Evolutionary Analysis of OAT Gene Family in River and Swamp Buffalo: Potential Role of SLCO3A1 Gene in Milk Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Identification of OAT Gene Family
2.3. Gene Duplication Analysis of OAT Gene Family
2.4. Sequence Analysis of OAT Gene Family
2.5. Comparative Transcriptomic Analysis of the OAT Genes in Buffalo Subspecies
3. Results
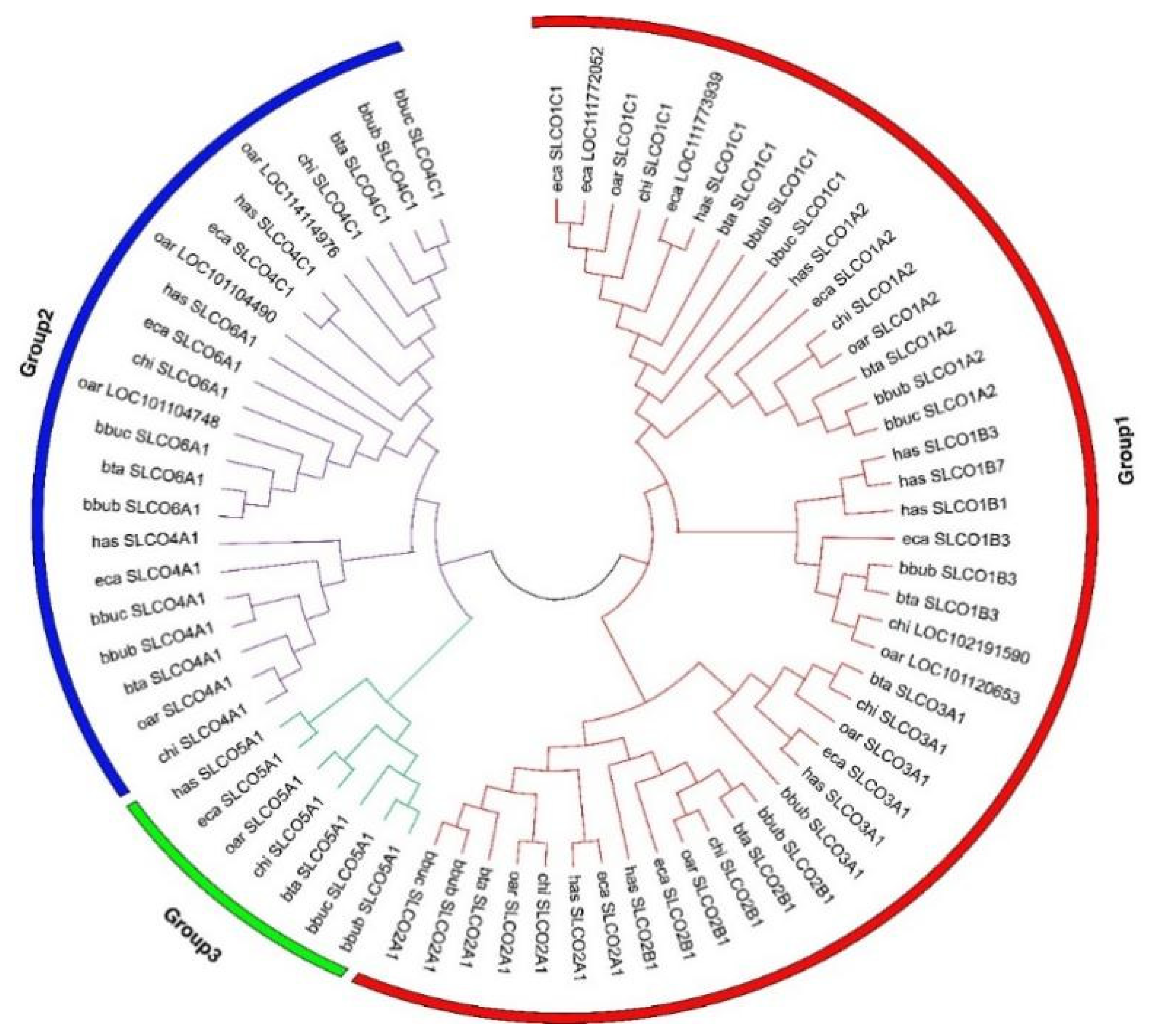
3.1. Identification and Phylogenetic Analysis of OAT Gene Family
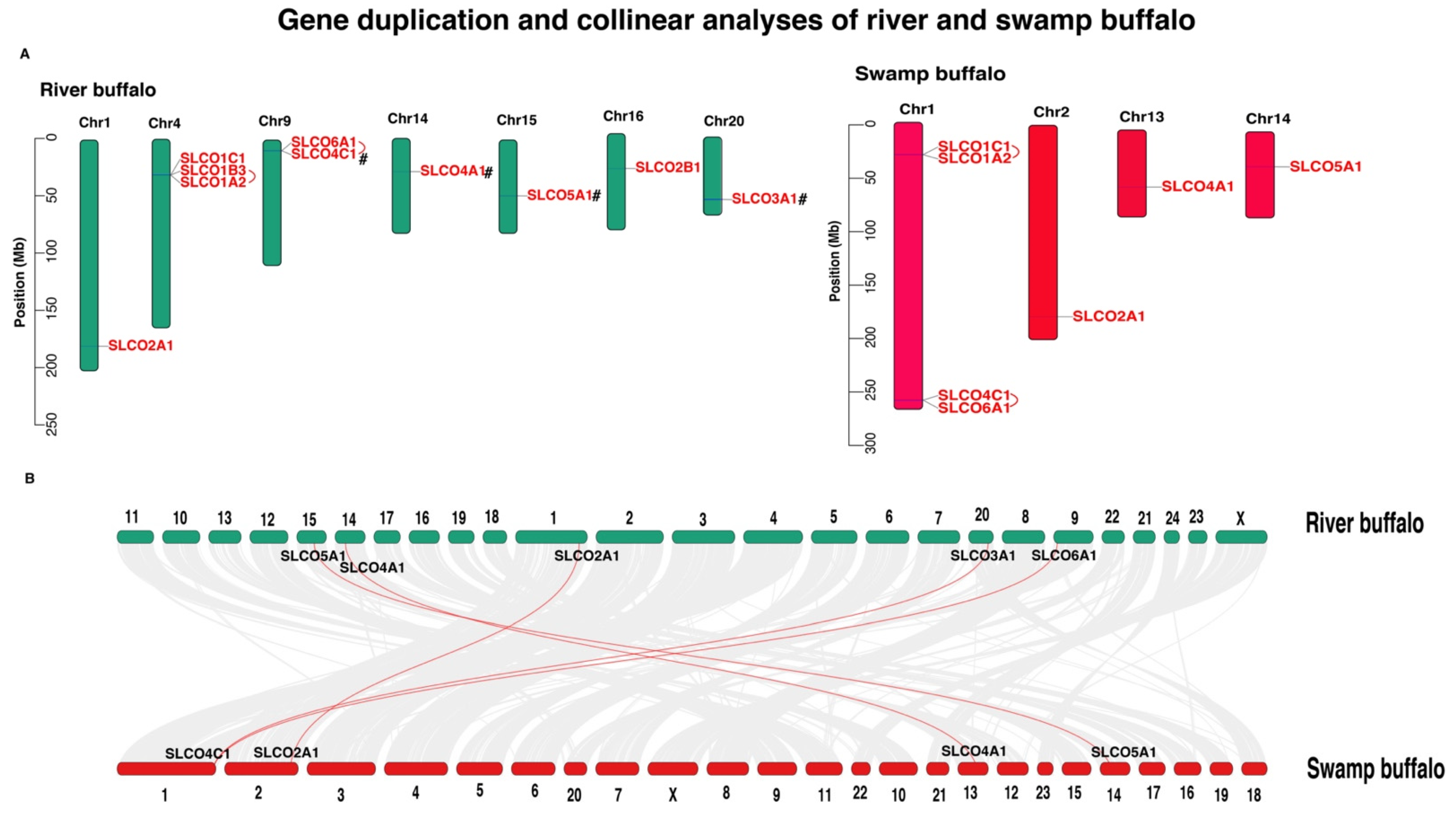
3.2. Chromosome Location and Gene Duplication Analysis of OAT Gene Family
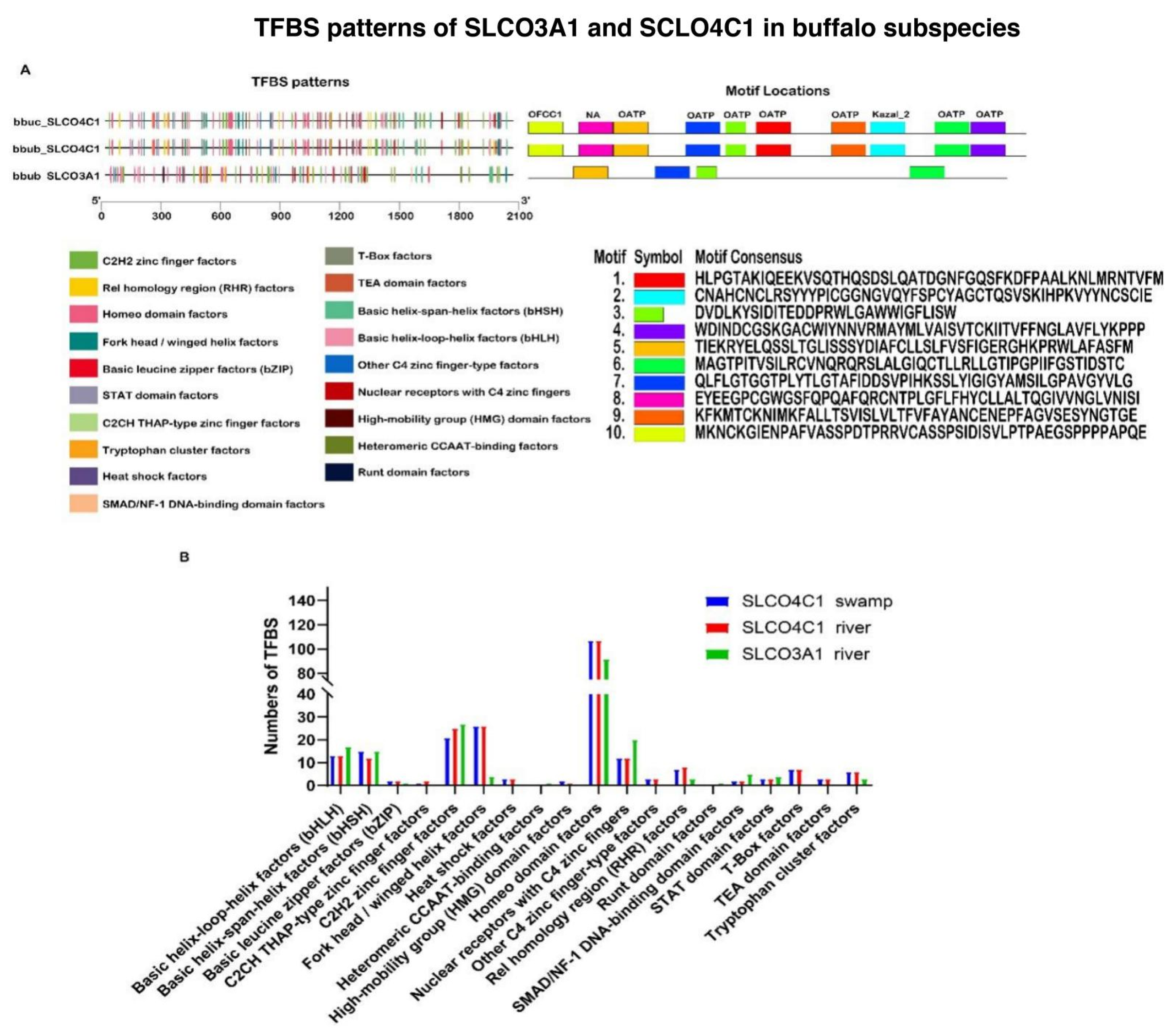
3.3. Sequence Characteristics of OAT Gene Family in Buffalo
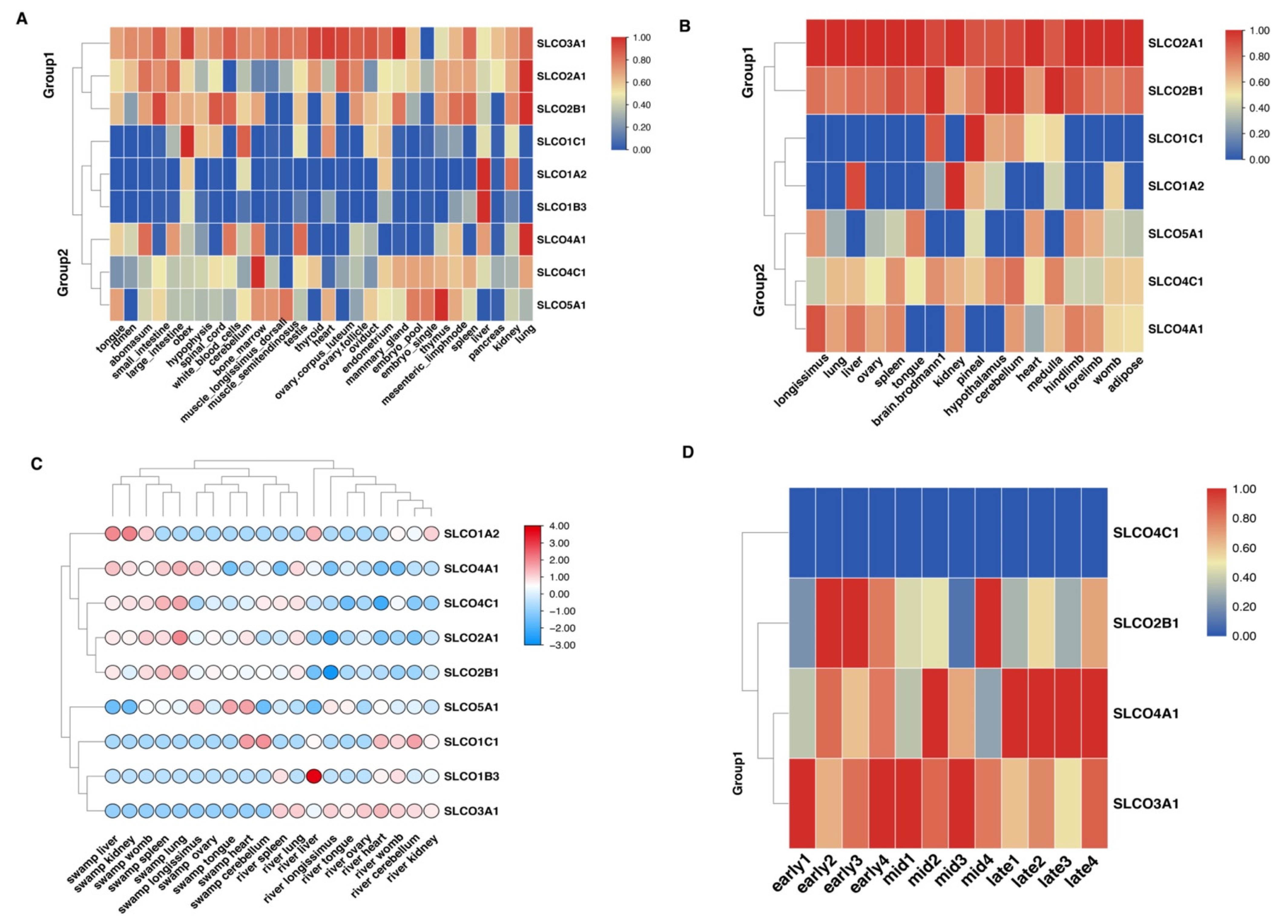
3.4. Expression Pattern Analyses of OAT Gene Family in Buffalo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nigam, S.K.; Bush, K.T.; Martovetsky, G.; Ahn, S.Y.; Liu, H.C.; Richard, E.; Bhatnagar, V.; Wu, W. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 2015, 95, 83–123. [Google Scholar] [CrossRef] [PubMed]
- García-Lino, A.M.; Álvarez-Fernández, I.; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the Mammary Gland-Contribution to Presence of Nutrients and Drugs into Milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibeagha-Awemu, E.M.; Peters, S.O.; Akwanji, K.A.; Imumorin, I.G.; Zhao, X. High density genome wide genotyping-by-sequencing and association identifies common and low frequency SNPs, and novel candidate genes