MicroRNA of Epithelial to Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. MicroRNA
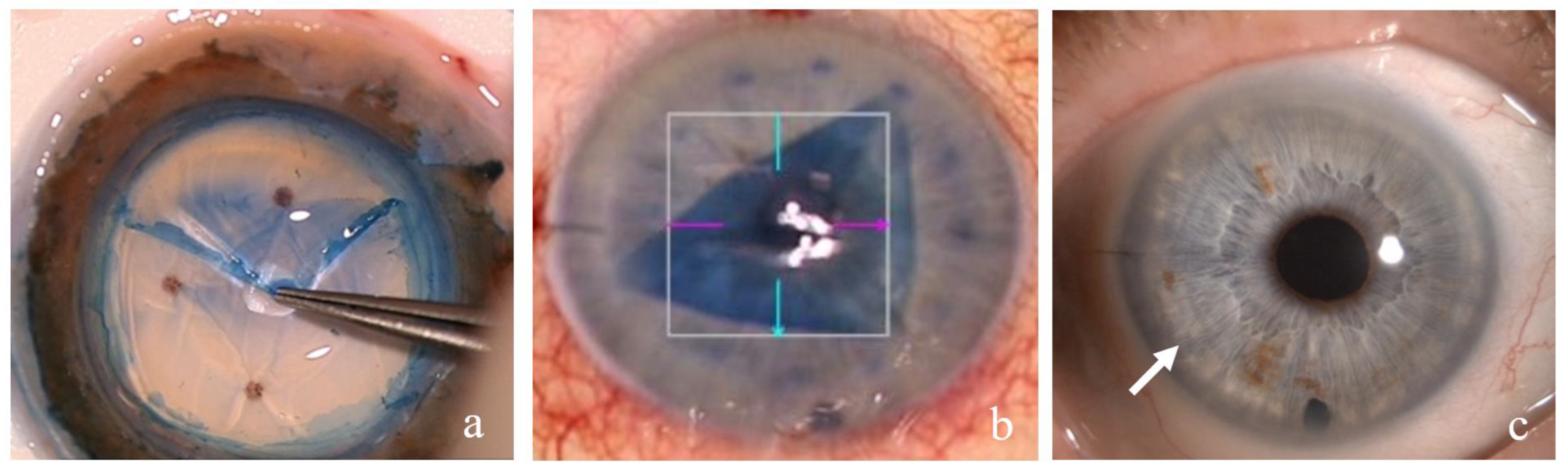
3.2. Epithelial-to-Mesenchymal Transition and Fuchs’ Endothelial Corneal Dystrophy
3.3. MiRNAs of the Epithelial-to-Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy
3.3.1. MiRNA and the Cornea
3.3.2. Corneal Endothelium Senescence and miRNA
3.3.3. Corneal Endothelium miRNA and Oxidative Stress in Fuchs’ Endothelial Corneal Dystrophy
3.3.4. miRNA in Fuchs’ Endothelial Corneal Dystrophy
3.3.5. miRNA, Endothelial Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGBL1 | ATP/GTP binding protein like 1 |
| Akt | protein kinase B |
| COL_A_ | collagen type _ α _chain |
| DGCR8 | DiGeorge syndrome critical region 8 gene |
| DMEK | Descemet membrane endothelial keratoplasty |
| DSO | Descemet stripping only |
| DWEK | Descemet without endothelial keratoplasty |
| EDICT syndrome | endothelial dystrophy–iris hypoplasia–congenital cataract–stromal thinning syndrome |
| EMT | epithelial mesenchymal transition |
| FECD | Fuchs’ endothelial corneal dystrophy |
| FGF-2 | fibroblast growth factor 2 |
| hCEC | human endothelial cell line |
| IL-1β | interleukin 1 β |
| INPPL1 | inositol polyphosphate phosphatase-like 1 gene |
| LAMC1 | laminin subunit γ 1 gene |
| LncRNA | long non-coding RNA |
| LOXHD1 | lipoxygenase homology domains 1 |
| MCP-1 | monocyte chemoattractant protein-1 |
| miRNA | microRNA |
| MMP | matrix metalloprotease |
| mRNA | messenger RNA |
| PPCD | posterior polymorphous corneal dystrophy |
| Rho A | Ras homolog gene-family member A |
| RISC | induced silencing complex |
| SLC4A11 | Solute-carrier family 4 sodium borate-transporter member 11 |
| SNAI 1 | Snail-family transcriptor repressor 1 |
| TCF4 | transcription factor 4 |
| TCF4 | transcription factor 4 gene |
| TCF8 | transcription factor 8 gene |
| TGF-β | transforming growth factor–β |
| TNF-α | tumor necrosis factor α |
| UTR | untranslated region |
| ZEB1 | Zinc-finger E-box-binding homeobox 1 |
References
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy. Ocul. Surf. 2010, 8, 173–184. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 2018, CD012097. [Google Scholar] [CrossRef]
- Price, M.O.; Giebel, A.W.; Fairchild, M.; Price, F.W., Jr. Descemet’s membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009, 116, 2361–2368. [Google Scholar] [CrossRef]
- Menzel-Severing, J.; Geerling, G. Descemet Stripping without Transplantation in Fuchs Endothelial Corneal Dystrophy. Klin. Mon. Augenheilkd. 2022, 239, 760–766. [Google Scholar] [CrossRef]
- Ong, H.S.; Ang, M.; Mehta, J. Evolution of therapies for the corneal endothelium: Past, present and future approaches. Br. J. Ophthalmol. 2021, 105, 454–467. [Google Scholar] [CrossRef]
- Kaufman, A.R.; Nose, R.M.; Pineda, R., 2nd. Descemetorhexis without endothelial keratoplasty (DWEK): Proposal for nomenclature standardization. Cornea 2018, 37, e20–e21. [Google Scholar] [CrossRef]
- Braunstein, R.E.; Airiani, S.; Chang, M.A.; Odrich, M.G. Corneal edema resolution after “descemetorhexis”. J. Cataract Refract. Surg. 2003, 29, 1436–1439. [Google Scholar] [CrossRef]
- Borkar, D.S.; Veldman, P.; Colby, K.A. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea 2016, 35, 1267–1273. [Google Scholar] [CrossRef]
- Wilson, S.E.; Bourne, W.M. Fuchs’ dystrophy. Cornea 1988, 7, 2–18. [Google Scholar] [CrossRef]
- Waring, G.O., 3rd; Bourne, W.M.; Edelhauser, H.F.; Kenyon, K.R. The corneal endothelium. Normal and pathologic structure and function. Ophthalmology 1982, 89, 531–590. [Google Scholar] [CrossRef]
