A Recurrent De Novo Terminal Duplication of 14q32 in Korean Siblings Associated with Developmental Delay and Intellectual Disability, Growth Retardation, Facial Dysmorphism, and Cerebral Infarction: A Case Report and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Duo Exome Sequencing of Two Affected Siblings
2.2. Chromosomal Microarray
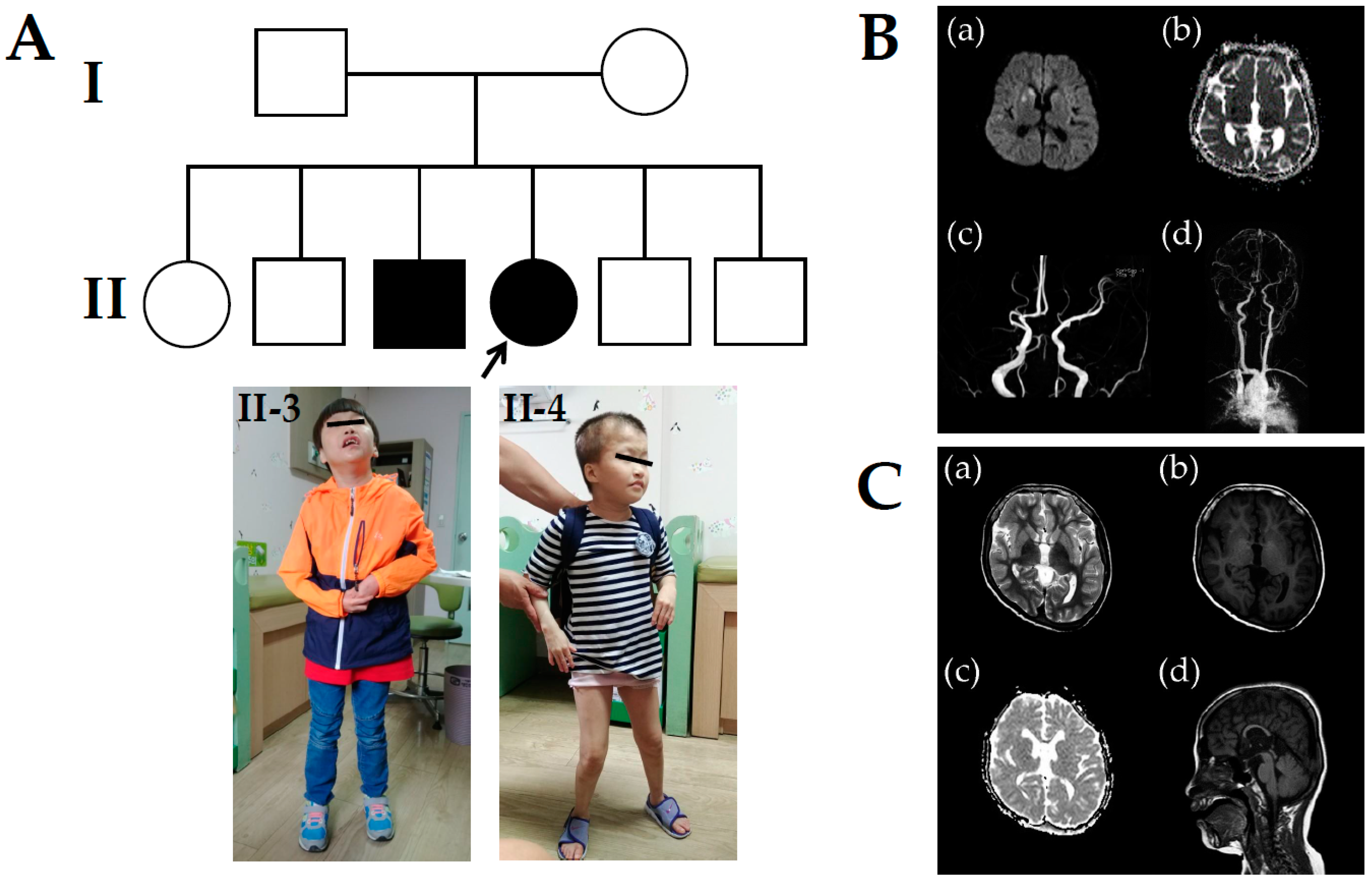
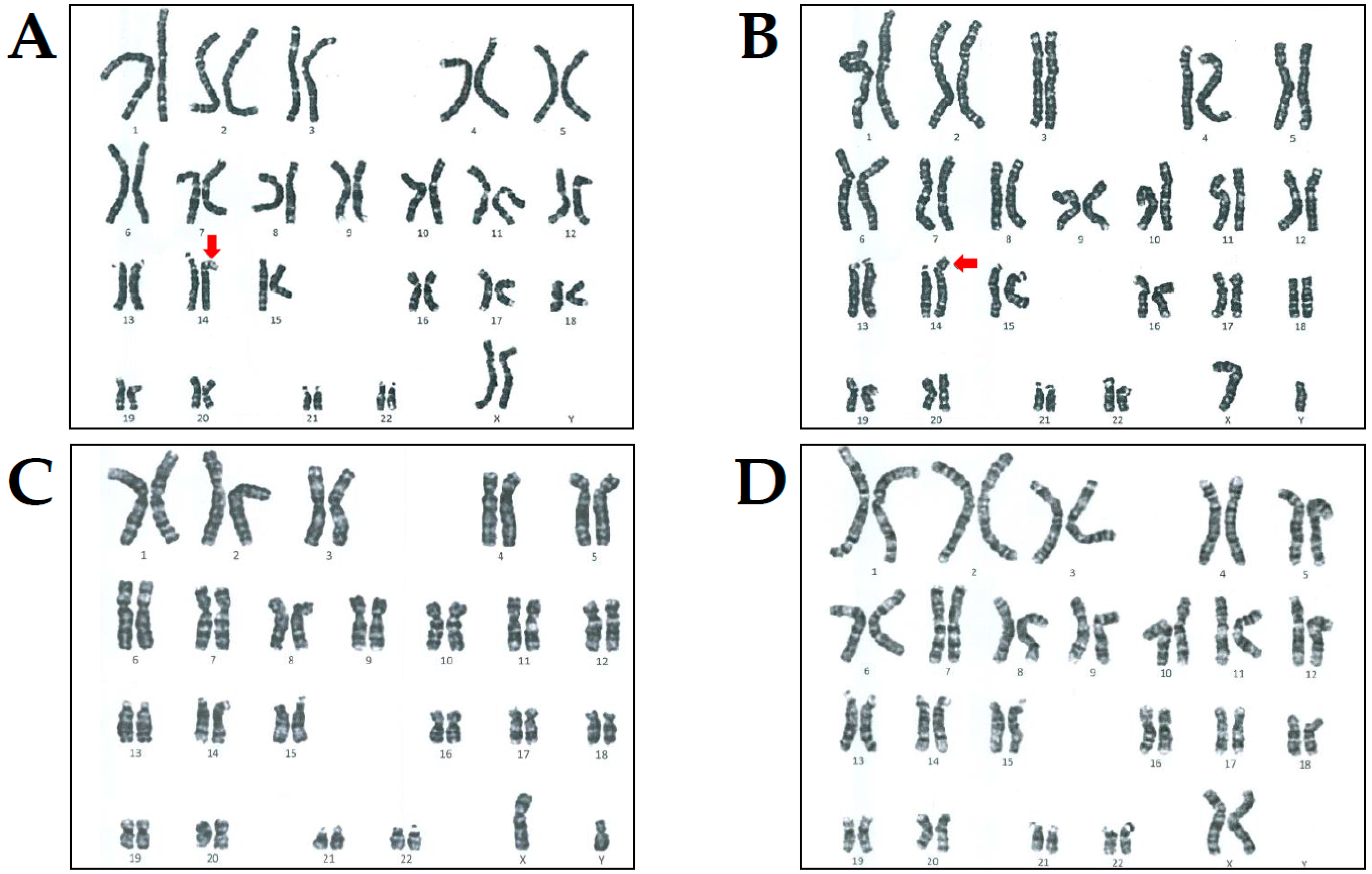
3. Case Presentation
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heilig, R.; Eckenberg, R.; Petit, J.L.; Fonknechten, N.; Da Silva, C.; Cattolico, L.; Levy, M.; Barbe, V.; de Berardinis, V.; Ureta-Vidal, A.; et al. The DNA sequence and analysis of human chromosome 14. Nature 2003, 421, 601–607. [Google Scholar] [CrossRef]
- Lupski, J.R.; Stankiewicz, P. Genomic disorders: Molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005, 1, e49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, P.R.; Carroll, A.J.; Korf, B.R. Overview of Clinical Cytogenetics. Curr. Protoc. Hum. Genet. 2016, 89, 8.1.1–8.1.13. [Google Scholar] [CrossRef] [PubMed]
- Harewood, L.; Fraser, P. The impact of chromosomal rearrangements on regulation of gene expression. Hum. Mol. Genet. 2014, 23, R76–R82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allderdice, P.W.; Miller, O.J.; Miller, D.A.; Breg, W.R.; Gendel, E.; Zelson, C. Familial translocation involving chromosomes 6, 14 and 20, identified by quinacrine fluorescence. Humangenetik 1971, 13, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.A.; Wyandt, H.E.; Magenis, R.E.; Lovrien, E.W.; Hecht, F. Mosaicism with translocation: Autoradiographic and fluorescent studies of an inherited reciprocal translocation t(2q+;14q-). J. Med. Genet. 1972, 9, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Short, E.M.; Solitare, G.B.; Breg, W.R. A case of partial 14 trisomy 47,XY,(14q-)+ and translocation t(9p+;14q-) in mother and brother. J. Med. Genet. 1972, 9, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, R.A.; Buttinghaus, K.; Struck, H. Partial trisomy 14 following a balanced reciprocal translocation t(14q-;21q+). Humangenetik 1973, 20, 187–189. [Google Scholar] [CrossRef]
- Fryns, J.P.; Cassiman, J.J.; Van den Berghe, H. Tertiary partial 14 trisomy 47, XX, plus 14q minus. Humangenetik 1974, 24, 71–77. [Google Scholar] [CrossRef]
- Wahlstrom, J. A prenatally discovered unbalanced translocation t(14;22) (q22 or 23;q13). Hereditas 1974, 78, 251–254. [Google Scholar] [CrossRef]
- Raoul, O.; Rethoré, M.O.; Dutriliaux, B.; Michon, L.; Lejeune, J. [Partial 14q trisomy. I. Partial 14q trisomy by maternal translocation t(10;14) (p15.2;q22)]. Ann. Genet. 1975, 18, 35–39. [Google Scholar] [PubMed]
