Molecular Evolution and Inheritance Pattern of Sox Gene Family among Bovidae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequence Retrieval and Multiple Sequence Alignment
2.2. Physicochemical Characterization of Sox Gene Family
2.3. Identification of Interacting Proteins, Functional Enrichment, and Pathway Analysis
2.4. Evolutionary Phylogenetic Analysis of Sox Gene Family
3. Results
3.1. Genome Wide Analysis of Sox Genes in Bovidae
3.2. Physicochemical Properties of Sox Genes
3.3. Evolutionary Dynamics and Phylogenetic Analysis
3.4. Conserved Motif Analysis of Sox Genes
3.5. Characterization of Functional Motifs
3.6. Biological, Molecular, and Cellular Function of Bos Sox Genes
3.7. Protein–Protein Interaction Cluster with Sox Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefebvre, V. Roles and regulation of SOX transcription factors in skeletogenesis. Curr. Top. Dev. Biol. 2019, 133, 171–193. [Google Scholar] [CrossRef]
- Stevanovic, M.; Drakulic, D.; Lazic, A.; Ninkovic, D.S.; Schwirtlich, M.; Mojsin, M. SOX Transcription Factors as Important Regulators of Neuronal and Glial Differentiation During Nervous System Development and Adult Neurogenesis. Front. Mol. Neurosci. 2021, 31, 654031. [Google Scholar] [CrossRef]
- Dy, P.; Penzo-Mendez, A.; Wang, H.; Pedraza, C.E.; Macklin, W.B.; Lefebvre, V. The three SoxC proteins–Sox4, Sox11 an Sox12–exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008, 36, 3101–3117. [Google Scholar] [CrossRef] [Green Version]
- Schepers, G.E.; Teasdale, R.D.; Koopman, P. Twenty pairs of Sox: Extent, homology, and nomenclature of the mouse and human Sox transcription factor gene families. Dev. Cell 2002, 3, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, H.; Kamachi, Y. SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int. J. Biochem. Cell Biol. 2010, 42, 391–399. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 2016, 17, 183–193. [Google Scholar] [CrossRef]
- Smith, A.N.; Miller, L.A.; Radice, G.; Ashery-Padan, R.; Lang, R.A. Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development 2009, 136, 2977–2985. [Google Scholar] [CrossRef] [Green Version]