- Waring, G.O., 3rd. Posterior collagenous layer of the cornea. Ultrastructural classification of abnormal collagenous tissue posterior to Descemet’s membrane in 30 cases. Arch. Ophthalmol. 1982, 100, 122–134. [Google Scholar] [CrossRef]
- Iwamoto, T.; Devoe, A.G. Electron microscopic studies on Fuchs combined dystrophy. 1. Posterior portion of cornea. Investig. Ophthalmol. 1971, 10, 9–28. [Google Scholar]
- Jun, A.S.; Meng, H.; Ramanan, N.; Matthaei, M.; Chakravarti, S.; Bonshek, R.; Black, G.C.; Grebe, R.; Kimos, M. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum. Mol. Genet. 2012, 21, 384–393. [Google Scholar] [CrossRef]
- Engler, C.; Kelliher, C.; Spitze, A.R.; Speck, C.L.; Eberhart, C.G.; Jun, A.S. Unfolded protein response in fuchs endothelial corneal dystrophy: A unifying pathogenic pathway? Am. J. Ophthalmol. 2010, 149, 194–202.e2. [Google Scholar] [CrossRef]
- Gottsch, J.D.; Sundin, O.H.; Liu, S.H.; Jun, A.S.; Broma, K.W.; Stark, W.J.; Vito, E.C.L.; Narang, A.K.; Thompson, J.M.; Magovern, M. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of Fuchs corneal dystrophy. Investig. Opthalmol. Vis. Sci. 2005, 46, 1934–1939. [Google Scholar] [CrossRef]
- Liskova, P.; Prescott, Q.; Bhattacharya, S.S.; Tuft, S.J. British family with early-onset Fuchs’ endothelial corneal dystrophy associated with p.L450W mutation in the COL8A2 gene. Br. J. Ophthalmol. 2007, 91, 1717–1718. [Google Scholar] [CrossRef]
- Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar]
- Alsbirk, P.H. Corneal thickness: II. Environmental and genetic factors. Acta Ophthalmol. 1978, 56, 105–113. [Google Scholar] [CrossRef]
- Charlesworth, J.; Kramer, P.L.; Dyer, T.; Diego, V.; Samples, J.R.; Craig, J.E.; Mackey, D.A.; Hewitt, A.W.; Blangero, J.; Wirtz, M.K. The path to open angle glaucoma gene discovery: Endophenotypic status of intraocular pressure, cup-to-disc ratio and central corneal thickness. Investig. Ophthalmol. Vis. Sci. 2010, 50, 4087–4090. [Google Scholar] [CrossRef]
- Landers, J.A.; Hewitt, A.W.; Dimasi, D.P.; Charlesworth, J.C.; Straga, T.; Mills, R.; Savarirayan, R.; Mackey, D.A.; Burdon, K.; Craig, J. Heritability of central corneal thickness in nuclear families. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4087–4090. [Google Scholar] [CrossRef]
- Louttit, M.D.; Kopplin, L.J.; Igo, R.P., Jr.; Fondran, J.R.; Tagliaferri, A.; Bardenstein, D.; Aldave, A.J.; Croasdale, C.R.; Price, M.O.; Rosenwasser, G.O. A multi-center study to map genes for Fuchs’ endothelial corneal dystrophy: Baseline characteristics and heritability. Cornea 2012, 31, 26–35. [Google Scholar] [CrossRef]
- Toh, T.; Liew, S.H.M.; MacKinnon, J.R.; Hewitt, A.W.; Poulsen, J.L.; Spector, T.D.; Gilbert, C.E.; Craig, J.; Hammond, C.J.; Mackey, D.A. Central corneal thickness is highly heritable: The Twin Eye Studies. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3718–3722. [Google Scholar] [CrossRef]
- Zheng, Y.; Ge, J.; Huang, G.; Zhang, J.; Liu, B.; Hur, Y.-M.; He, M. Heritability of central corneal thickness in Chinese: The Guangzhou Twin Eye Study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4303–4307. [Google Scholar] [CrossRef]
- Biswas, S.; Munier, F.L.; Yardley, J.; Hart-Holden, N.; Perveen, R.; Cousin, P.; Sutphin, J.E.; Noble, B.; Batterbury, M.; Kielty, C.; et al. Missense mutations in COL8A2, the gene encoding the α2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum. Mol. Genet. 2001, 10, 2415–2423. [Google Scholar] [CrossRef]
- Baratz, K.H.; Tosakulwong, N.; Ryu, E.; Brown, W.L.; Branham, K.; Chen, W.; Tran, K.D.; Schmid-Kubista, K.E.; Heckenlively, J.R.; Swaroop, A.; et al. E2-2 protein and Fuchs’s corneal dystrophy. N. Engl. J. Med. 2010, 363, 1016–1024. [Google Scholar] [CrossRef]