- Fryns, J.P.; Van Eygen, M.; Tanghe, W.; Van Den Berghe, H. Partial trisomy 14q due to familial t(14q-,11q+) translocation. Hum. Genet. 1977, 37, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Zellweger, H. Partial trisomy 14q -- and parental translocation of No. 14 chromosome. Report of a case and review of the literature. J. Med. Genet. 1977, 14, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Trunca, C.; Opitz, J.M. Pericentric inversion of chromosome 14 and the risk of partial duplication of 14q (14q31 leads to 14qter). Am. J. Med. Genet. 1977, 1, 217–228. [Google Scholar] [CrossRef]
- Pfeiffer, R.A.; Kessel, E. Balanced and unbalanced pericentric inversion of a chromosome 14. Hum. Genet. 1978, 43, 103–106. [Google Scholar] [CrossRef]
- Bridgman, G.; Butler, L.J. A child trisomic for the distal part of chromosome 14q. Arch. Dis. Child. 1980, 55, 474–477. [Google Scholar] [CrossRef] [Green Version]
- Geormaneanu, M.; Geormaneanu, C.; Walter-Rosianu, A.; Papuc, M. [Distal trisomy 14q associated with agenesis of the corpus callosus and truncus arteriosus due to the maternal translocation t(5;14)(q13;q23q32) (author's transl)]. Ann. Genet. 1981, 24, 176–178. [Google Scholar]
- Sklower, S.; Jenkins, E.; Nolin, S.; Warburton, D.; Yaboa, K.; Merkrebs, A.; Schwartz, R.; Wisniewski, K.; Stimson, C.; Brown, T. Familial distal trisomy 14q. Am. J. Hum. Genet. 1982, 34, 145A. [Google Scholar]
- Atkin, J.F.; Patil, S. Duplication of the distal segment of 14q. Am. J. Med. Genet. 1983, 16, 357–366. [Google Scholar] [CrossRef]
- Cohen, M.M.; Charrow, J.; Balkin, N.E.; Harris, C.J. Partial trisomy 14 (q23 leads to qter) via segregation of a 14/X translocation. Am. J. Hum. Genet. 1983, 35, 635–644. [Google Scholar]
- Nikolis, J.; Ivanovic, K.; Kosanovic, M. Tandem duplication of chromosome 14 (q24 leads to q32) in male newborn with congenital malformations. Clin. Genet. 1983, 23, 321–324. [Google Scholar] [PubMed]
- Orye, E.; Van Bever, H.; Desimpel, H. Distal trisomy 14q due to tandem duplication (q24 leads to q32). Ann. Genet. 1983, 26, 238–239. [Google Scholar] [PubMed]
- Romain, D.R.; Columbano-Green, L.M.; Smythe, R.H.; Parfitt, R.G.; Gebbie, O.; Chapman, C.J.; Wall, M. Partial trisomy 14q24 leads to qter. J. Med. Genet. 1983, 20, 466–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turleau, C.; de Grouchy, J.; Chavin-Colin, F.; Denavit, M.F.; Le Touze, P. [Distal 14q trisomy]. Ann. Genet. 1983, 26, 165–170. [Google Scholar]
- Kaiser, P.; Forster, W.; Steuernagel, P.; Hillig, U.; Herberg, K.P. Familial pericentric inversion (14) (p11;q24) with a rec dup(q) in one offspring. Clin. Genet. 1984, 26, 73–76. [Google Scholar] [CrossRef]