- Ekonomou, A.; Kazanis, I.; Malas, S.; Wood, H.; Alifragis, P.; Denaxa, M.; Karagogeos, D.; Constanti, A.; Lovell-Badge, R.; Episkopou, V. Neuronal migration and ventral subtype identity in the telencephalon depend on SOX1. PloS Biol. 2005, 3, e186-10. [Google Scholar] [CrossRef] [PubMed]
- Rizzoti, K.; Brunelli, S.; Carmignac, D.; Thomas, P.Q.; Robinson, I.C.; Lovell-Badge, R. SOX3 is required during the formation of the hypothalamopituitary axis. Nat. Genet. 2004, 36, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Smits, P.; Li, P.; Mandel, J.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B.; Lefebvre, V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev. Cell 2001, 1, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, D.; Gardner, J.; Chambers, D.; Hosking, B.; Peters, J.; Muscat, G.; Abbott, C.; Koopman, P. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. Genet. 2000, 24, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, Y.; Kondoh, H. Sox proteins: Regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144. [Google Scholar] [CrossRef] [Green Version]
- Barrionuevo, F.; Naumann, A.; Bagheri-Fam, S.; Speth, V.; Taketo, M.M.; Scherer, G.; Neubüser, A. Sox9 is required for invagination of the otic placode in mice. Dev. Biol. 2008, 317, 213–224. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, Y.; Hara, K.; Kanai-Azuma, M.; Matsui, T.; Miura, Y.; Tsunekawa, N.; Kurohmaru, M.; Saijoh, Y.; Koopman, P.; Kanai, Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem. Biophys. Res. Commun. 2007, 360, 539–544. [Google Scholar] [CrossRef]
- François, M.; Caprini, A.; Hosking, B.; Orsenigo, F.; Wilhelm, D.; Browne, C.; Paavonen, K.; Karnezis, T.; Shayan, R.; Downes, M.; et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 2008, 456, 643–647. [Google Scholar] [CrossRef]
- Kanai-Azuma, M.; Kanai, Y.; Gad, J.M.; Tajima, Y.; Taya, C.; Kurohmaru, M.; Sanai, Y.; Yonekawa, H.; Yazaki, K.; Tam, P.P.; et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 2002, 129, 2367–2379. [Google Scholar] [CrossRef]