- Li, Y.-J.; Minear, M.A.; Rimmler, J.; Zhao, B.; Balajonda, E.; Hauser, M.A.; Allingham, R.R.; Eghrari, A.O.; Riazuddin, S.A.; Katsanis, N.; et al. Replication of TCF4 through association and linkage studies in late-onset Fuchs endothelial corneal dystrophy. PLoS ONE 2011, 6, e18044. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; McGlumphy, E.J.; Yeo, W.S.; Wang, J.; Katsanis, N.; Gottsch, J.D. Replication of the TCF4 intronic variant in late-onset Fuchs corneal dystrophy and evidence of independence from the FCD2 locus. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2825–2829. [Google Scholar] [CrossRef]
- Thalamuthu, A.; Khor, C.C.; Venkataraman, D.; Koh, L.W.; Tan, D.T.H.; Aung, T.; Mehta, J.S.; Vithana, E.N. Association of TCF4 gene polymorphisms with Fuchs corneal dystrophy in the Chinese. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5573–5578. [Google Scholar] [CrossRef]
- Kuot, A.; Hewitt, A.W.; Griggs, K.; Klebe, S.; Mills, R.; Jhanji, V.; Craig, J.; Sharma, S.; Burdon, K.P. Association of TCF4 and CLU polymorphisms with Fuchs’ endothelial dystrophy and implication of CLU and TGFB1 proteins in the disease process. Eur. J. Hum. Genet. 2012, 20, 632–638. [Google Scholar] [CrossRef]
- Kobayashi, A.; Fujiki, K.; Murakami, A.; Kato, T.; Chen, L.Z.; Onoe, H.; Nakayasu, K.; Sakurai, M.; Takahashi, M.; Sugiyama, K.; et al. Analysis of COL8A2 gene mutation in Japanese patients with Fuchs’ endothelial dystrophy and posterior polymorphous dystrophy. Jpn. J. Ophthalmol. 2004, 48, 195–198. [Google Scholar] [CrossRef]
- Desronvil, T.; Logan-Wyatt, D.; Abdrabou, W.; Triana, M.; Jones, R.; Taheri, S.; Del Bono, E.; Pasquale, L.; Olivier, M.; Haines, J.; et al. Distribution of COL8A2 and COL8A1gene variants in Caucasian primary open angle glaucoma patients with thin central corneal thickness. Mol. Vis. 2010, 16, 2185–2191. [Google Scholar] [PubMed]
- Vithana, E.N.; Aung, T.; Khor, C.C.; Cornes, B.K.; Tay, W.-T.; Sim, X.; Lavanya, R.; Wu, R.; Zheng, Y.; Hibberd, M.; et al. Collagen-related genes influence the glaucoma risk factor, central corneal thickness. Hum. Mol. Genet. 2011, 20, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Puk, O.; Dalke, C.; Calzada-Wack, J.; Ahmad, N.; Klaften, M.; Wagner, S.; de Angelis, M.H.; Graw, J. Reduced corneal thickness and enlarged anterior chamber in a novel ColVIIIa2G257D mutant mouse. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Hopfer, U.; Fukai, N.; Hopfer, H.; Wolf, G.; Joyce, N.; Li, E.; Olsen, B.R. Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye. FASEB J. 2005, 19, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Igo, R.P., Jr.; Kopplin, L.J.; Joseph, P.; Truitt, B.; Fondran, J.; Bardenstein, D.; Aldave, A.J.; Croasdale, C.R.; Price, M.O.; Rosenwasser, M.; et al. Differing roles for TCF4 and COL8A2 in central corneal thickness and fuchs endothelial corneal dystrophy. PLoS ONE 2012, 7, e46742. [Google Scholar] [CrossRef] [PubMed]
- Hamill, C.E.; Schmedt, T.; Jurkunas, U. Fuchs endothelial cornea dystrophy: A review of the genetics behind disease development. Semin. Ophthalmol. 2013, 28, 281–286. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Vithana, E.N.; Seet, L.F.; Liu, Y.; Al-Saif, A.; Koh, L.W.; Heng, Y.M.; Aung, T.; Meadows, D.N.; Eghrari, A.O.; et al. Missense mutations in the sodium borate cotransporter SLC4A11 cause late-onset Fuchs corneal dystrophy. Hum. Mutat. 2010, 31, 1261–1268. [Google Scholar] [CrossRef]