- Markkanen, A.; Somer, M.; Nordstrom, A.M. Distal trisomy 14q syndrome; a case report. Clin. Genet. 1984, 26, 231–234. [Google Scholar] [CrossRef]
- Sklower, S.L.; Jenkins, E.C.; Nolin, S.L.; Duncan, C.J.; Warburton, D.; Yeboa, K.A.; Merkrebs, A.; Schwartz, R.; Wisniewski, K.; Stimson, C.; et al. Distal duplication 14q: Report of three cases and further delineation of the syndrome. Hum. Genet. 1984, 68, 159–164. [Google Scholar] [CrossRef]
- Carr, D.M.; Jones-Quartey, K.; Vartanian, M.V.; Moore-Kaplan, H. Duplication 14(q31----qter). J. Med. Genet. 1987, 24, 372–374. [Google Scholar] [CrossRef]
- Mikelsaar, R.V.; Ilus, T.A.; Lurie, I.W. Distal trisomy 14q. J. Med. Genet. 1987, 24, 380–381. [Google Scholar] [CrossRef] [Green Version]
- Wakita, Y.; Narahara, K.; Kikkawa, K.; Namba, H.; Hiramoto, K.; Eguchi, K.; Matsubara, T.; Kimoto, H. Distal 14q trisomy syndrome in two siblings: Further delineation of its phenotype. Jinrui idengaku zasshi. Jpn. J. Hum. Genet. 1988, 33, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Masada, C.T.; Olney, A.H.; Fordyce, R.; Sanger, W.G. Partial deletion of 14q and partial duplication of 14q in sibs: Testicular mosaicism for t(14q;14q) as a common mechanism. Am. J. Med. Genet. 1989, 34, 528–534. [Google Scholar] [CrossRef]
- Gilgenkrantz, S.; Vigneron, J.; Peter, M.O.; Dufier, J.L.; Teboul, M.; Chery, M.; Keyeux, G.; Lefranc, M.P. Distal trisomy 14q. I. Clinical and cytogenetical studies. Hum. Genet. 1990, 85, 612–616. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sakai, K.; Sakuma, S.; Sato, E.; Maruyama, M.; Hashimoto, T.; Fukuda, S.; Nishimi, Y.; Nakagome, Y.; Nakahori, Y.; et al. Partial trisomy of the distal segment 14q. Hum. Genet. 1990, 84, 574–576. [Google Scholar] [CrossRef]
- Carter, N.P.; Ferguson-Smith, M.A.; Perryman, M.T.; Telenius, H.; Pelmear, A.H.; Leversha, M.A.; Glancy, M.T.; Wood, S.L.; Cook, K.; Dyson, H.M.; et al. Reverse chromosome painting: A method for the rapid analysis of aberrant chromosomes in clinical cytogenetics. J. Med. Genet. 1992, 29, 299–307. [Google Scholar] [CrossRef]
- Mignon-Ravix, C.; Mugneret, F.; Stavropoulou, C.; Depetris, D.; Khau Van Kien, P.; Mattei, M.G. Maternally inherited duplication of the possible imprinted 14q31 region. J. Med. Genet. 2001, 38, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Orellana, C.; Martinez, F.; Badia, L.; Millan, J.M.; Montero, M.R.; Andres, J.; Prieto, F. Trisomy rescue by postzygotic unbalanced (X;14) translocation in a girl with dysmorphic features. Clin. Genet. 2001, 60, 206–211. [Google Scholar] [CrossRef]