influencing cow milk traits. Sci. Rep. 2016, 6, 31109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, H.E.; Jui, G.; Joyce, D.A.; Reilly, C.R., 3rd; Lunt, D.H.; Renn, S.C. Gene duplication in an African cichlid adaptive radiation. BMC Genom. 2014, 15, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Jiang, J.; Padhi, A.; Ning, C.; Fu, J.; Wang, A.; Mrode, R.; Liu, J.F. Characterization of genome-wide segmental duplications reveals a common genomic feature of association with immunity among domestic animals. BMC Genom. 2017, 18, 293. [Google Scholar] [CrossRef] [Green Version]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef] [Green Version]
- Katju, V.; Lynch, M. On the formation of novel genes by duplication in the Caenorhabditis elegans genome. Mol. Biol. Evol. 2006, 23, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.L. Gene duplication and the origin of novel proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 8791–8792. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Nadeem, A.; Javed, M.; Hassan, F.-U.; Luo, X.; Khalid, R.B.; Liu, Q. Genomic Identification, Evolution and Sequence Analysis of the Heat-Shock Protein Gene Family in Buffalo. Genes 2020, 11, 1388. [Google Scholar] [CrossRef]
- Meng, D.; Cao, Y.; Chen, T.; Abdullah, M.; Jin, Q.; Fan, H.; Lin, Y.; Cai, Y. Evolution and functional divergence of MADS-box genes in Pyrus. Sci. Rep. 2019, 9, 1266. [Google Scholar] [CrossRef]
- Wahid, H.; Rosnina, Y. Buffalo: Asia. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef]
