Srs2 and Pif1 as Model Systems for Understanding Sf1a and Sf1b Helicase Structure and Function
Abstract
:1. Introduction
2. Helicase Molecular Mechanisms
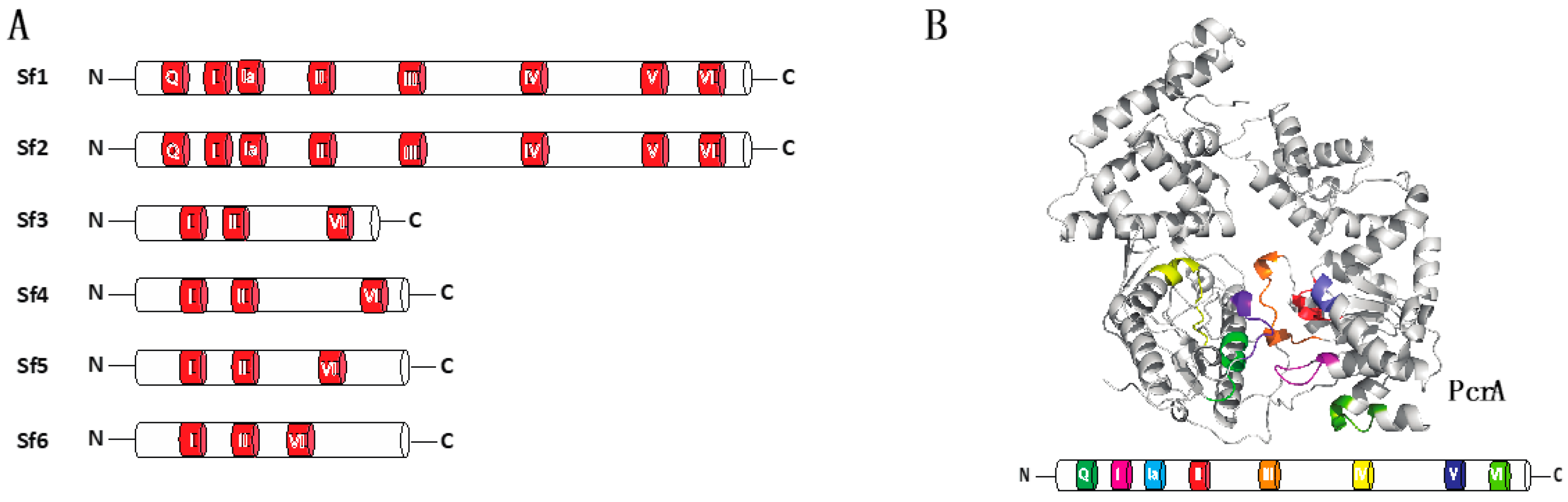
2.1. Helicase Domains and Motifs
2.2. General Aspects of Helicase Translocation
2.3. Nucleic Acid Unwinding
3. Structural Features of Sf1a and Sf1b Helicases
3.1. Structural Organization of Sf1a and Sf1b Helicases
3.2. Mechanism of Sf1a and Sf1b Helicase Translocation
4. Srs2 and Pif1 as Model Systems for Understanding Sf1a and Sf1b Helicases
4.1. Srs2 Is an Sf1a Helicase That Regulates Homologous Recombination
4.2. Srs2 as Prototypical “Antirecombinase”
4.3. Single-Molecule Studies of Srs2 Antirecombinase Activity
4.4. Regulation of Srs2 Antirecombinase Activity
4.5. Pif1 Is an Sf1b Helicase with Multifaceted Roles in DNA Replication
4.6. Pif1 Has Multifaceted Roles in DNA Replication
4.7. Pif1 and Telomere Length Regulation
4.8. Pif1 and Replication Fork Convergence
4.9. Pif1 Acts as a “Pseudo-Replicative” DNA Helicase during BIR
4.10. Single-Molecule Studies of Pif1 Activity
5. Future Directions
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Niu, H.; Klein, H.L. Multifunctional Roles of Saccharomyces cerevisiae Srs2 protein in Replication, Recombination and Repair. FEMS Yeast Res. 2016, 17, fow111. [Google Scholar] [CrossRef] [Green Version]
- Pyle, A.M. Translocation and unwinding mechanisms of RNA and DNA helicases. Annu. Rev. Biophys. 2008, 37, 317–336. [Google Scholar] [CrossRef]
- Singleton, M.R.; Dillingham, M.; Wigley, D.B. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007, 76, 23–50. [Google Scholar] [CrossRef]
- Brosh, R.M., Jr. DNA helicases involved in DNA repair and their roles in cancer. Nat. Rev. Cancer 2013, 13, 542–558. [Google Scholar] [CrossRef]
- Bernstein, K.A.; Gangloff, S.; Rothstein, R. The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 2010, 44, 393–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branzei, D.; Szakal, B. Building up and breaking down: Mechanisms controlling recombination during replication. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 381–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosh, R.M., Jr.; Bohr, V.A. Human premature aging, DNA repair and RecQ helicases. Nucleic Acids Res. 2007, 35, 7527–7544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croteau, D.L.; Popuri, V.; Opresko, P.; Bohr, V.A. Human RecQ helicases in DNA repair, recombination, and replication. Annu. Rev. Biochem. 2014, 83, 519–552. [Google Scholar] [CrossRef] [Green Version]
- Enemark, E.J.; Joshua-Tor, L. On helicases and other motor proteins. Curr. Opin. Struct. Biol. 2008, 18, 243–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohman, T.M.; Tomko, E.J.; Wu, C.G. Non-hexameric DNA helicases and translocases: Mechanisms and regulation. Nat. Rev. Mol. Cell Biol. 2008, 9, 391–401. [Google Scholar] [CrossRef]
- Modrich, P. Mismatch repair, genetic stability, and cancer. Science 1994, 266, 1959–1960. [Google Scholar] [CrossRef]
- Sancar, A. Mechanisms of DNA excision repair. Science 1994, 266, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Ellis, N.A. DNA helicases in inherited human disorders. Curr. Opin. Genet. Dev. 1997, 7, 354–363. [Google Scholar] [CrossRef]
- Andressoo, J.; Hoeijmakers, J. Transcription-coupled repair and premature ageing. Mutat. Res. Mol. Mech. Mutagen. 2005, 577, 179–194. [Google Scholar] [CrossRef]
- Ouyang, K.J.; Woo, L.L.; Ellis, N.A. Homologous recombination and maintenance of genome integrity: Cancer and aging through the prism of human RecQ helicases. Mech. Ageing Dev. 2008, 129, 425–440. [Google Scholar] [CrossRef]
- Andressoo, J.-O.; Hoeijmakers, J.H.; Waard, H. Nucleotide Excision Repair and its Connection with Cancer and Ageing. Adv. Exp. Med. Biol. 2006, 570, 45–83. [Google Scholar] [CrossRef]