- Perrin, Y.; Addor, M.C.; Sekarski, N.; Gaide, A.C.; Schorderet, D.F. Distal trisomy 14 (q24 --> qter) and aorto-pulmonary window: A case report and review of the literature. Ann. Genet. 2002, 45, 173–175. [Google Scholar] [CrossRef]
- Sonoda, T.; Kouno, K.; Sawada, K.; Sugimoto, T. Distal 14q trisomy due to a maternal derivative chromosome 14. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2001, 43, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Sutton, V.R.; Coveler, K.J.; Lalani, S.R.; Kashork, C.D.; Shaffer, L.G. Subtelomeric FISH uncovers trisomy 14q32: Lessons for imprinted regions, cryptic rearrangements and variant acrocentric short arms. Am. J. Med. Genet. 2002, 112, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Chern, S.R.; Lin, S.P.; Lin, C.C.; Li, Y.C.; Wang, T.H.; Lee, C.C.; Pan, C.W.; Hsieh, L.J.; Wang, W. A paternally derived inverted duplication of distal 14q with a terminal 14q deletion. Am. J. Med. Genet. Part A 2005, 139a, 146–150. [Google Scholar] [CrossRef]
- Šliužas, V.; Utkus, A.; Kučinskas, V. Recombinant chromosome 14 due to maternal pericentric inversion. J. Appl. Genet. 2008, 49, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.T.; Dorr, H.G.; Trautmann, U.; Hoyer, J.; Hofmann, K.; Kraus, C.; Ekici, A.B.; Reis, A.; Rauch, A. A de novo 7.6Mb tandem duplication of 14q32.2-qter associated with primordial short stature with neurosecretory growth hormone dysfunction, distinct facial anomalies and mild developmental delay. Eur. J. Med. Genet. 2008, 51, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Pallister, P.D.; Pallister, A.B.; South, S.; Toydemir, R.; Johnson, J.P.; Beischel, L.; Opitz, J.M. A deletion 13q34/duplication 14q32.2-14q32.33 syndrome diagnosed 50 years after neonatal presentation as infantile hypercalcemia. Am. J. Med. Genet. Part A 2011, 155a, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Hwang, K.S.; Su, H.Y.; Lin, S.P.; Su, Y.N.; Chern, S.R.; Su, J.W.; Chen, Y.T.; Chen, W.L.; Wang, W. Prenatal diagnosis and molecular cytogenetic characterization of a de novo interstitial duplication of 14q (14q31.3-->q32.12) associated with abnormal maternal serum biochemistry. Taiwan. J. Obstet. Gynecol. 2013, 52, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgardioli, I.C.; Simioni, M.; Viguetti-Campos, N.L.; Prota, J.R.; Gil-da-Silva-Lopes, V.L. A new case of partial 14q31.3-qter trisomy due to maternal pericentric inversion. Gene 2013, 523, 192–194. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.P.; Lin, C.J.; Chern, S.R.; Wu, P.S.; Chen, Y.N.; Chen, S.W.; Lee, C.C.; Chen, L.F.; Yang, C.W.; Wang, W. Prenatal diagnosis and molecular cytogenetic characterization of a de novo unbalanced reciprocal translocation of der(9)t(9;14)(p24.2;q32.11) associated with 9p terminal deletion and 14q distal duplication. Taiwan. J. Obstet. Gynecol. 2016, 55, 596–601. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Dong, C.; Li, J.; Hu, D.; Yao, L.; Wu, Y. A Familial 14q32.32q32.33 Duplication/17p13.3 Deletion Syndrome with Facial Anomalies and Moderate Intellectual Disability. Cytogenet. Genome Res. 2016, 148, 262–267. [Google Scholar] [CrossRef]
- Santoro, S.; Bao, L.; Saal, H.M. Terminal 14q Deletion and Duplication with Gastrointestinal and Pulmonary Disease. Pediatr Neonatal Care 2016, 5, 00174. [Google Scholar] [CrossRef]
- Villa, N.; Scatigno, A.; Redaelli, S.; Conconi, D.; Cianci, P.; Farina, C.; Fossati, C.; Dalpra, L.; Maitz, S.; Selicorni, A. 14q32.3-qter trisomic segment: A case report and literature review. Mol. Cytogenet. 2016, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, B.; Yang, L.; Wang, H.; Wu, B.; Liu, R.; Chen, H.; Chen, X.; Yu, S.; Chen, B.; et al. Clinical exome sequencing as the first-tier test for diagnosing developmental disorders covering both CNV and SNV: A Chinese cohort. J. Med. Genet. 2020, 57, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Yamada, M.; Uehara, T.; Takenouchi, T.; Kosaki, K. Parallel detection of single nucleotide variants and copy number variants with exome analysis: Validation in a cohort of 700 undiagnosed patients. Am. J. Med. Genet. Part A 2020, 182, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Vandersluis, S.; Holubowich, C.; Ungar, W.J.; Goh, E.S.; Boycott, K.M.; Sikich, N.; Dhalla, I.; Ng, V. Cost-effectiveness of genome-wide sequencing for unexplained developmental disabilities and multiple congenital anomalies. Genet. Med. 2021, 23, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.J.; Amr, S.S.; Bowser, M.J.; Gowrisankar, S.; Hynes, E.; Mahanta, L.M.; Rehm, H.L.; Funke, B.; Lebo, M.S. VisCap: Inference and visualization of germ-line copy-number variants from targeted clinical sequencing data. Genet. Med. 2016, 18, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Shaw-Smith, C.; Redon, R.; Rickman, L.; Rio, M.; Willatt, L.; Fiegler, H.; Firth, H.; Sanlaville, D.; Winter, R.; Colleaux, L.; et al. Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. J. Med. Genet. 2004, 41, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Kim, Y.; Han, E.; Park, J.; Chae, H.; Kwon, A.; Choi, H.; Kim, J.; Son, J.O.; Lee, S.J.; et al. Chromosomal Microarray Analysis as a First-Tier Clinical Diagnostic Test in Patients With Developmental Delay/Intellectual Disability, Autism Spectrum Disorders, and Multiple Congenital Anomalies: A Prospective Multicenter Study in Korea. Ann. Lab. Med. 2019, 39, 299–310. [Google Scholar] [CrossRef]