- Borghese, A.; Moioli, B. Buffalo: Mediterranean Region. Ref. Modul. Food Sci. 2016. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Shen, J.; Achilli, A.; Chen, N.; Chen, Q.; Dang, R.; Zheng, Z.; Zhang, H.; Zhang, X.; Wang, S.; et al. Genomic analyses reveal distinct genetic architectures and selective pressures in buffaloes. GigaScience 2020, 9, giz166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tan, G.; Lenhard, B. TFBSTools: An R/bioconductor package for transcription factor binding site analysis. Bioinformatics 2016, 32, 1555–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wang, G.D.; Ruan, J.; Chen, Y.B.; Yang, C.P.; Cao, X.; Wu, H.; Liu, Y.H.; Du, Z.L.; Wang, X.P.; et al. Genomic analysis of snub-nosed monkeys (Rhinopithecus) identifies genes and processes related to high-altitude adaptation. Nat. Genet. 2016, 48, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Montalbetti, N.; Dalghi, M.G.; Albrecht, C.; Hediger, M.A. Nutrient transport in the mammary gland: Calcium, trace minerals and water soluble vitamins. J. Mammary Gland Biol. Neoplasia 2014, 19, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.K.; Schweizer, U.; Köhrle, J. Transport of thyroid hormone in brain. Front. Endocrinol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.H.; Glover, K.P.; Han, X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol. Sci. 2010, 117, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.; Tamai, I. Roles of Organic Anion Transporting Polypeptide 2A1 (OATP2A1/SLCO2A1) in Regulating the Pathophysiological Actions of Prostaglandins. AAPS J. 2017, 20, 13. [Google Scholar] [CrossRef]