- Stevnsner, T.; Muftuoglu, M.; Aamann, M.D.; Bohr, V.A. The role of cockayne syndrome group B (CSB) protein in base excision repair and aging. Mech. Ageing Dev. 2008, 129, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Singleton, M.; Wigley, D.B. Modularity and specialization in superfamily 1 and 2 helicases. J. Bacteriol. 2002, 184, 1819–1826. [Google Scholar] [CrossRef] [Green Version]
- Caruthers, J.M.; McKay, D.B. Helicase structure and mechanism. Curr. Opin. Struct. Biol. 2002, 12, 123–133. [Google Scholar] [CrossRef]
- Iyer, L.M.; Leipe, D.D.; Koonin, E.V.; Aravind, L. Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 2004, 146, 11–31. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Koonin, E.V. One more conserved sequence motif in helicases. Nucleic Acids Res. 1988, 16, 7734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbalenya, A.; Koonin, E.V.; Donchenko, A.P.; Blinov, V.M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989, 17, 4713–4730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbalenya, A.; Koonin, E.V.; Donchenko, A.P.; Blinov, V.M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988, 235, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Gorbalenya, A.E.; Koonin, E.V.; Donchenko, A.P.; Blinov, V.M. A conserved NTP-motif in putative helicases. Nature 1988, 333, 22. [Google Scholar] [CrossRef] [PubMed]
- Hodgman, T.C.; Hodgman, C. A new superfamily of replicative proteins. Nature 1988, 333, 22–23. [Google Scholar] [CrossRef]
- Tuteja, N.; Tuteja, R. Unraveling DNA helicases. Motif, structure, mechanism and function. Eur. J. Biochem. 2004, 271, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Hsieh, J.; Brendza, K.M.; Lohman, T.M. E. coli Rep oligomers are required to initiate DNA unwinding in vitro. J. Mol. Biol. 2001, 310, 327–350. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.; Rasnik, I.; Cheng, W.; Babcock, H.; Gauss, G.H.; Lohman, T.M.; Chu, S. Initiation and re-initiation of DNA unwinding by the Escherichia coli Rep helicase. Nature 2002, 419, 638–641. [Google Scholar] [CrossRef]
- Maluf, N.K.; Ali, J.A.; Lohman, T.M. Kinetic mechanism for formation of the active, dimeric UvrD helicase-DNA complex. J. Biol. Chem. 2003, 278, 31930–31940. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.; Raney, K.D. Protein displacement by an assembly of helicase molecules aligned along single-stranded DNA. Nat. Struct. Mol. Biol. 2004, 11, 531–538. [Google Scholar] [CrossRef]
- Kaniecki, K.; De Tullio, L.; Gibb, B.; Kwon, Y.; Sung, P.; Greene, E.C. Dissociation of Rad51 presynaptic complexes and heteroduplex DNA joints by tandem assemblies of Srs. Cell Rep. 2017, 21, 3166–3177. [Google Scholar] [CrossRef] [Green Version]
- Yang, W. Lessons learned from UvrD helicase: Mechanism for directional movement. Annu. Rev. Biophys. 2010, 39, 367–385. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Yang, W. UvrD Helicase unwinds DNA one base pair at a time by a two-part power stroke. Cell 2006, 127, 1349–1360. [Google Scholar] [CrossRef] [Green Version]
- Soultanas, P.; Wigley, D.B. DNA helicases: Inching forward. Curr. Opin. Struct. Biol. 2000, 10, 124–128. [Google Scholar] [CrossRef]
- Velankar, S.; Soultanas, P.; Dillingham, M.; Subramanya, H.S.; Wigley, D.B. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell 1999, 97, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Singleton, M.; Dillingham, M.; Gaudier, M.; Kowalczykowski, S.C.; Wigley, D.B. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature 2004, 432, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.M.; Fazio, N.T. How does a helicase unwind DNA? Insights from RecBCD helicase. BioEssays 2018, 40, 1800009. [Google Scholar] [CrossRef] [PubMed]