- Sock, E.; Rettig, S.D.; Enderich, J.; Bosl, M.R.; Tamm, E.R.; Wegner, M. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol. Cell. Biol. 2004, 24, 6635–6644. [Google Scholar] [CrossRef] [Green Version]
- Matthee, C.A.; Davis, S.K. Molecular Insights into the Evolution of the Family Bovidae: A Nuclear DNA Perspective. Mol. Biol. Evol. 2001, 18, 1220–1230. [Google Scholar] [CrossRef]
- Escudeiro, A.; Ferreira, D.; Mendes-da-Silva, A.; Heslop-Harrison, J.; Adega, F.; Chaves, R. Bovine satellite DNAs–a history of the evolution of complexity and its impact in the Bovidae family. Eur. Zool. J. 2019, 86, 20–37. [Google Scholar] [CrossRef]
- Hepp, D.; Gonçalves, G.L.; de Freitas, T.R. Prediction of the damage-associated non-synonymous single nucleotide polymorphisms in the human MC1R gene. PLoS ONE 2015, 10, e0121812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morenikeji, O.B.; Thomas, B.N. In silico analyses of CD14 molecule reveal significant evolutionary diversity, potentially associated with speciation and variable immune response in mammals. PeerJ 2019, 7, e7325. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; De Kumar, B.; Paulson, A.; Parrish, M.E.; Zhang, Y.; Florens, L.; Conaway, J.W.; Si, K.; Krumlauf, R. A six-amino-acid motif is a major determinant in functional evolution of HOX1 proteins. Genes Dev. 2020, 34, 1680–1696. [Google Scholar] [CrossRef] [PubMed]
- Soremekun, O.S.; Omolabi, K.F.; Soliman, M.E.S. Identification and classification of differentially expressed genes reveal potential molecular signature associated with SARS-CoV-2 infection in lung adenocarcinomal cells. Inform. Med. Unlocked 2020, 20, 100384. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ciccarelli, F.D.; Doerks, T.; von Mering, C.; Creevey, C.J.; Snel, B.; Bork, P. Toward automatic reconstruction of a highly resolved tree of life. Science 2006, 311, 1283–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef]
- Jiang, L.; Bi, D.; Ding, H.; Wu, X.; Zhu, R.; Zeng, J.; Yang, X.; Kan, X. Systematic Identification and Evolution Analysis of Sox Genes in Coturnix japonica Based on Comparative Genomics. Genes 2019, 10, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Wang, B.; Du, H. A review on Sox genes in fish. Rev. Aquac. 2021, 13, 1986–2003. [Google Scholar] [CrossRef]