- Malhotra, D.; Loganathan, S.K.; Chiu, A.M.; Lukowski, C.M.; Casey, J.R. Human corneal expression of SLC4A11, a gene mutated in endothelial corneal dystrophies. Sci. Rep. 2019, 9, 9681. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Zaghloul, N.A.; Al-Saif, A.; Davey, L.; Diplas, B.H.; Meadows, D.N.; Eghrari, A.; Minear, M.A.; Li, Y.-J.; Klintworth, G.K.; et al. Missense mutations in TCF8 cause late-onset Fuchs corneal dystrophy and interact with FCD4 on chromosome 9p. Am. J. Hum. Genet. 2010, 86, 45–53. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Parker, D.S.; McGlumphy, E.J.; Oh, E.C.; Iliff, B.W.; Schmedt, T.; Jurkunas, U.; Schleif, R.; Katsanis, N.; Gottsch, J.D. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am. J. Hum. Genet. 2012, 90, 533–539. [Google Scholar] [CrossRef]
- Sarnicola, C.; Farooq, A.V.; Colby, K. Fuchs endothelial corneal dystrophy: Update on pathogenesis and future directions. Eye Contact Lens 2019, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Fraga, M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P.; Chen, C.Z. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004, 5, 396–400. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- van Rooij, E. The art of microRNA research. Circ. Res. 2011, 108, 219–234. [Google Scholar] [CrossRef]
- Pukl, S.S. Are miRNAs Dynamic Biomarkers in Keratoconus? A Review of the Literature. Genes 2022, 13, 588. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Gong, A.; Dong, Y.; Hao, X.; Wang, X.; Zheng, J.; Ma, W.; Song, Y.; Zhang, J.; et al. The Long Noncoding RNA LINC00963 Inhibits Corneal Fibrosis Scar Formation by Targeting miR-143-3p. DNA Cell Biol. 2022, 41, 400–409. [Google Scholar] [CrossRef]
- Jayachandran, J.; Srinivasan, H.; Mani, K.P. Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 2021, 710, 108984. [Google Scholar] [CrossRef]
- Iliff, B.W.; Riazuddin, S.A.; Gottsch, J.D. The genetics of Fuchs’ corneal dystrophy. Expert Rev. Ophthalmol. 2012, 7, 363–375. [Google Scholar] [CrossRef]
- Lee, J.G.; Kay, E.P. NF-kappa B is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1530–1538. [Google Scholar] [CrossRef]
- Joko, T.; Shiraishi, A.; Akune, Y.; Tokumaru, S.; Kobayashi, T.; Miyata, K.; Ohashi, Y. Involvement of P38MAPK in human corneal endothelial cell migration induced by TGF-β(2). Exp. Eye Res. 2013, 108, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Chen, H.C.; Chen, S.Y.; Tseng, S.C.G. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J. Cell Sci. 2012, 125, 3636–3648. [Google Scholar] [CrossRef]
- Li, C.; Dong, F.; Jia, Y.N.; Du, H.; Dong, N.; Xu, Y.; Wang, S.; Wu, H.; Liu, Z.; Li, W. Notch signal regulates corneal endothelial-to-mesenchymal transition. Am. J. Pathol. 2013, 183, 786–795. [Google Scholar] [CrossRef]
- Kawai, M.; Inoue, T.; Inatani, M.; Tsuboi, N.; Shobayashi, K.; Matsukawa, A.; Yoshida, A.; Tanihara, H. Elevated levels of monocyte chemoattractant protein-1 in the aqueous humor after phacoemulsification. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7951–7960. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de la Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Sun, H.M.; Yuan, J.Q. Extracellular matrix production of lens epithelial cells. J. Cataract Refract. Surg. 2001, 27, 1303–1309. [Google Scholar] [CrossRef]
- Okumura, N.; Kay, E.P.; Nakahara, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Inhibition of TGF-β signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS ONE 2013, 8, e58000. [Google Scholar] [CrossRef]