- Subramanya, H.S.; Bird, L.; Brannigan, J.A.; Wigley, D.B. Crystal structure of a DExx box DNA helicase. Nature 1996, 384, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Saikrishnan, K.; Powell, B.; Cook, N.J.; Webb, M.R.; Wigley, D.B. Mechanistic basis of 5′-3′ translocation in SF1B helicases. Cell 2009, 137, 849–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, B.; George, N.P.; Thurmes, T.M.; Zhou, R.; Jani, N.; Wessel, S.R.; Sandler, S.J.; Ha, T.; Keck, J.L. Structural mechanisms of PriA-mediated DNA replication restart. Proc. Natl. Acad. Sci. USA 2014, 111, 1373–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korolev, S.; Hsieh, J.; Gauss, G.H.; Lohman, T.M.; Waksman, G. Major domain swiveling revealed by the crystal structures of complexes of E. coli rep helicase bound to single-stranded DNA and ADP. Cell 1997, 90, 635–647. [Google Scholar] [CrossRef] [Green Version]
- Singleton, M.; Scaife, S.; Wigley, D.B. Structural analysis of DNA replication fork reversal by RecG. Cell 2001, 107, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, D.A.; Zittel, M.C.; Keck, J.L. High-resolution structure of the E. coli RecQ helicase catalytic core. EMBO J. 2003, 22, 4910–4921. [Google Scholar] [CrossRef] [Green Version]
- Büttner, K.; Nehring, S.; Hopfner, K.-P. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat. Struct. Mol. Biol. 2007, 14, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Bird, L.E.; Subramanya, H.S.; Wigley, D.B. Helicases: A unifying structural theme? Curr. Opin. Struct. Biol. 1998, 8, 14–18. [Google Scholar] [CrossRef]
- Raney, K.D.; Byrd, A.K.; Aarattuthodiyil, S. Structure and mechanisms of SF1 DNA helicases. Adv. Exp. Med. Biol. 2012, 767, 17–46. [Google Scholar] [CrossRef]
- Saikrishnan, K.; Griffiths, S.P.; Cook, N.; Court, R.; Wigley, D.B. DNA binding to RecD: Role of the 1B domain in SF1B helicase activity. EMBO J. 2008, 27, 2222–2229. [Google Scholar] [CrossRef] [Green Version]
- Marini, V.; Krejci, L. Srs2: The “odd-job man” in DNA repair. DNA Repair Amst. 2010, 9, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Muellner, J.; Schmidt, K.H. Yeast genome maintenance by the multifunctional PIF1 DNA helicase family. Genes 2020, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.K.; Raney, K.D. Structure and function of Pif1 helicase. Biochem. Soc. Trans. 2017, 45, 1159–1171. [Google Scholar] [CrossRef]
- Geronimo, C.L.; Zakian, V.A. Getting it done at the ends: Pif1 family DNA helicases and telomeres. DNA Repair 2016, 44, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Pohl, T.J.; Zakian, V.A. Pif1 family DNA helicases: A helpmate to RNase H? DNA Repair Amst. 2019, 84, 102633. [Google Scholar] [CrossRef]
- Aguilera, A.; Klein, H.L. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics 1988, 119, 779–790. [Google Scholar] [CrossRef]
- Rong, L.; Palladino, F.; Aguilera, A.; Klein, H. The hyper-gene conversion hpr5-1 mutation of Saccharomyces cerevisiae is an allele of the SRS2/RADH gene. Genetics 1991, 127, 75–85. [Google Scholar] [CrossRef]
- Elango, R.; Sheng, Z.; Jackson, J.; Decata, J.; Ibrahim, Y.; Pham, N.T.; Liang, D.H.; Sakofsky, C.J.; Vindigni, A.; Lobachev, K.S.; et al. Break-induced replication promotes formation of lethal joint molecules dissolved by Srs. Nat. Commun. 2017, 8, 1790. [Google Scholar] [CrossRef]
- Ira, G.; Malkova, A.; Liberi, G.; Foiani, M.; Haber, J.E. Srs2 and Sgs1–top3 suppress crossovers during double-strand break repair in yeast. Cell 2003, 115, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, A. Modulation of meiotic homologous recombination by DNA helicases. Yeast 2016, 34, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Palladino, F.; Klein, H. Analysis of mitotic and meiotic defects in Saccharomyces cerevisiae SRS2 DNA helicase mutants. Genetics 1992, 132, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Aboussekhra, A.; Chanet, R.; Zgaga, Z.; Cassier-Chauvat, C.; Heude, M.; Fabre, F. RADH, a gene of Saccharomyces cerevisiae encoding a putative DNA helicase involved in DNA repair. Characteristics of radH mutants and sequence of the gene. Nucleic Acids Res. 1989, 17, 7211–7219. [Google Scholar] [CrossRef] [Green Version]