- Ugele, B.; Bahn, A.; Rex-Haffner, M. Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J. Steroid Biochem. Mol. Biol. 2008, 111, 1–6. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nojima, J.; Ohbayashi, M.; Kohyama, N.; Yamamoto, T. Molecular cloning and functional characterization of a novel gene encoding human prostaglandin carrier, hPrC. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2011, 131, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Ban, D.; Zhang, C.; Zhang, Y. Expression profiles and association between OATP4A1 and PGE (2) in adenomyosis tissue. Int. J. Clin. Exp. Pathol. 2019, 12, 3367–3375. [Google Scholar]
- Kuo, K.L.; Zhu, H.; McNamara, P.J.; Leggas, M. Localization and functional characterization of the rat Oatp4c1 transporter in an in vitro cell system and rat tissues. PLoS ONE 2012, 7, e39641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fietz, D.; Bakhaus, K.; Wapelhorst, B.; Grosser, G.; Günther, S.; Alber, J.; Döring, B.; Kliesch, S.; Weidner, W.; Galuska, C.E.; et al. Membrane transporters for sulfated steroids in the human testis—Cellular localization, expression pattern and functional analysis. PLoS ONE 2013, 8, e62638. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, K.; Detro-Dassen, S.; Rinis, N.; Fahrenkamp, D.; Müller-Newen, G.; Merk, H.F.; Schmalzing, G.; Zwadlo-Klarwasser, G.; Baron, J.M. Characterization of SLCO5A1/OATP5A1, a solute carrier transport protein with non-classical function. PLoS ONE 2013, 8, e83257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Chopra, S.; Peterson, T. A segmental gene duplication generated differentially expressed myb-homologous genes in maize. Plant Cell 2000, 12, 2311–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, P.W.; Marlétaz, F.; Maeso, I.; Dunwell, T.L.; Paps, J. New genes from old: Asymmetric divergence of gene duplicates and the evolution of development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20150480. [Google Scholar] [CrossRef]
- Banerjee-Basu, S.; Baxevanis, A.D. Molecular evolution of the homeodomain family of transcription factors. Nucleic Acids Res. 2001, 29, 3258–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Dai, S.; Chen, X.; Liang, X.; Qu, L.; Jiang, L.; Guo, M.; Zhou, Z.; Wei, H.; Zhang, H.; et al. Mechanism of forkhead transcription factors binding to a novel palindromic DNA site. Nucleic Acids Res. 2021, 49, 3573–3583. [Google Scholar] [CrossRef] [PubMed]
- Alam, I.; Batool, K.; Cui, D.L.; Yang, Y.Q.; Lu, Y.H. Comprehensive genomic survey, structural classification and expression analysis of C2H2 zinc finger protein gene family in Brassica rapa L. PLoS ONE 2019, 14, e0216071. [Google Scholar] [CrossRef]
- Porter, B.A.; Ortiz, M.A.; Bratslavsky, G.; Kotula, L. Structure and Function of the Nuclear Receptor Superfamily and Current Targeted Therapies of Prostate Cancer. Cancers 2019, 11, 1852. [Google Scholar] [CrossRef] [Green Version]