- Heenan, P.; Zondag, L.; Wilson, M.J. Evolution of the Sox gene family within the chordate phylum. Gene 2016, 575, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.A.; Rubenstein, E. Proline: The distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS ONE 2013, 8, e53785. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chen, X.; Wang, M.; Zhang, W.; Pan, J.; Qin, Q.; Zhong, L.; Shao, J.; Sun, M.; Jiang, H.; et al. Genome-wide identification, phylogeny and expressional profile of the Sox gene family in channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. 2018, 28, 17–26. [Google Scholar] [CrossRef]
- Roy, S.; Maheshwari, N.; Chauhan, R.; Sen, N.K.; Sharma, A. Structure prediction and functional characterization of secondary metabolite proteins of Ocimum. Bioinformation 2011, 6, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Kadel, S.; Persico, M.; Thibodeau, J.; Lainé, C.; Bazinet, L. Use of redundancy analysis and multivariate regression models to select the significant membrane properties affecting peptide migration during electrodialysis with filtration membranes. Sep. Purif. Technol. 2019, 221, 114–125. [Google Scholar] [CrossRef]
- Larney, C.; Bailey, T.L.; Koopman, P. Switching on sex: Transcriptional regulation of the testis-determining gene Sry. Development 2014, 141, 2195–2205. [Google Scholar] [CrossRef] [Green Version]
- Herpin, A.; Schart, M. Plasticity of gene-regulatory networks controlling sex determination: Of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015, 16, 1260–1274. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Liu, S.; Gao, X.; Jiang, Y.; Perera, D.; Wang, X.; Li, C.; Sun, L.; Zhang, J.; Kaltenboeck, L. Male-biased genes in catfish as revealed by RNA-Seq analysis of the testis transcriptome. PLoS ONE 2013, 8, e68452. [Google Scholar] [CrossRef]