- Flores, M.J.; Bidnenko, V.; Michel, B. The DNA repair helicase UvrD is essential for replication fork reversal in replication mutants. EMBO Rep. 2004, 5, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veaute, X.; Delmas, S.; Selva, M.; Jeusset, J.; Le Cam, E.; Matic, I.; Fabre, F.; Petit, M.-A. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 2004, 24, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangloff, S.; Soustelle, C.; Fabre, F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nat. Genet. 2000, 25, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.L. Mutations in recombinational repair and in checkpoint control genes suppress the lethal combination of srs2Delta with other DNA repair genes in Saccharomyces cerevisiae. Genetics 2001, 157, 557–565. [Google Scholar] [CrossRef]
- Ooi, S.L.; Shoemaker, D.D.; Boeke, J. DNA helicase gene interaction network defined using synthetic lethality analyzed by microarray. Nat. Genet. 2003, 35, 277–286. [Google Scholar] [CrossRef]
- Tong, A.H.Y.; Lesage, G.; Bader, G.; Ding, H.; Xu, H.; Xin, X.; Young, J.; Berriz, G.F.; Brost, R.L.; Chang, M.; et al. Global mapping of the yeast genetic interaction network. Science 2004, 303, 808–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Boone, C.; Klein, H.L. Mrc1 Is required for sister chromatid cohesion to aid in recombination repair of spontaneous damage. Mol. Cell. Biol. 2004, 24, 7082–7090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Ye, P.; Yuan, D.S.; Wang, X.; Bader, J.; Boeke, J.D. A DNA integrity network in the yeast saccharomyces cerevisiae. Cell 2006, 124, 1069–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, L.; Klein, H. Purification and characterization of the SRS2 DNA helicase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1993, 268, 1252–1259. [Google Scholar] [CrossRef]
- Van Komen, S.; Reddy, M.S.; Krejci, L.; Klein, H.; Sung, P. ATPase and DNA helicase activities of the saccharomyces cerevisiae anti-recombinase Srs. J. Biol. Chem. 2003, 278, 44331–44337. [Google Scholar] [CrossRef] [Green Version]
- Krejci, L.; Macris, M.; Li, Y.; Van Komen, S.; Villemain, J.; Ellenberger, T.; Klein, H.; Sung, P. Role of ATP hydrolysis in the antirecombinase function of Saccharomyces cerevisiae Srs2 protein. J. Biol. Chem. 2004, 279, 23193–23199. [Google Scholar] [CrossRef] [Green Version]
- Veaute, X.; Jeusset, J.; Soustelle, C.; Kowalczykowski, S.C.; Le Cam, E.; Fabre, F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 2003, 423, 309–312. [Google Scholar] [CrossRef]
- Krejci, L.; Van Komen, S.; Li, Y.; Villemain, J.; Reddy, M.S.; Klein, H.; Ellenberger, T.; Sung, P. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 2003, 423, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Crickard, J.B.; Xue, C.; Wang, W.; Kwon, Y.; Sung, P.; Greene, E.C. The RecQ helicase Sgs1 drives ATP-dependent disruption of Rad51 filaments. Nucleic Acids Res. 2019, 47, 4694–4706. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Raynard, S.; Sehorn, M.G.; Lu, X.; Bussen, W.; Zheng, L.; Stark, J.M.; Barnes, E.L.; Chi, P.; Janscak, P.; et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007, 21, 3073–3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, C.; Molnarova, L.; Steinfeld, J.B.; Zhao, W.; Ma, C.; Spirek, M.; Kaniecki, K.; Kwon, Y.; Beláň, O.; Krejci, K.; et al. Single-molecule visualization of human RECQ5 interactions with single-stranded DNA recombination intermediates. Nucleic Acids Res. 2020, 49, 285–305. [Google Scholar] [CrossRef]
- Bugreev, D.V.; Yu, X.; Egelman, E.H.; Mazin, A.V. Novel pro- and anti-recombination activities of the Bloom’s syndrome helicase. Genes Dev. 2007, 21, 3085–3094. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, A.; Osman, F.; Folkyte, V.; Sofueva, S.; Whitby, M.C. Fbh1 limits Rad51-dependent recombination at blocked replication forks. Mol. Cell. Biol. 2009, 29, 4742–4756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simandlova, J.; Zagelbaum, J.; Payne, M.; Chu, W.K.; Shevelev, I.; Hanada, K.; Chatterjee, S.; Reid, D.A.; Liu, Y.; Janscak, P.; et al. FBH1 helicase disrupts RAD51 filaments in vitro and modulates homologous recombination in mammalian cells. J. Biol. Chem. 2013, 288, 34168–34180. [Google Scholar] [CrossRef] [Green Version]