- Leach, N.T.; Cole, S.M.; Sandstrom, D.J.; Weremowicz, S. A novel pericentric inversion of chromosome 14 involving the rRNA gene cluster. Prenat. Diagn. 2005, 25, 620–621. [Google Scholar] [CrossRef]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009, 84, 524–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessel, D.; Gehbauer, C.; Bramswig, N.C.; Schluth-Bolard, C.; Venkataramanappa, S.; van Gassen, K.L.I.; Hempel, M.; Haack, T.B.; Baresic, A.; Genetti, C.A.; et al. BCL11B mutations in patients affected by a neurodevelopmental disorder with reduced type 2 innate lymphoid cells. Brain 2018, 141, 2299–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, F.; Wang, C.; Luo, C.; Wang, Y.; Shao, B.; Tan, J.; Hu, P.; Xu, Z. A De Novo heterozygous frameshift mutation identified in BCL11B causes neurodevelopmental disorder by whole exome sequencing. Mol. Genet. Genomic Med. 2019, 7, e897. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Yin, W.; Hu, B.; Kline, A.D.; Zhang, V.W.; Liang, D.; Sun, Y.; Wang, L.; Tang, S.; Powis, Z.; et al. De Novo Mutations of CCNK Cause a Syndromic Neurodevelopmental Disorder with Distinctive Facial Dysmorphism. Am. J. Hum. Genet. 2018, 103, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, M.; Vulto-van Silfhout, A.T.; Germain, P.L.; Vitriolo, A.; Kumar, R.; Douglas, E.; Haan, E.; Kosaki, K.; Takenouchi, T.; Rauch, A.; et al. YY1 Haploinsufficiency Causes an Intellectual Disability Syndrome Featuring Transcriptional and Chromatin Dysfunction. Am. J. Hum. Genet. 2017, 100, 907–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemsen, M.H.; Vissers, L.E.; Willemsen, M.A.; van Bon, B.W.; Kroes, T.; de Ligt, J.; de Vries, B.B.; Schoots, J.; Lugtenberg, D.; Hamel, B.C.; et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J. Med. Genet. 2012, 49, 179–183. [Google Scholar] [CrossRef]
- Poirier, K.; Lebrun, N.; Broix, L.; Tian, G.; Saillour, Y.; Boscheron, C.; Parrini, E.; Valence, S.; Pierre, B.S.; Oger, M.; et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 2013, 45, 639–647. [Google Scholar] [CrossRef]
- Olson, H.E.; Jean-Marçais, N.; Yang, E.; Heron, D.; Tatton-Brown, K.; van der Zwaag, P.A.; Bijlsma, E.K.; Krock, B.L.; Backer, E.; Kamsteeg, E.J.; et al. A Recurrent De Novo PACS2 Heterozygous Missense Variant Causes Neonatal-Onset Developmental Epileptic Encephalopathy, Facial Dysmorphism, and Cerebellar Dysgenesis. Am. J. Hum. Genet. 2018, 102, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beygo, J.; Küchler, A.; Gillessen-Kaesbach, G.; Albrecht, B.; Eckle, J.; Eggermann, T.; Gellhaus, A.; Kanber, D.; Kordaß, U.; Lüdecke, H.J.; et al. New insights into the imprinted MEG8-DMR in 14q32 and clinical and molecular description of novel patients with Temple syndrome. Eur. J. Hum. Genet. 2017, 25, 935–945. [Google Scholar] [CrossRef] [PubMed]