- Matthaei, M.; Hu, J.; Kallay, L.; Eberhart, C.G.; Cursiefen, C.; Qian, J.; Lackner, E.-M.; Jun, A.S. Endothelial cell microRNA expression in human late-onset Fuchs dystrophy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 216–225. [Google Scholar] [CrossRef]
- Matthaei, M.; Zhu, A.Y.; Kallay, L.; Eberhart, C.G.; Cursiefen, C.; Jun, A.S. Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy. Exp. Eye Res. 2014, 129, 13–17. [Google Scholar] [CrossRef]
- De Roo, A.K.; Struyf, S.; Foets, B.; van den Oord, J.J. Transforming Growth Factor Beta Switch in Aqueous Humor of Patients with Fuchs’ Endothelial Corneal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, M.; Gillessen, J.; Muether, P.S.; Hoerster, R.; Bachmann, B.O.; Hueber, A.; Cursiefen, C.; Heindl, L.M. Epithelial-Mesenchymal Transition (EMT)-Related Cytokines in the Aqueous Humor of Phakic and Pseudophakic Fuchs’ Dystrophy Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2749–2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reneker, L.W.; Bloch, A.; Xie, L.; Overbeek, P.A.; Ash, J.D. Induction of corneal myofibroblasts by lens-derived transforming growth factor beta1 (TGFbeta1): A transgenic mouse model. Brain Res. Bull. 2010, 81, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [CrossRef]
- Okumura, N.; Minamiyama, R.; Ho, L.T.; Kay, E.P.; Kawasaki, S.; Tourtas, T.; Schlötzer-Schrehardt, U.; Kruse, F.; Young, R.D.; Quantock, A.J.; et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Lab. Investig. 2015, 95, 1291–1304. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Chen, Y.; Liu, J.Y.; Lu, H.; Wang, W.; Lu, X.; Dean, K.C.; Gao, L.; Kaplan, H.J.; et al. Sphere-induced reprogramming of RPE cells into dual-potential RPE stem-like cells. EBioMedicine 2020, 52, 102618. [Google Scholar] [CrossRef]
- Gupta, R.; Kumawat, B.L.; Paliwal, P.; Tandon, R.; Sharma, N.; Sen, S.; Kashyap, S.; Nag, T.C.; Vajpayee, R.B.; Sharma, A. Association of ZEB1 and TCF4 rs613872 changes with late onset Fuchs endothelial corneal dystrophy in patients from northern India. Mol. Vis. 2015, 21, 1252–1260. [Google Scholar]
- Vandewalle, C.; Van Roy, F.; Berx, G. The role of the ZEB family of transcription factors in development and disease. Cell. Mol. Life Sci. 2009, 66, 773–787. [Google Scholar] [CrossRef]
- Lechner, J.; Dash, D.P.; Muszynska, D.; Hosseini, M.; Segev, F.; George, S.; Frazer, D.G.; Moore, J.E.; Kaye, S.B.; Young, T.; et al. Mutational spectrum of the ZEB1 gene in corneal dystrophies supports a genotype-phenotype correlation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3215–3223. [Google Scholar] [CrossRef]
- Mehta, J.S.; Vithana, E.N.; Tan, D.T.; Yong, V.H.K.; Yam, G.H.F.; Law, R.W.K.; Chong, W.G.W.; Pang, C.P.; Aung, T. Analysis of the posterior polymorphous corneal dystrophy 3 gene, TCF8, in late-onset Fuchs endothelial corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 184–188. [Google Scholar] [CrossRef]
- Sanchez-Tillo, E.; Liu, Y.; de Barrios, O.; Siles, L.; Fanlo, L.; Cuatrecasas, M.; Darling, D.S.; Dean, D.C.; Castells, A.; Postigo, A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell. Mol. Life Sci. 2012, 69, 3429–3456. [Google Scholar] [CrossRef] [PubMed]
- Yellore, V.S.; Rayner, S.A.; Nguyen, C.K.; Gangalum, R.K.; Jing, Z.; Bhat, S.P.; Aldave, A.J. Analysis of the role of ZEB1 in the pathogenesis of posterior polymorphous corneal dystrophy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Krafchak, C.M.; Pawar, H.; Moroi, S.E.; Sugar, A.; Lichter, P.R.; Mackey, D.A.; Mian, S.; Nairus, T.; Elner, V.; Schteingart, M.T.; et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am. J. Hum. Genet. 2005, 77, 694–708. [Google Scholar] [CrossRef] [Green Version]