- Mankouri, H.; Chu, W.K.; Hickson, I.D. A novel antirecombinase gains PARIty. Mol. Cell 2012, 45, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, G.-L.; Dejsuphong, D.; Petalcorin, M.; Hofmann, K.; Takeda, S.; Boulton, S.J.; D’Andrea, A.D. Inhibition of homologous recombination by the PCNA-interacting protein PARI. Mol. Cell 2012, 45, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-K.; Johnson, R.E.; Yu, S.-L.; Prakash, L.; Prakash, S. Requirement of Yeast SGS1 and SRS2 genes for replication and transcription. Science 1999, 286, 2339–2342. [Google Scholar] [CrossRef] [PubMed]
- Kowalczykowski, S.C. An overview of the molecular mechanisms of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a016410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.; Plank, J.; Dombrowski, C.C.; Kowalczykowski, S.C. Direct imaging of RecA nucleation and growth on single molecules of SSB-coated ssDNA. Nature 2012, 491, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Bianco, P.R.; Tracy, R.B.; Kowalczykowski, S.C. DNA strand exchange proteins: A biochemical and physical comparison. Front. Biosci. 1998, 3, D570–D603. [Google Scholar]
- Galletto, R.; Amitani, I.; Baskin, R.J.; Kowalczykowski, S.C. Direct observation of individual RecA filaments assembling on single DNA molecules. Nature 2006, 443, 875–878. [Google Scholar] [CrossRef]
- Lindsley, J.E.; Cox, M.M. Dissociation pathway for recA nucleoprotein filaments formed on linear duplex DNA. J. Mol. Biol. 1989, 205, 695–711. [Google Scholar] [CrossRef]
- van Mameren, J.; Modesti, M.; Kanaar, R.; Wyman, C.; Peterman, E.J.; Wuite, G.J. Counting RAD51 proteins disassembling from nucleoprotein filaments under tension. Nature 2009, 457, 745–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antony, E.; Tomko, E.J.; Xiao, Q.; Krejci, L.; Lohman, T.M.; Ellenberger, T. Srs2 Disassembles Rad51 Filaments by a Protein-Protein Interaction Triggering ATP turnover and dissociation of Rad51 from DNA. Mol. Cell 2009, 35, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Fung, C.W.; Fortin, G.S.; Peterson, S.; Symington, L.S. The rad51-K191R ATPase-defective mutant is impaired for presynaptic filament formation. Mol. Cell. Biol. 2006, 26, 9544–9554. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.; Shinohara, A.; Sonoda, E.; Yamaguchi-Iwai, Y.; Takata, M.; Weichselbaum, R.R.; Takeda, S. The essential functions of human Rad51 are independent of ATP hydrolysis. Mol. Cell. Biol. 1999, 19, 6891–6897. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Antony, E.; Doganay, S.; Koh, H.R.; Lohman, T.M.; Myong, S. Srs2 prevents Rad51 filament formation by repetitive motion on DNA. Nat. Commun. 2013, 4, 2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myong, S.; Rasnik, I.; Joo, C.; Lohman, T.M.; Ha, T. Repetitive shuttling of a motor protein on DNA. Nature 2005, 437, 1321–1325. [Google Scholar] [CrossRef]
- De Tullio, L.; Kaniecki, K.; Kwon, Y.; Crickard, J.B.; Sung, P.; Greene, E.C. Yeast Srs2 helicase promotes redistribution of single-stranded DNA-bound RPA and Rad52 in homologous recombination regulation. Cell Rep. 2017, 21, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, B.; Hengel, S.R.; Grundy, M.K.; Bernstein, K.A. RAD51 gene family structure and function. Annu. Rev. Genet. 2020, 54, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Zhang, Y.; Feng, W.; Jasin, M. Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol. 2015, 7, a016600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, S.L.; Firmenich, A.A.; Berg, P. Complex formation in yeast double-strand break repair: Participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 6925–6929. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.D.; Symington, L.S. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol. Cell. Biol. 1995, 15, 4843–4850. [Google Scholar] [CrossRef] [Green Version]