| Species | Gene | CHR | Protein ID | Length | Mw (kDa) | pI |
|---|---|---|---|---|---|---|
| river | SLCO1A2 | Chr4 | XP_006067688.1 | 666 | 73.79 | 7.09 |
| river | SLCO1C1 | Chr4 | XP_006067684.2 | 712 | 78.46 | 9.02 |
| river | SLCO1B3 | Chr4 | XP_025139143.1 | 689 | 75.45 | 8.71 |
| river | SLCO3A1 | Chr20 | XP_025127060.1 | 692 | 74.38 | 6.94 |
| river | SLCO2A1 | Chr1 | XP_006051482.1 | 644 | 70.2 | 8.81 |
| river | SLCO2B1 | Chr16 | XP_006074131.1 | 708 | 76.67 | 7.93 |
| river | SLCO4C1 | Chr9 | XP_006069687.1 | 719 | 77.4 | 7.00 |
| river | SLCO5A1 | Chr15 | XP_006073179.2 | 846 | 92.13 | 7.89 |
| river | SLCO4A1 | Chr14 | XP_006072365.2 | 761 | 81.19 | 7.55 |
| river | SLCO6A1 | Chr9 | XP_025147965.1 | 731 | 79.8 | 8.52 |
| swamp | SLCO1C1 | Chr1 | GWHPAAJZ000564 | 1222 | 133.66 | 8.31 |
| swamp | SLCO1A2 | Chr1 | GWHPAAJZ000565 | 666 | 73.81 | 7.09 |
| swamp | SLCO2A1 | Chr2 | GWHPAAJZ009028 | 644 | 70.2 | 8.81 |
| swamp | SLCO4C1 | Chr1 | GWHPAAJZ002472 | 719 | 77.49 | 6.82 |
| swamp | SLCO5A1 | Chr14 | GWHPAAJZ005023 | 846 | 92.13 | 7.89 |
| swamp | SLCO4A1 | Chr13 | GWHPAAJZ004480 | 761 | 81.2 | 7.64 |
| swamp | SLCO6A1 | Chr1 | GWHPAAJZ002473 | 933 | 100.78 | 7.97 |
| Subspecies | Seq1 | Seq2 | Type | Ka | Ks | Ka/Ks | Date (Mya) |
|---|---|---|---|---|---|---|---|
| River | SLCO1B3 | SLCO1A2 | tandem | 0.573 | 1.664 | 0.344 | 66.014 |
