.M., 3rd; Kim, D.; Berquist, B.R.; Sigurdson, A.J. Variation in base excision repair capacity. Mutat. Res. 2011, 711, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Bernal, I.; Becerril-Perez, F.; Aguilar-Arnal, L. Circadian rhythms in the three-dimensional genome: Implications of chromatin interactions for cyclic transcription. Clin. Epigenetics 2019, 11, 79. [Google Scholar] [CrossRef]
- Hood, S.; Cassidy, P.; Cossette, M.P.; Weigl, Y.; Verwey, M.; Robinson, B.; Stewart, J.; Amir, S. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J. Neurosci. 2010, 30, 14046–14058. [Google Scholar] [CrossRef]
- Ripperger, J.A.; Shearman, L.P.; Reppert, S.M.; Schibler, U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000, 14, 679–689. [Google Scholar]
- Slamberova, R.; Nohejlova, K.; Ochozkova, A.; Mihalcikova, L. What is the role of subcutaneous single injections on the behavior of adult male rats exposed to drugs? Physiol. Res. 2018, 67, S665–S672. [Google Scholar] [CrossRef]
- Robinson, S.L.; Thiele, T.E. The Role of Neuropeptide Y (NPY) in Alcohol and Drug Abuse Disorders. Int. Rev. Neurobiol. 2017, 136, 177–197. [Google Scholar] [PubMed]
- Yi, M.; Li, H.; Wu, Z.; Yan, J.; Liu, Q.; Ou, C.; Chen, M. A Promising Therapeutic Target for Metabolic Diseases: Neuropeptide Y Receptors in Humans. Cell Physiol. Biochem. 2018, 45, 88–107. [Google Scholar] [CrossRef]
- Field, M.D.; Maywood, E.S.; O’Brien, J.A.; Weaver, D.R.; Reppert, S.M.; Hastings, M.H. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 2000, 25, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Perreau-Lenz, S.; Spanagel, R. Clock genes x stress x reward interactions in alcohol and substance use disorders. Alcohol 2015, 49, 351–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uz, T.; Ahmed, R.; Akhisaroglu, M.; Kurtuncu, M.; Imbesi, M.; Dirim Arslan, A.; Manev, H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience 2005, 134, 1309–1316. [Google Scholar] [CrossRef]
- Lynch, W.J.; Girgenti, M.J.; Breslin, F.J.; Newton, S.S.; Taylor, J.R. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: A focus on circadian genes. Brain Res. 2008, 1213, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wise, R.A.; Bozarth, M.A. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987, 94, 469–492. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [Green Version]
- Lanteri, C.; Salomon, L.; Torrens, Y.; Glowinski, J.; Tassin, J.P. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology 2008, 33, 1724–1734. [Google Scholar] [CrossRef] [Green Version]
- De Backer, J.F.; Monlezun, S.; Detraux, B.; Gazan, A.; Vanopdenbosch, L.; Cheron, J.; Cannazza, G.; Valverde, S.; Cantacorps, L.; Nassar, M.; et al. Deletion of Maged1 in mice abolishes locomotor and reinforcing effects of cocaine. EMBO Rep. 2018, 19, e45089. [Google Scholar] [CrossRef]
- Badiani, A.; Belin, D.; Epstein, D.; Calu, D.; Shaham, Y. Opiate versus psychostimulant addiction: The differences do matter. Nat. Rev. Neurosci. 2013, 12, 685–700. [Google Scholar] [CrossRef]
- Nutt, D.J.; Lingford-Hughes, A.; Erritzoe, D.; Stokes, P.R. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 2015, 16, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, A.C.; Grunder, G.; Hirth, N.; Noori, H.R.; Spanagel, R.; Sommer, W.H. Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neurosci. Biobehav. Rev. 2019, 106, 141–164. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W.L. Sugar addiction: Is it real? A narrative review. Br. J. Sports Med. 2017, 52, 910–913. [Google Scholar] [CrossRef]
- Wei, S.; Bilbao, R.; Spanagel, R. Sugar-addictive phenotypes in mice. Eur. Neuropsychopharmacol. 2018, 25, S23–S24. [Google Scholar] [CrossRef]