- Fortin, G.S.; Symington, L.S. Mutations in yeast Rad51 that partially bypass the requirement for Rad55 and Rad57 in DNA repair by increasing the stability of Rad51–DNA complexes. EMBO J. 2004, 23, 4876. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Renault, L.; Veaute, X.; Fabre, F.; Stahlberg, H.; Heyer, W.-D. Rad51 paralogues Rad55–Rad57 balance the antirecombinase Srs2 in Rad51 filament formation. Nature 2011, 479, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Roy, U.; Kwon, Y.; Marie, L.; Symington, L.; Sung, P.; Lisby, M.; Greene, E.C. The Rad51 paralog complex Rad55-Rad57 acts as a molecular chaperone during homologous recombination. Mol. Cell 2021, 81, 1043–1057.e8. [Google Scholar] [CrossRef]
- Bernstein, K.; Reid, R.; Sunjevaric, I.; DeMuth, K.; Burgess, R.C.; Rothstein, R. The Shu complex, which contains Rad51 paralogues, promotes DNA repair through inhibition of the Srs2 anti-recombinase. Mol. Biol. Cell 2011, 22, 1599–1607. [Google Scholar] [CrossRef]
- Burgess, R.C.; Lisby, M.; Altmannova, V.; Krejci, L.; Sung, P.; Rothstein, R. Localization of recombination proteins and Srs2 reveals anti-recombinase function in vivo. J. Cell Biol. 2009, 185, 969–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, C.; Colavito, S.; Kwon, Y.; Sung, P.; Krejci, L. Regulation of Rad51 recombinase presynaptic filament assembly via interactions with the Rad52 mediator and the Srs2 anti-recombinase. J. Biol. Chem. 2009, 284, 24363–24371. [Google Scholar] [CrossRef] [Green Version]
- Ma, E.; Dupaigne, P.; Maloisel, L.; Guerois, R.; Le Cam, E.; Coïc, E. Rad52-Rad51 association is essential to protect Rad51 filaments against Srs2, but facultative for filament formation. eLife 2018, 7, e32744. [Google Scholar] [CrossRef]
- Lisby, M.; Barlow, J.H.; Burgess, R.C.; Rothstein, R. Choreography of the DNA damage response: Spatiotemporal relationships among checkpoint and repair proteins. Cell 2004, 118, 699–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foury, F.; Kolodynski, J. pif mutation blocks recombination between mitochondrial rho+ and rho- genomes having tandemly arrayed repeat units in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1983, 80, 5345–5349. [Google Scholar] [CrossRef] [Green Version]
- Schulz, V.; Zakian, V.A. The saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 1994, 76, 145–155. [Google Scholar] [CrossRef]
- Boulé, J.-B.; Zakian, V.A. Roles of Pif1-like helicases in the maintenance of genomic stability. Nucleic Acids Res. 2006, 34, 4147–4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessler, J.B.; Torres, J.Z.; Zakian, V.A. The Pif1p subfamily of helicases: Region-specific DNA helicases? Trends Cell Biol. 2001, 11, 60–65. [Google Scholar] [CrossRef]
- Zhang, D.H.; Zhou, B.; Huang, Y.; Xu, L.X.; Zhou, J.Q. The human Pif1 helicase, a potential Escherichia coli RecD homologue, inhibits telomerase activity. Nucleic Acids Res. 2006, 34, 1393–1404. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, K.H.; Kolodner, R.D. Requirement of Rrm3 helicase for repair of spontaneous DNA lesions in cells lacking Srs2 or Sgs1 helicase. Mol. Cell. Biol. 2004, 24, 3213–3226. [Google Scholar] [CrossRef] [Green Version]
- Bochman, M.L.; Judge, C.P.; Zakian, V.A. The Pif1 family in prokaryotes: What are our helicases doing in your bacteria? Mol. Biol. Cell. 2011, 22, 1955–1959. [Google Scholar] [CrossRef]
- Bochman, M.; Sabouri, N.; Zakian, V.A. Unwinding the functions of the Pif1 family helicases. DNA Repair 2010, 9, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-F.; Dai, Y.-X.; Duan, X.-L.; Liu, N.-N.; Shi, W.; Li, N.; Li, M.; Dou, S.-X.; Dong, Y.; Rety, S.; et al. Crystal structures of the BsPif1 helicase reveal that a major movement of the 2B SH3 domain is required for DNA unwinding. Nucleic Acids Res. 2016, 44, 2949–2961. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Ren, W.; Bharath, S.R.; Tang, X.; He, Y.; Chen, C.; Liu, Z.; Li, D.; Song, H. Structural and functional insights into the unwinding mechanism of bacteroides sp Pif. Cell Rep. 2016, 14, 2030–2039. [Google Scholar] [CrossRef] [Green Version]