| DD Genes | Morbidity | Diseases | OMIM | Inheritance |
|---|---|---|---|---|
| CCDC88C | Yes | Hydrocephalus, congenital, 1 | # 236600 | AR |
| TRIP11 | Yes | Achondrogenesis, type IA Odontochondrodysplasia 1 | # 200600 # 184260 | AR |
| SLC24A4 | Yes | Amelogenesis imperfecta, type IIA5 | # 615887 | AR |
| TMEM251 | Yes | Dysostosis multiplex, Ain-Naz type | # 619345 | AR |
| UBR7 | Yes | Li-Campeau syndrome | # 619189 | AR |
| VRK1 | Yes | Pontocerebellar hypoplasia type 1A | # 607596 | AR |
| BCL11B | Yes | Intellectual developmental disorder with dysmorphic facies, speech delay, and T-cell abnormalities | # 618092 | AD |
| CCNK | Yes | Intellectual developmental disorder with hypertelorism and distinctive facies | # 618147 | AD |
| YY1 | Yes | Gabriele-de Vries syndrome | # 617557 | AD |
| DYNC1H1 | Yes | Mental retardation, autosomal dominant 13 | # 614563 | AD |
| TECPR2 | Yes | Spastic paraplegia 49, autosomal recessive | # 615031 | AR |
| APOPT1 | Yes | Mitochondrial complex IV deficiency, nuclear type 17 | # 619061 | AR |
| AKT1 | Yes | Cowden syndrome 6 | # 615109 | AD |
| BRF1 | Yes | Cerebellofaciodental syndrome | # 616202 | AR |
| PACS2 | Yes | Developmental and epileptic encephalopathy 66 | # 618067 | AD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.Y.; Park, J. A Recurrent De Novo Terminal Duplication of 14q32 in Korean Siblings Associated with Developmental Delay and Intellectual Disability, Growth Retardation, Facial Dysmorphism, and Cerebral Infarction: A Case Report and Literature Review. Genes 2021, 12, 1388. https://doi.org/10.3390/genes12091388
Han JY, Park J. A Recurrent De Novo Terminal Duplication of 14q32 in Korean Siblings Associated with Developmental Delay and Intellectual Disability, Growth Retardation, Facial Dysmorphism, and Cerebral Infarction: A Case Report and Literature Review. Genes. 2021; 12(9):1388. https://doi.org/10.3390/genes12091388
Chicago/Turabian StyleHan, Ji Yoon, and Joonhong Park. 2021. "A Recurrent De Novo Terminal Duplication of 14q32 in Korean Siblings Associated with Developmental Delay and Intellectual Disability, Growth Retardation, Facial Dysmorphism, and Cerebral Infarction: A Case Report and Literature Review" Genes 12, no. 9: 1388. https://doi.org/10.3390/genes12091388