- Westwater, M.L.; Fletcher, P.C.; Ziauddeen, H. Sugar addiction: The state of the science. Eur. J. Nutr. 2016, 55, 55–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markus, C.R.; Rogers, P.J.; Brouns, F.; Schepers, R. Eating dependence and weight gain; no human evidence for a ’sugar-addiction’ model of overweight. Appetite 2017, 114, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, G.; Wortwein, G.; Fink-Jensen, A.; Woldbye, D.P. Neuropeptide Y Y5 receptor antagonism causes faster extinction and attenuates reinstatement in cocaine-induced place preference. Pharmacol. Biochem. Behav. 2013, 105, 151–156. [Google Scholar] [CrossRef]
- Pantazis, C.B.; James, M.H.; Bentzley, B.S.; Aston-Jones, G. The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addict. Biol. 2019, 25, e12795. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Espana, R.A. Hypocretin/orexin regulation of dopamine signaling: Implications for reward and reinforcement mechanisms. Front. Behav. Neurosci. 2012, 6, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandre, C.; Andermann, M.L.; Scammell, T.E. Control of arousal by the orexin neurons. Curr. Opin. Neurobiol. 2013, 23, 752–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.L.; Badve, P.S.; Barson, J.R.; Bass, C.E.; Espana, R.A. Hypocretin receptor 1 knockdown in the ventral tegmental area attenuates mesolimbic dopamine signaling and reduces motivation for cocaine. Addict. Biol. 2018, 23, 1032–1045. [Google Scholar] [CrossRef]
- Tan, L.; Shi, Y.G. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 2012, 139, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef]
- Chen, Z.X.; Riggs, A.D. DNA methylation and demethylation in mammals. J. Biol. Chem. 2011, 286, 18347–18353. [Google Scholar] [CrossRef] [Green Version]
- Fragou, D.; Zanos, P.; Kouidou, S.; Njau, S.; Kitchen, I.; Bailey, A.; Kovatsi, L. Effect of chronic heroin and cocaine administration on global DNA methylation in brain and liver. Toxicol. Lett. 2013, 218, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Shao, N.; Szulwach, K.E.; Vialou, V.; Huynh, J.; Zhong, C.; Le, T.; Ferguson, D.; Cahill, M.E.; Li, Y.; et al. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat. Neurosci. 2015, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Massart, R.; Barnea, R.; Dikshtein, Y.; Suderman, M.; Meir, O.; Hallett, M.; Kennedy, P.; Nestler, E.J.; Szyf, M.; Yadid, G. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J. Neurosci. 2015, 35, 8042–8058. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, A.; Beuckmann, C.T.; Willie, J.T.; Hara, J.; Tsujino, N.; Mieda, M.; Tominaga, M.; Yagami, K.; Sugiyama, F.; Goto, K.; et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003, 38, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Burdakov, D.; Jensen, L.T.; Alexopoulos, H.; Williams, R.H.; Fearon, I.M.; O’Kelly, I.; Gerasimenko, O.; Fugger, L.; Verkhratsky, A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 2006, 50, 711–722. [Google Scholar] [CrossRef]
- Sheng, Z.; Santiago, A.M.; Thomas, M.P.; Routh, V.H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell Neurosci. 2014, 62, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Wang, Q.; Elsarelli, M.M.; Brown, R.L.; Ritter, S. Hindbrain Catecholamine Neurons Activate Orexin Neurons During Systemic Glucoprivation in Male Rats. Endocrinology 2015, 156, 2807–2820. [Google Scholar] [CrossRef]
- Franco-Enzastiga, U.; Garcia, G.; Murbartian, J.; Gonzalez-Barrios, R.; Salinas-Abarca, A.B.; Sanchez-Hernandez, B.; Tavares-Ferreira, D.; Herrera, L.A.; Barragan-Iglesias, P.; Delgado-Lezama, R.; et al. Sex-dependent pronociceptive role of spinal alpha5 -GABAA receptor and its epigenetic regulation in neuropathic rodents. J. Neurochem. 2020, 156, 897–916. [Google Scholar] [CrossRef]