- Wilhelm, D.; Palmer, S.; Koopman, P. Sex determination and gonadal development in mammals. Physiol. Rev 2007, 87, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, S.; Silva Casey, E.; Bell, D.; Harland, R.; Lovell-Badge, R. The expression of Sox3 throughout the developing central nervous system is dependent on the combined action of discrete, evolutionarily conserved regulatory elements. Genesis 2003, 36, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Meeks, J.J.; Hurley, L.; Raverot, G.; Frassetto, A.; Jameson, J.L. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol. Cell. Biol. 2003, 23, 8084–8091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrides, N.; Panayiotou, E.; Fanis, P.; Karaiskos, C.; Lapathitis, G.; Malas, S. Sequential Role of SOXB2 Factors in GABAergic Neuron Specification of the Dorsal Midbrain. Front. Mol. Neurosci. 2018, 11, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Lefebvre, V. L-Sox5 and Sox6 drive expression of the aggrecan gene in cartilage by securing binding of Sox9 to a far-upstream enhancer. Mol. Cell. Biol. 2008, 28, 4999–5013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.F.; Lefebvre, V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015, 43, 8183–8203. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, A.; Lefebvre, V. The SOXE transcription factors-SOX8, SOX9 and SOX10-share a bi-partite transactivation mechanism. Nucleic Acids Res. 2019, 47, 6917–6931. [Google Scholar] [CrossRef]
- Frietze, S.; Farnham, P.J. Transcription factor effector domains. Sub. Cell Biochem. 2011, 52, 261–277. [Google Scholar]
- Akiyama, H.; Lyons, J.P.; Mori-Akiyama, Y.; Yang, X.; Zhang, R.; Zhang, Z.; Deng, J.M.; Taketo, M.M.; Nakamura, T.; Behringer, R.R.; et al. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kim, I.K.; Yang, J.M.; Lee, E.; Koh, B.I.; Song, S.; Park, J.; Lee, S.; Choi, C.; Kim, J.W.; et al. SoxF Transcription Factors Are Positive Feedback Regulators of VEGF Signaling. Circ. Res. 2016, 119, 839–852. [Google Scholar] [CrossRef] [Green Version]