- Lahaye, A.; Leterme, S.; Foury, F. PIF1 DNA helicase from Saccharomyces cerevisiae. Biochemical characterization of the enzyme. J. Biol. Chem. 1993, 268, 26155–26161. [Google Scholar] [CrossRef]
- Ribeyre, C.; Lopes, J.; Boulé, J.-B.; Piazza, A.; Guédin, A.; Zakian, V.A.; Mergny, J.-L.; Nicolas, A. The Yeast Pif1 Helicase Prevents Genomic Instability Caused by G-Quadruplex-forming CEB1 sequences in vivo. PLoS Genet. 2009, 5, e1000475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paeschke, K.; Bochman, M.; Garcia, P.D.; Cejka, P.; Friedman, K.L.; Kowalczykowski, S.C.; Zakian, V.A. Pif1 family helicases suppress genome instability at G-quadruplex motifs. Nature 2013, 497, 458–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Zhang, J.; Bochman, M.L.; Zakian, V.A.; Ha, T. Periodic DNA patrolling underlies diverse functions of Pif1 on R-loops and G-rich DNA. eLife 2014, 3, e02190. [Google Scholar] [CrossRef]
- Boulé, J.-B.; Zakian, V.A. The yeast Pif1p DNA helicase preferentially unwinds RNA DNA substrates. Nucleic Acids Res. 2007, 35, 5809–5818. [Google Scholar] [CrossRef] [Green Version]
- Chib, S.; Byrd, A.; Raney, K.D. Yeast Helicase Pif1 Unwinds RNA:DNA hybrids with higher processivity than DNA:DNA duplexes. J. Biol. Chem. 2016, 291, 5889–5901. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-Q.; Monson, E.K.; Teng, S.-C.; Schulz, V.P.; Zakian, V.A. Pif1p Helicase, a Catalytic Inhibitor of Telomerase in Yeast. Science 2000, 289, 771–774. [Google Scholar] [CrossRef]
- Budd, M.E.; Reis, C.C.; Smith, S.; Myung, K.; Campbell, J.L. Evidence suggesting that Pif1 helicase functions in DNA replication with the Dna2 helicase/nuclease and DNA polymerase delta. Mol. Cell. Biol. 2006, 26, 2490–2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, J.E.; Burgers, P.M.J.; Campbell, J.L.; Bambara, R.A. Pif1 Helicase Lengthens Some Okazaki Fragment Flaps Necessitating Dna2 nuclease/helicase action in the two-nuclease processing pathway. J. Biol. Chem. 2009, 284, 25170–25180. [Google Scholar] [CrossRef] [Green Version]
- Paeschke, K.; Capra, J.A.; Zakian, V.A. DNA Replication through G-Quadruplex motifs is promoted by the saccharomyces cerevisiae Pif1 DNA helicase. Cell 2011, 145, 678–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, P.L.T.; Pohl, T.J.; Chen, C.-F.; Chan, A.; Pott, S.; Zakian, V.A. PIF1 family DNA helicases suppress R-loop mediated genome instability at tRNA genes. Nat. Commun. 2017, 8, 15025. [Google Scholar] [CrossRef] [PubMed]
- Osmundson, J.S.; Kumar, J.; Yeung, R.; Smith, J.S.O.J.K.R.Y.D.J. Pif1-family helicases cooperatively suppress widespread replication-fork arrest at tRNA genes. Nat. Struct. Mol. Biol. 2016, 24, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivessa, A.; Zhou, J.-Q.; Zakian, V.A. The saccharomyces Pif1p DNA Helicase and the Highly Related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell 2000, 100, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Deegan, T.D.; Baxter, J.; Bazán, M.O.; Yeeles, J.T.; Labib, K.P. Pif1-Family Helicases Support Fork Convergence during DNA Replication Termination in Eukaryotes. Mol. Cell 2019, 74, 231–244.e9. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.A.; Kwon, Y.; Xu, Y.; Chung, W.H.; Chi, P.; Niu, H.; Mayle, R.; Chen, X.; Malkova, A.; Sung, P.; et al. Pif1 helicase and Poldelta promote recombination-coupled DNA synthesis via bubble migration. Nature 2013, 502, 393–396. [Google Scholar] [CrossRef]
- Saini, N.; Ramakrishnan, S.; Elango, R.; Ayyar, S.; Zhang, Y.; Deem, A.; Ira, G.; Haber, J.E.; Lobachev, K.S.; Malkova, A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 2013, 502, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulé, J.-B.; Vega, L.; Zakian, V.A. The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 2005, 438, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mangahas, J.L.; Alexander, M.K.; Sandell, L.; Zakian, V.A. Repair of chromosome ends after telomere loss in saccharomyces. Mol. Biol. Cell 2001, 12, 4078–4089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myung, K.; Chen, C.; Kolodner, R.D. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 2001, 411, 1073–1076. [Google Scholar] [CrossRef]