- Chen, B.T.; Bowers, M.S.; Martin, M.; Hopf, F.W.; Guillory, A.M.; Carelli, R.M.; Chou, J.K.; Bonci, A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 2008, 59, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker-Andresen, D.; Zhao, Q.; Li, X.; Jupp, B.; Chesworth, R.; Lawrence, A.J.; Bredy, T. Persistent variations in neuronal DNA methylation following cocaine self-administration and protracted abstinence in mice. Neuroepigenetics 2015, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Werner, C.T.; Altshuler, R.D.; Shaham, Y.; Li, X. Epigenetic Mechanisms in Drug Relapse. Biol. Psychiatry 2020, 14, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Challen, G.A.; Sun, D.; Mayle, A.; Jeong, M.; Luo, M.; Rodriguez, B.; Mallaney, C.; Celik, H.; Yang, L.; Xia, Z.; et al. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell Stem Cell 2014, 15, 350–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Karnik, R.; Gu, H.; Ziller, M.J.; Clement, K.; Tsankov, A.M.; Akopian, V.; Gifford, C.A.; Donaghey, J.; Galonska, C.; et al. Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells. Nat. Genet. 2015, 47, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, B.; Pantoja, C.R.G.; Sosa, M.H.; Vitullo, A.D.; Bisagno, V.; Gonzalez, C.R. Cocaine alters the mouse testicular epigenome with direct impact on histone acetylation and DNA methylation marks. Reprod. Biomed. Online 2018, 37, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018, 4, dvy016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazar-Contes, I.; Roszkowski, M.; Tanwar, D.K.; Mansuy, I.M. Symposium summary: Epigenetic inheritance-impact for biology and society 26-28 August 2019, Zurich, Switzerland. Environ. Epigenet 2020, 6, dvaa004. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, A.; Kerek, R.; Pourie, G.; Helle, D.; Gueant, J.L.; Daval, J.L.; Bossenmeyer-Pourie, C. Late Maternal Folate Supplementation Rescues from Methyl Donor Deficiency-Associated Brain Defects by Restoring Let-7 and miR-34 Pathways. Mol. Neurobiol. 2017, 54, 5017–5033. [Google Scholar] [CrossRef]


| Gene | Primer sequence (5′–3′) | GeneBank Acc. # |
|---|---|---|
| Dnmt3a | FP:GCTGAAGGAGAGGGAACTGA RP:TGCCTGGAAGGTGAGTCTTG | NM_001003958 |
| Dnmt3b | FP:TGCGGTAAGAAGAACCCTGT RP:CTGATAGCCGTCCTCATCGT | NM_001003959 |
| Tet1 | FP: CTGTGGGGAATGCACCTACT RP: TGGCTTCTTTTTGAGCACCT | XM_008774952.1 |
| Tet2 | FP: AGAAGCGTAAGAAGCGCAGT RP:TCTTTTTCATTTGACCGTCTCTTCC | XM_006224264.2 |
| Tet3 | FP: TGTGTGCAAGAGGACTTTCG RP: TACTGACGGGTGGTTTCTCC | XM_006224966 |
| CLOCK | FP: GTCATCCTTCAGTAGTCAGTCCA RP: ACATGCCTTGTGGGATTGGT | NM_021856.2 |
| Bmal 1 | FP: ACTGTCTAGGTGGAGGATTTTGG RP: CTGGTCACCTCAAAGCGACT | NM_024362.2 |
| Per1 | FP: TACCAGCCATTCCGCCTAAC RP: CGGGGAGCTTCATAACCAGA | NM_001034125.1 |
| Per2 | FP: CACCCTGAAAAGAAAGTGCGA RP: CAACGCCAAGGAGCTCAAGT | NM_031678.1 |
| Cry1 | FP: AGGACGCACAGAGTGTTGG RP: TCCTCCCGCATGCTTTCGTATC | NM_198750.2 |
| Cry2 | FP: GGGGACTACATCCGGCGATA RP: ATGATGCACTTAGCGGCCTT | NM_133405.2 |
| Rev-erbβ | FP: GGGAGGATGCATCTGGTTTG RP: CACCTCTTTTACTGCTGGGG | NM_147210.2 |
| NPY | FP: TGGCCAGATACTACTCCGCT RP: GCTGGATCTCTTGCCATATCTCT | NM_012614.2 |
| DBP1 | FP: AAGGCAAGGAAAGTCCAGGT RP: TGGCTGCTTCATTGTTCTTG | NM_012543.3 |
| Orx | TCCTTGGGTATTTGGACCAC CCCAGGGAACCTTTGTAGAAG | NM_013179 |
| 36B4 | FP:GTGCCTCACTCCATCATCAA RP:TCCGACTCTTCCTTTGCTTC | NM_022402 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, L.; Kalsbeek, A.; Zwiller, J.; Anglard, P. Rhythmic Regulation of DNA Methylation Factors and Core-Clock Genes in Brain Structures Activated by Cocaine or Sucrose: Potential Role of Chromatin Remodeling. Genes 2021, 12, 1195. https://doi.org/10.3390/genes12081195
Saad L, Kalsbeek A, Zwiller J, Anglard P. Rhythmic Regulation of DNA Methylation Factors and Core-Clock Genes in Brain Structures Activated by Cocaine or Sucrose: Potential Role of Chromatin Remodeling. Genes. 2021; 12(8):1195. https://doi.org/10.3390/genes12081195
Chicago/Turabian StyleSaad, Lamis, Andries Kalsbeek, Jean Zwiller, and Patrick Anglard. 2021. "Rhythmic Regulation of DNA Methylation Factors and Core-Clock Genes in Brain Structures Activated by Cocaine or Sucrose: Potential Role of Chromatin Remodeling" Genes 12, no. 8: 1195. https://doi.org/10.3390/genes12081195
APA StyleSaad, L., Kalsbeek, A., Zwiller, J., & Anglard, P. (2021). Rhythmic Regulation of DNA Methylation Factors and Core-Clock Genes in Brain Structures Activated by Cocaine or Sucrose: Potential Role of Chromatin Remodeling. Genes, 12(8), 1195. https://doi.org/10.3390/genes12081195