- Ito, M. Function and molecular evolution of mammalian Sox15, a singleton in the SoxG group of transcription factors. Int. J. Biochem. Cell Biol. 2010, 42, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.C.; Soufi, A.; Pollard, S.M. Post-translational modification of SOX family proteins: Key biochemical targets in cancer? Semin. Cancer Biol. 2020, 67, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Ichisaka, T.; Nakagawa, M.; Yamanaka, S. Differential roles for Sox15 and Sox2 in transcriptional control in mouse embryonic stem cells. J. Biol. Chem. 2005, 280, 24371–24379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, H.; Nakamura, A.; Urata, M.; Shirae-Kurabayashi, M.; Kuraku, S.; Russell, S.; Ohtsuka, S. The evolutionally conserved function of group B1 Sox family members confers the unique role of Sox2 in mouse ES cells. BMC Evol. Biol. 2016, 16, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienz, M. β-catenin: A pivot between cell adhesion and Wnt signaling. Curr. Biol. 2005, 127, 2061–2069. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Zhang, C.; Li, X.; Jia, C.; Chen, L.; Yuan, Y.; Gao, Q.; Lu, Z.; Feng, Y.; Zhao, R.; et al. LEF1 Enhances the Progression of Colonic Adenocarcinoma via Remodeling the Cell Motility Associated Structures. Int. J. Mol. Sci. 2021, 22, 10870. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Chen, F.; Milewski, R.; Li, J.; Lu, M.; Epstein, J.A. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J. Clin. Invest. 2000, 106, 963–971. [Google Scholar] [CrossRef]
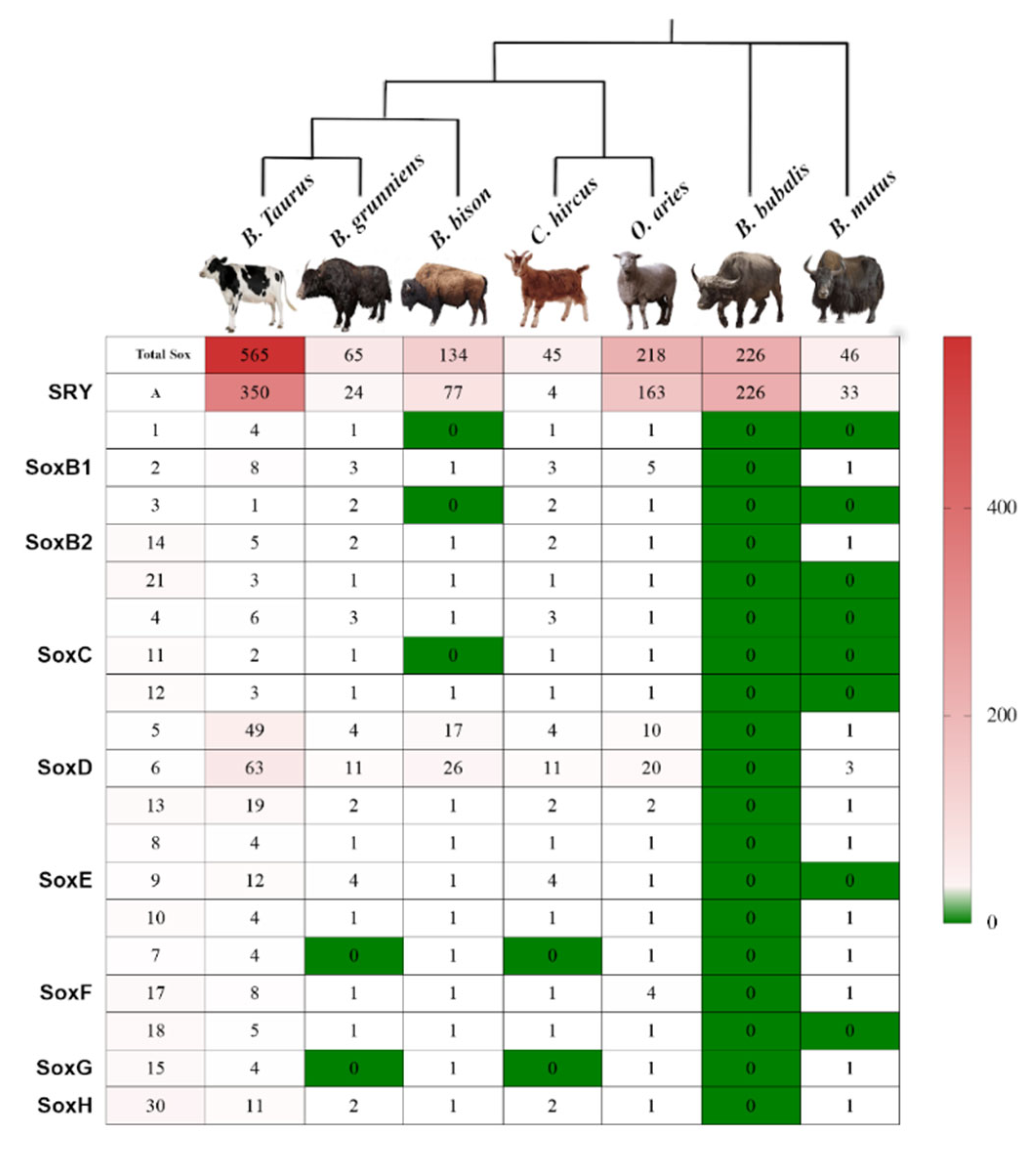


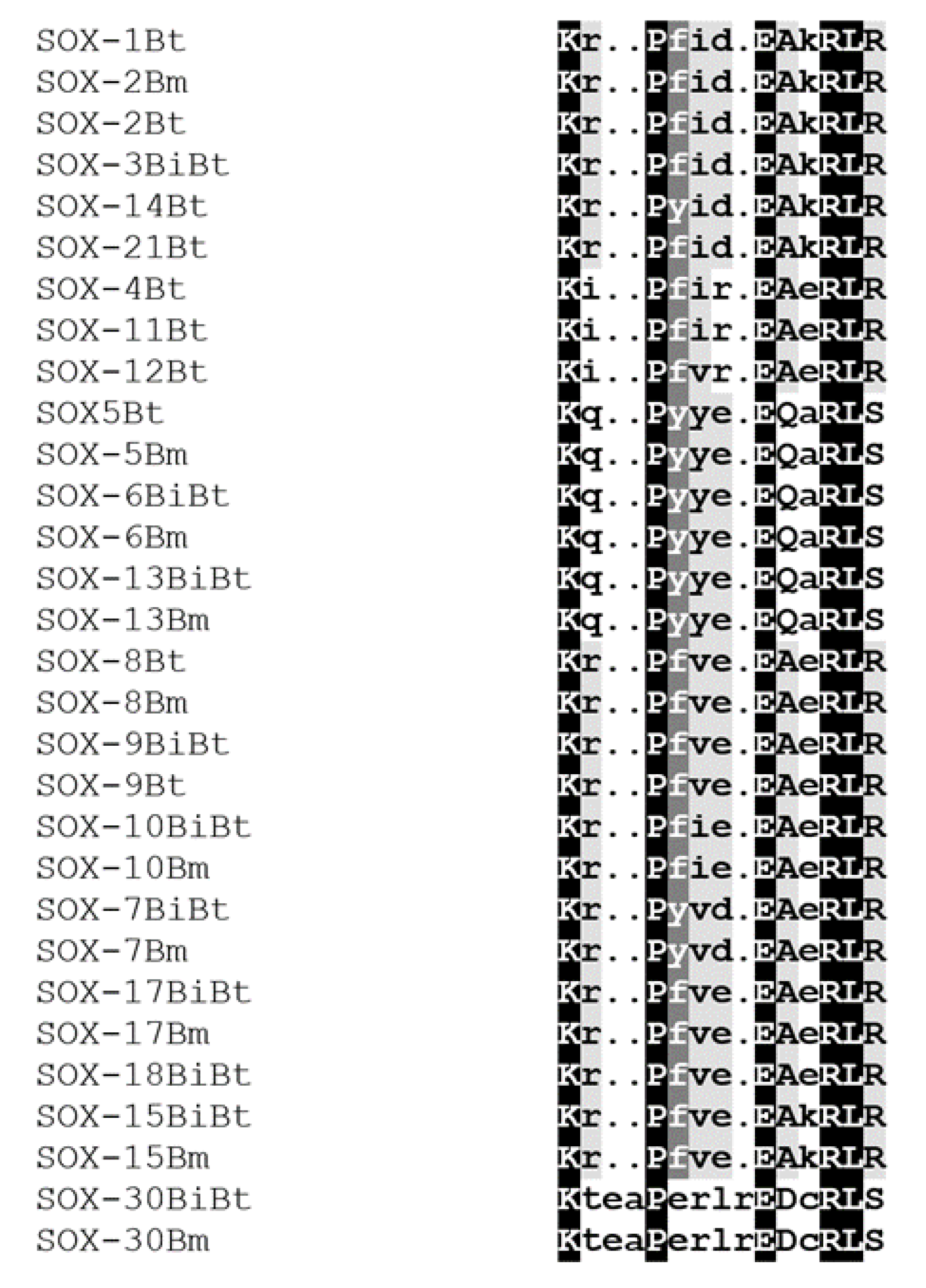

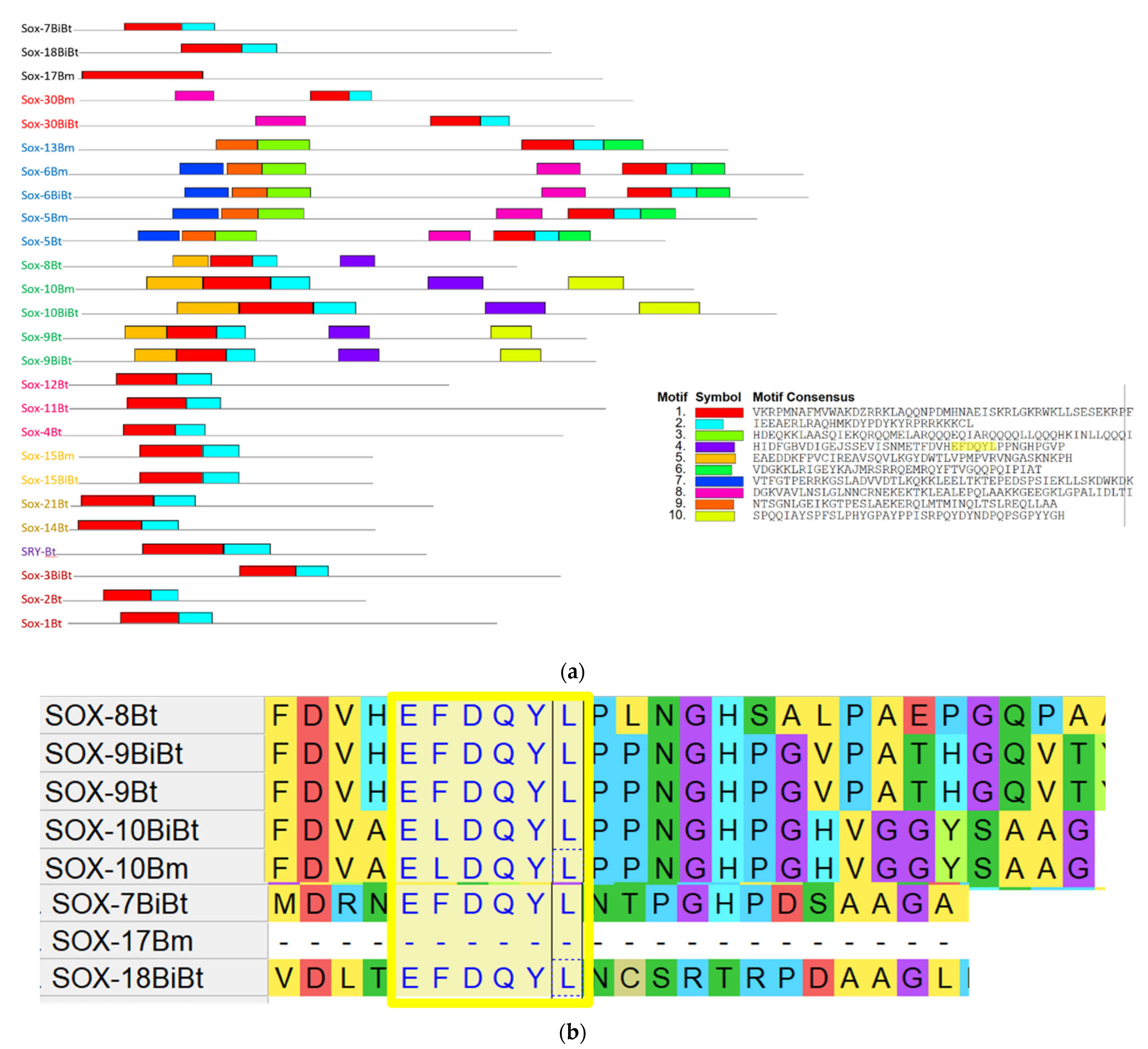
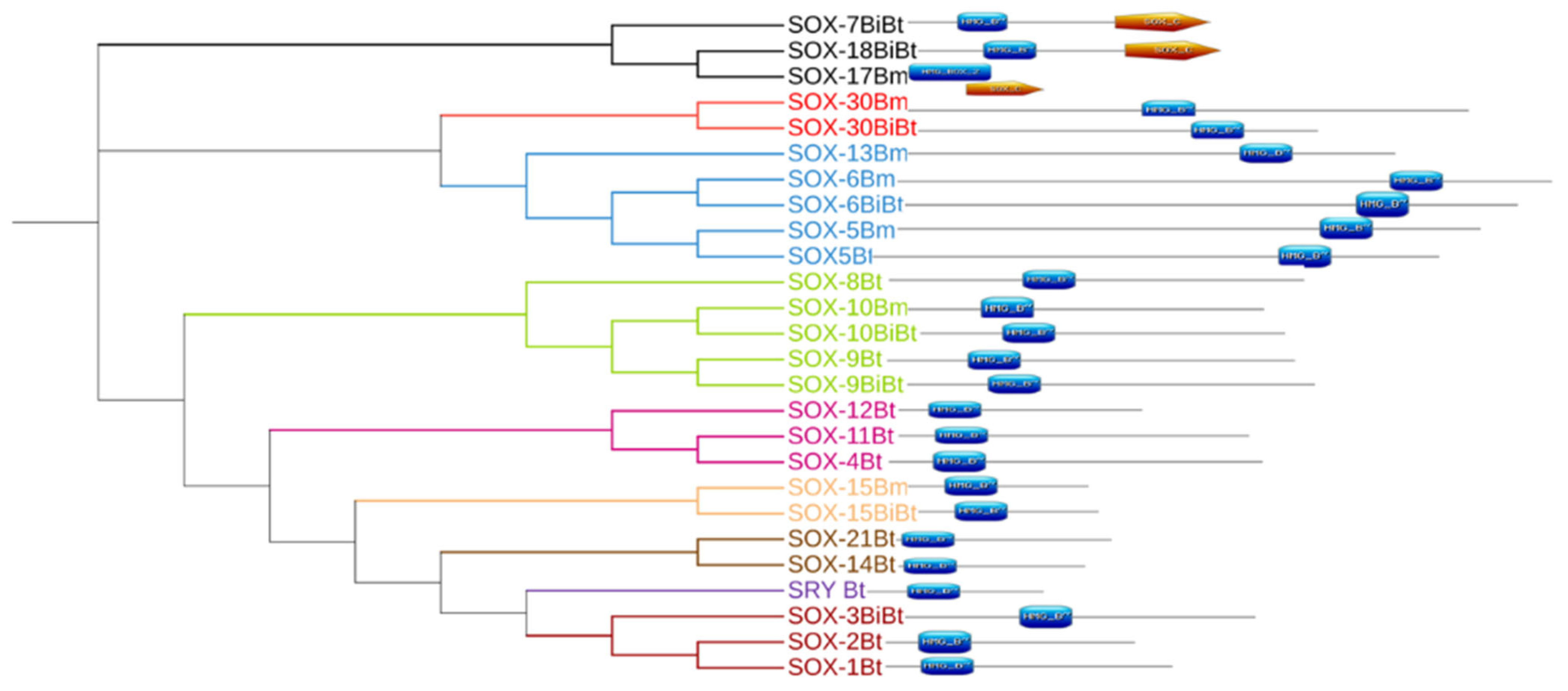


| Gene Symbol | Size | MW (Da) | pI | Chromosome | AI | II | GRAVY | Extinction Coefficient | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Accession Number | ||||||||||
| SoxA | SRY | ABY19364.1 | 229 | 26,627.11 | 9.53 | Y | 62.62 | 60.71 | −0.858 | 30,285 |
| SoxB1 | Sox1 | XP_005214070.1 | 369 | * | * | 1 | 44.44 | 41.96 | −0.472 | 35,870 |
| Sox2 | NP_001098933.1 | 320 | 34,480.95 | 9.74 | 1 | 48.25 | 59.43 | −0.739 | 37,360 | |
| Sox3 | XP_027390716.1 | 451 | 45,113.00 | 9.78 | X | 61.18 | 69.35 | −0.314 | 38,850 | |
| SoxB2 | Sox14 | NP_001157253.1 | 240 | 26,485.39 | 9.68 | 1 | 63.17 | 53.51 | −0.585 | 33,015 |