- Aldave, A.J.; Bourla, N.; Yellore, V.S.; Rayner, S.A.; Khan, M.A.; Salem, A.K.; Sonmez, B. Keratoconus is not associated with mutations in COL8A1 and COL8A2. Cornea 2007, 26, 963–965. [Google Scholar] [CrossRef]
- Drewry, M.; Helwa, I.; Allingham, R.R.; Hauser, M.A.; Liu, Y. miRNA Profile in Three Different Normal Human Ocular Tissues by miRNA-Seq. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; Oliveira-Fernandes, M.; Lavker, R.M. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol. Vis. 2006, 12, 1175–1184. [Google Scholar]
- Abu-Amero, K.K.; Helwa, I.; Al-Muammar, A.; Strickland, S.; Hauser, M.A.; Allingham, R.R.; Liu, Y. Screening of the Seed Region of MIR184 in Keratoconus Patients from Saudi Arabia. BioMed Res. Int. 2015, 2015, 604508. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Karali, M.; Peluso, I.; Marigo, V.; Banfi, S. Identification and characterization of microRNAs expressed in the mouse eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 509–515. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Y.; Wang, Y.; Chen, P.; Yu, Y.; Song, Z. MicroRNA profile comparison of the corneal endothelia of young and old mice: Implications for senescence of the corneal endothelium. Mol. Vis. 2013, 19, 1815–1825. [Google Scholar]
- Bae, Y.; Hwang, J.S.; Shin, Y.J. miR-30c-1 encourages human corneal endothelial cells to regenerate through ameliorating senescence. Aging 2021, 13, 9348–9372. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Middleton, J.; Jeon, Y.J.; Magee, P.; Veneziano, D.; Lagana, A.; Leong, H.S.; Sahoo, S.; Fassan, M.; Booton, R.; et al. KRAS induces lung tumorigenesis through microRNAs modulation. Cell Death Dis. 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Peng, Y.; Hu, X.; Xu, J.; Zhu, S.; Yu, Z.; Han, S. miR-30c regulates proliferation, apoptosis and differentiation via the Shh signaling pathway in P19 cells. Exp. Mol. Med. 2016, 48, e248. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Bitar, M.S.; Funaki, T.; Azizi, B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am. J. Pathol. 2010, 177, 2278–2289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Handa, J.T.; Green, W.R.; Stark, W.J.; Weinberg, R.S.; Jun, A.S. Advanced glycation end products and receptors in Fuchs’ dystrophy corneas undergoing Descemet’s stripping with endothelial keratoplasty. Ophthalmology 2007, 114, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of oxidative stress in human corneal diseases. J. Histochem. Cytochem. 2002, 50, 341–351. [Google Scholar] [CrossRef]
- Li, X.; Karki, P.; Lei, L.; Wang, H.; Fliegel, L. Na+/H+ exchanger isoform 1 facilitates cardiomyocyte embryonic stem cell differentiation. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H159–H170. [Google Scholar] [CrossRef]
- Hamuro, J.; Asada, K.; Ueno, M.; Yamashita, T.; Mukai, A.; Fujita, T.; Ito, E.; Hiramoto, N.; Toda, M.; Sotozono, C.; et al. Repressed miR-34a Expression Dictates the Cell Fate to Corneal Endothelium Failure. Investig. Ophthalmol. Vis. Sci. 2022, 63, 22. [Google Scholar] [CrossRef]
- Ueno, M.; Asada, K.; Toda, M.; Nagata, K.; Sotozono, C.; Kosaka, N.; Ochiya, T.; Kinoshita, S.; Hamuro, J. Concomitant evaluation of a panel of exosome proteins and MiRs for qualification of cultured human cornea endothelial cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4393–4402. [Google Scholar] [CrossRef]
- Kriegel, A.J.; Liu, Y.; Fang, Y.; Ding, X.; Liang, M. The miR-29 family: Genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol. Genom. 2012, 44, 237–244. [Google Scholar] [CrossRef]