| SLCO6A1 | SLCO4C1 | tandem | 0.546 | 1.047 | 0.522 | 41.545 | |
| SLCO3A1 | SLCO4C1 | Segment | 0.746 | 1.538 | 0.485 | 61.032 | |
| SLCO4A1 | SLCO5A1 | Segment | 0.718 | 2.180 | 0.329 | 86.518 | |
| Swamp | SLCO1C1 | SLCO1A2 | tandem | 0.512 | 2.423 | 0.211 | 96.155 |
| SLCO4C1 | SLCO6A1 | tandem | 0.564 | 1.063 | 0.530 | 42.198 |
| Seq1/River Buffalo | Seq2/Swamp Buffalo | Ka | Ks | Ka/Ks | Date (Mya) |
|---|---|---|---|---|---|
| SLCO2A1 | SLCO2A1 | 0.001 | 0.008 | 0.118 | 0.302 |
| SLCO6A1 | SLCO4C1 | 0.861 | 1.391 | 0.619 | 55.210 |
| SLCO4A1 | SLCO4A1 | 0.723 | 1.165 | 0.620 | 46.238 |
| SLCO5A1 | SLCO5A1 | 0.000 | 0.002 | 0.000 | 0.063 |
| SLCO3A1 | SLCO4C1 | 0.811 | 1.181 | 0.686 | 46.857 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Liang, S.; Liang, A.; Rushdi, H.E.; Deng, T. Evolutionary Analysis of OAT Gene Family in River and Swamp Buffalo: Potential Role of SLCO3A1 Gene in Milk Performance. Genes 2021, 12, 1394. https://doi.org/10.3390/genes12091394
Ma X, Liang S, Liang A, Rushdi HE, Deng T. Evolutionary Analysis of OAT Gene Family in River and Swamp Buffalo: Potential Role of SLCO3A1 Gene in Milk Performance. Genes. 2021; 12(9):1394. https://doi.org/10.3390/genes12091394
Chicago/Turabian StyleMa, Xiaoya, Shasha Liang, Aixin Liang, Hossam E. Rushdi, and Tingxian Deng. 2021. "Evolutionary Analysis of OAT Gene Family in River and Swamp Buffalo: Potential Role of SLCO3A1 Gene in Milk Performance" Genes 12, no. 9: 1394. https://doi.org/10.3390/genes12091394