| Sox21 | XP_024855919.1 | 277 | 28,666.92 | 9.74 | 12 | 69.13 | 58.51 | −0.208 | 31,525 | |
| SoxC | Sox4 | NP_001071596.1 | 481 | 47,785.11 | 7.20 | 23 | 54.22 | 58.41 | −0.478 | 35,660 |
| Sox11 | XP_024855327.1 | 451 | 47,147.03 | 4.95 | 11 | 58.16 | 65.27 | −0.652 | 37,150 | |
| Sox12 | XP_010809935.2 | 314 | 33,997.76 | 5.14 | 13 | 49.87 | 67.74 | −0.982 | 40,575 | |
| SoxD | Sox5 | AAI14659.1 | 728 | 80,127.28 | 6.13 | 5 | 69.48 | 63.71 | −0.746 | 43,445 |
| Sox6 | XP_027419183.1 | 858 | 95,541.56 | 7.93 | 15 | 64.52 | 61.11 | −0.794 | 64,790 | |
| Sox13 | XP_027419995.1 | 623 | 69,061.04 | 6.14 | 16 | 71.88 | 69.67 | −0.742 | 41,870 | |
| SoxE | Sox8 | XP_002698019.3 | 534 | 56,239.99 | 7.77 | 25 | 52.25 | 59.49 | −0.708 | 63,175 |
| Sox9 | XP_027374751.1 | 524 | 57,173.49 | 6.31 | 19 | 46.98 | 80.78 | −0.984 | 55,935 | |
| Sox10 | XP_027396627.1 | 469 | 50,020.20 | 6.19 | 5 | 53.30 | 58.52 | −0.822 | 54,445 | |
| SoxF | Sox7 | XP_027404803.1 | 534 | 57,216.30 | 9.62 | 8 | 55.66 | 63.44 | −0.834 | 58,830 |
| Sox17 | XP_027416795.1 | 410 | 43,060.76 | 5.91 | 14 | 60.63 | 65.47 | −0.509 | 39,225 | |
| Sox18 | XP_027415027.1 | 389 | 41,337.00 | 8.42 | 13 | 62.96 | 78.07 | −0.573 | 40,255 | |
| SoxG | Sox15 | XP_027374313.1 | 233 | 25,140.18 | 9.85 | 19 | 49.06 | 69.49 | −0.875 | 38,515 |
| SoxH | Sox30 | XP_027401619.1 | 757 | 82,746.76 | 8.62 | 7 | 70.01 | 68.76 | −0.603 | 48,415 |
| SRY | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOX-1Bt | 50 | 100 | |||||||||||||||||
| SOX-2Bt | 51.80 | 74.05 | 100 | ||||||||||||||||
| SOX-3BiBt | 51.80 | 68.62 | 68.20 | 100 | |||||||||||||||
| SOX-14Bt | 47.59 | 46.42 | 43.75 | 47.76 | 100 | ||||||||||||||
| SOX-21Bt | 42.77 | 45.88 | 44.35 | 43.92 | 71.42 | 100 | |||||||||||||
| SOX-4Bt | 36.14 | 33.20 | 34.30 | 32.54 | 34.37 | 37.25 | 100 | ||||||||||||
| SOX-11Bt | 36.74 | 31.12 | 33.05 | 32.54 | 34.82 | 32.15 | 54.47 | 100 | |||||||||||
| SOX-12Bt | 30.72 | 38.46 | 38.46 | 41.75 | 34.61 | 38.46 | 59.89 | 56.04 | 100 | ||||||||||
| SOX5Bt | 16.86 | 14.12 | 12.13 | 13.33 | 12.05 | 12.54 | 12.87 | 13.22 | 16.48 | 100 | |||||||||