- Llorente, B.; Smith, C.E.; Symington, L.S. Break-induced replication: What is it and what is it for? Cell Cycle 2008, 7, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Kockler, Z.; Osia, B.; Lee, R.; Musmaker, K.; Malkova, A. Repair of DNA breaks by break-induced replication. Annu. Rev. Biochem. 2021, 90, 165–191. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Z.; Osia, B.A.; Twarowski, J.; Sun, L.; Kramara, J.; Lee, R.S.; Kumar, S.; Elango, R.; Li, H.; et al. Tracking break-induced replication shows that it stalls at roadblocks. Nature 2021, 590, 655–659. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Jehi, S.; Li, J.; Liu, S.; Wang, Z.; Truong, L.; Chiba, T.; Wang, Z.; Wu, X. PIF1 helicase promotes break-induced replication in mammalian cells. EMBO J. 2021, 40, e104509. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Soranno, A.; Sparks, M.A.; Galletto, R. Branched unwinding mechanism of the Pif1 family of DNA helicases. Proc. Natl. Acad. Sci. USA 2019, 116, 24533–24541. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-X.; Chen, W.-F.; Liu, N.-N.; Teng, F.-Y.; Guo, H.-L.; Hou, X.-M.; Dou, S.-X.; Rety, S.; Xi, X.-G. Structural and functional studies of SF1B Pif1 from Thermus oshimai reveal dimerization-induced helicase inhibition. Nucleic Acids Res. 2021, 49, 4129–4143. [Google Scholar] [CrossRef]
- Li, J.-H.; Lin, W.-X.; Zhang, B.; Nong, D.-G.; Ju, H.-P.; Ma, J.-B.; Xu, C.-H.; Ye, F.-F.; Xi, X.G.; Li, M.; et al. Pif1 is a force-regulated helicase. Nucleic Acids Res. 2016, 44, 4330–4339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; LE, S.; Chen, J.; Byrd, A.K.; Rhodes, D.; Raney, K.D.; Yan, J. Direct quantification of the translocation activities of Saccharomyces cerevisiae Pif1 helicase. Nucleic Acids Res. 2019, 47, 7494–7501. [Google Scholar] [CrossRef] [PubMed]
- Schauer, G.D.; Spenkelink, L.M.; Lewis, J.S.; Yurieva, O.; Mueller, S.H.; van Oijen, A.M.; O’Donnell, M.E. Replisome bypass of a protein-based R-loop block by Pif. Proc. Natl. Acad. Sci. USA 2020, 117, 30354–30361. [Google Scholar] [CrossRef] [PubMed]
- Crickard, J.B.; Kaniecki, K.; Kwon, Y.; Sung, P.; Greene, E.C. Meiosis-specific recombinase Dmc1 is a potent inhibitor of the Srs2 antirecombinase. Proc. Natl. Acad. Sci. USA 2018, 115, E10041–E10048. [Google Scholar] [CrossRef] [Green Version]
- Chiolo, I.; Carotenuto, W.; Maffioletti, G.; Petrini, J.; Foiani, M.; Liberi, G. Srs2 and Sgs1 DNA Helicases Associate with Mre11 in different subcomplexes following checkpoint activation and CDK1-mediated Srs2 phosphorylation. Mol. Cell. Biol. 2005, 25, 5738–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiolo, I.; Saponaro, M.; Baryshnikova, A.; Kim, J.-H.; Seo, Y.-S.; Liberi, G. The Human F-Box DNA Helicase FBH1 faces saccharomyces cerevisiae Srs2 and postreplication repair pathway roles. Mol. Cell. Biol. 2007, 27, 7439–7450. [Google Scholar] [CrossRef] [Green Version]
- Foury, F.; Dyck, E.V. A PIF-dependent recombinogenic signal in the mitochondrial DNA of yeast. EMBO J. 1985, 4, 3525–3530. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meir, A.; Greene, E.C. Srs2 and Pif1 as Model Systems for Understanding Sf1a and Sf1b Helicase Structure and Function. Genes 2021, 12, 1319. https://doi.org/10.3390/genes12091319
Meir A, Greene EC. Srs2 and Pif1 as Model Systems for Understanding Sf1a and Sf1b Helicase Structure and Function. Genes. 2021; 12(9):1319. https://doi.org/10.3390/genes12091319
Chicago/Turabian StyleMeir, Aviv, and Eric C. Greene. 2021. "Srs2 and Pif1 as Model Systems for Understanding Sf1a and Sf1b Helicase Structure and Function" Genes 12, no. 9: 1319. https://doi.org/10.3390/genes12091319
APA StyleMeir, A., & Greene, E. C. (2021). Srs2 and Pif1 as Model Systems for Understanding Sf1a and Sf1b Helicase Structure and Function. Genes, 12(9), 1319. https://doi.org/10.3390/genes12091319