- Villarreal, G., Jr.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, J.D.; Zhang, C.; Sundin, O.H.; Bell, W.R.; Stark, W.J.; Green, W.R. Fuchs corneal dystrophy: Aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Komers, R.; Carew, R.; Winbanks, C.E.; Xu, B.; Herman-Edelstein, M.; Koh, P.; Thomas, M.; Jandeleit-Dahm, K.; Gregorevic, P.; et al. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Okumura, N.; Ueno, M.; Nakagawa, H.; Hamuro, J.; Kinoshita, S. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea 2013, 32, 1167–1170. [Google Scholar] [CrossRef]
- Iliff, B.W.; Riazuddin, S.A.; Gottsch, J.D. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Investig. Ophthalmol. Vis. Sci. 2012, 53, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Akpek, E.K.; Jun, A.S.; Goodman, D.F.; Green, W.R.; Gottsch, J.D. Clinical and ultrastructural features of a novel hereditary anterior segment dysgenesis. Ophthalmology 2002, 109, 513–519. [Google Scholar] [CrossRef]
- Jun, A.S.; Broman, K.W.; Do, D.V.; Akpek, E.K.; Stark, W.J.; Gottsch, J.D. Endothelial dystrophy, iris hypoplasia, congenital cataract, and stromal thinning (EDICT) syndrome maps to chromosome 15q22.1–q25.3. Am. J. Ophthalmol. 2002, 134, 172–176. [Google Scholar] [CrossRef]
- Hughes, A.E.; Bradley, D.T.; Campbell, M.; Lechner, J.; Dash, D.P.; Simpson, D.A.; Willoughby, C.E. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 2011, 89, 628–633. [Google Scholar] [CrossRef]
- Graff, J.W.; Powers, L.S.; Dickson, A.M.; Kim, J.; Reisetter, A.C.; Hassan, I.H.; Kremens, K.; Gross, T.J.; Wilson, M.E.; Monick, M.M. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS ONE 2012, 7, e44066. [Google Scholar] [CrossRef]
- Schembri, F.; Sridhar, S.; Perdomo, C.; Gustafson, A.M.; Zhang, X.; Ergun, A.; Lu, J.; Liu, G.; Zhang, X.; Bowers, J.; et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 2319–2324. [Google Scholar] [CrossRef]
- Izzotti, A.; Calin, G.A.; Arrigo, P.; Steele, V.E.; Croce, C.M.; De Flora, S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2009, 23, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Chung, A.C.; Huang, X.R.; Meng, X.M.; Hui, D.S.; Yu, C.M.; Sung, J.J.; Lan, H.Y. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J. Am. Soc. Nephrol. 2011, 22, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, C.; Lin, X.; Li, J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie 2013, 95, 1355–1359. [Google Scholar] [CrossRef]
- Roderburg, C.; Urban, G.W.; Bettermann, K.; Vucur, M.; Zimmermann, H.; Schmidt, S.; Janssen, J.; Koppe, C.; Knolle, P.; Castoldi, M.; et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 2011, 53, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pandit, K.V.; Milosevic, J.; Kaminski, N. MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 2011, 157, 191–199. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef]
- Li, N.; Cui, J.; Duan, X.; Chen, H.; Fan, F. Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway in human Tenon’s fibroblasts. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1670–1678. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.R.; Qiu, J.M.; Epstein, D.L.; Gonzalez, P. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3567–3572. [Google Scholar] [CrossRef]
- Dunmire, J.J.; Lagouros, E.; Bouhenni, R.A.; Jones, M.; Edward, D.P. MicroRNA in aqueous humor from patients with cataract. Exp. Eye Res. 2013, 108, 68–71. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuda, S.; Kunikata, H.; Sato, J.; Kokubun, T.; Yasuda, M.; Nishiguchi, K.M.; Inada, T.; Nakazawa, T. Profiles of extracellular miRNAs in the aqueous humor of glaucoma patients assessed with a microarray system. Sci. Rep. 2014, 4, 5089. [Google Scholar] [CrossRef]