| SOX-5Bm | 16.86 | 14.12 | 12.13 | 13.33 | 12.05 | 12.54 | 12.83 | 13.22 | 16.48 | 100 | 100 | ||||||||
| SOX-6BiBt | 19.27 | 14.88 | 14.22 | 13.72 | 15.62 | 11.37 | 12.35 | 14.78 | 15.38 | 81.43 | 81.13 | 100 | |||||||
| SOX-6Bm | 19.27 | 14.88 | 14.22 | 13.72 | 15.62 | 11.37 | 12.35 | 14.78 | 15.38 | 81.43 | 81.13 | 100 | 100 | ||||||
| SOX-13Bm | 15.66 | 13.33 | 12.97 | 14.50 | 14.73 | 13.33 | 13.72 | 11.76 | 14.83 | 70.58 | 70.58 | 65.49 | 65.49 | 100 | |||||
| SOX-8Bt | 37.34 | 30.15 | 33.05 | 30.19 | 35.71 | 32.15 | 31.32 | 32.68 | 39.01 | 12.50 | 12.45 | 12.45 | 12.45 | 12.54 | 100 | ||||
| SOX-9BiBt | 39.75 | 27.86 | 32.21 | 29.01 | 33.48 | 28.62 | 27.27 | 29.57 | 39.56 | 11.36 | 11.36 | 11.36 | 11.36 | 12.54 | 62.50 | 100 | |||
| SOX-9Bt | 39.75 | 27.86 | 32.21 | 29.01 | 33.48 | 28.62 | 27.27 | 29.57 | 39.56 | 11.36 | 11.36 | 11.36 | 11.36 | 12.54 | 62.50 | 100 | 100 | ||
| SOX-10BiBt | 36.74 | 28.73 | 33.05 | 29.80 | 34.82 | 30.58 | 30.26 | 33.07 | 39.56 | 12.64 | 12.64 | 12.64 | 12.64 | 11.37 | 59.77 | 72.03 | 72.03 | 100 | |
| SOX-10Bm | 36.74 | 28.73 | 33.05 | 29.80 | 34.82 | 30.58 | 30.26 | 33.07 | 39.56 | 12.64 | 12.64 | 12.64 | 12.64 | 11.37 | 59.77 | 72.03 | 72.03 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyemi, M.O.; Finucan, J.; Grytsay, A.; Osaiyuwu, O.H.; Adegbaju, M.S.; Ogunade, I.M.; Thomas, B.N.; Peters, S.O.; Morenikeji, O.B. Molecular Evolution and Inheritance Pattern of Sox Gene Family among Bovidae. Genes 2022, 13, 1783. https://doi.org/10.3390/genes13101783
Akinyemi MO, Finucan J, Grytsay A, Osaiyuwu OH, Adegbaju MS, Ogunade IM, Thomas BN, Peters SO, Morenikeji OB. Molecular Evolution and Inheritance Pattern of Sox Gene Family among Bovidae. Genes. 2022; 13(10):1783. https://doi.org/10.3390/genes13101783
Chicago/Turabian StyleAkinyemi, Mabel O., Jessica Finucan, Anastasia Grytsay, Osamede H. Osaiyuwu, Muyiwa S. Adegbaju, Ibukun M. Ogunade, Bolaji N. Thomas, Sunday O. Peters, and Olanrewaju B. Morenikeji. 2022. "Molecular Evolution and Inheritance Pattern of Sox Gene Family among Bovidae" Genes 13, no. 10: 1783. https://doi.org/10.3390/genes13101783