- Kosaka, N.; Izumi, H.; Sekine, K.; Ochiya, T. microRNA as a new immune-regulatory agent in breast milk. Silence 2010, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Peplow, P.V. MicroRNAs as biomarkers in glaucoma and potential therapeutic targets. Neural Regen. Res. 2022, 17, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Wecker, T.; Hoffmeier, K.; Plötner, A.; Grüning, B.A.; Horres, R.; Backofen, R.; Reinhard, T.; Schlunck, G. MicroRNA Profiling in Aqueous Humor of Individual Human Eyes by Next-Generation Sequencing. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.R.; Segu, Z.M.; Price, M.O.; Lai, X.; Witzmann, F.A.; Mechref, Y.; Yoder, M.C.; Price, F.W. Alterations in the aqueous humor proteome in patients with Fuchs endothelial corneal dystrophy. Mol. Vis. 2010, 16, 2376–2383. [Google Scholar]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp. Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; O’Brien, K.; Kelleher, F.C.; Corcoran, C.; Germano, S.; Radomski, M.W.; Crown, J.; O’Driscoll, L. Isolation of exosomes for subsequent mRNA, MicroRNA, and protein profiling. Methods Mol. Biol. 2011, 784, 181–195. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Conde-Vancells, J.; Falcon-Perez, J.M. Isolation of urinary exosomes from animal models to unravel noninvasive disease biomarkers. Methods Mol. Biol. 2012, 909, 321–340. [Google Scholar] [CrossRef]
- Pavlič, A.; Hauptman, N.; Boštjančič, E.; Zidar, N. Long Non-Coding RNAs as Potential Regulators of EMT-Related Transcription Factors in Colorectal Cancer—A Systematic Review and Bioinformatics Analysis. Cancers 2022, 14, 2280. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Luo, H.; Chen, L.; Li, J.; Zhu, X.; Huang, K. Identification of an epithelial-mesenchymal transition related long non-coding RNA (LncRNA) signature in Glioma. Bioengineered 2021, 12, 4016–4031. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Qiu, J.; Hua, K. Long non-coding RNAs as emerging regulators of epithelial to mesenchymal transition in gynecologic cancers. Biosci. Trends 2018, 12, 342–353. [Google Scholar] [CrossRef]
- Cao, M.X.; Jiang, Y.P.; Tang, Y.L.; Liang, X.H. The crosstalk between lncRNA and microRNA in cancer metastasis: Orchestrating the epithelial-mesenchymal plasticity. Oncotarget 2017, 8, 12472–12483. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.J.; Liu, J.Y.; Zhang, Y.Y.; Li, X.M.; Wang, L.N.; Yao, J.; Jiang, Q.; Yan, B. Identification of corneal neovascularization-related long noncoding RNAs through microarray analysis. Cornea 2015, 34, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.H.; Lv, Y.; Wang, W.Q.; Sun, G.L.; Zhang, H.H. LncRNA NEAT1 promotes inflammatory response and induces corneal neovascularization. J. Mol. Endocrinol. 2018, 61, 231–239. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stunf Pukl, S. MicroRNA of Epithelial to Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy. Genes 2022, 13, 1711. https://doi.org/10.3390/genes13101711
Stunf Pukl S. MicroRNA of Epithelial to Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy. Genes. 2022; 13(10):1711. https://doi.org/10.3390/genes13101711
Chicago/Turabian StyleStunf Pukl, Spela. 2022. "MicroRNA of Epithelial to Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy" Genes 13, no. 10: 1711. https://doi.org/10.3390/genes13101711
APA StyleStunf Pukl, S. (2022). MicroRNA of Epithelial to Mesenchymal Transition in Fuchs’ Endothelial Corneal Dystrophy. Genes, 13(10), 1711. https://doi.org/10.3390/genes13101711




