Influence of Chinese Herbal Formula on Bone Characteristics of Cobb Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimentation Ethical Statement
2.2. Chicken, Diet and Housing
2.3. Extraction of Chinese Herbal Formula
2.4. Feeding Experiment and Slaughter Sampling
2.5. Determination Index and Method
2.5.1. Live Weight Determination
2.5.2. Bone Strength Determination
2.5.3. Analysis of Bone Tissue Microstructure
2.5.4. Determination of Blood Biochemical Indicators
2.5.5. RNA Preparation and RT-qPCR
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Comparative Analysis of Live Weight and Survival Rate
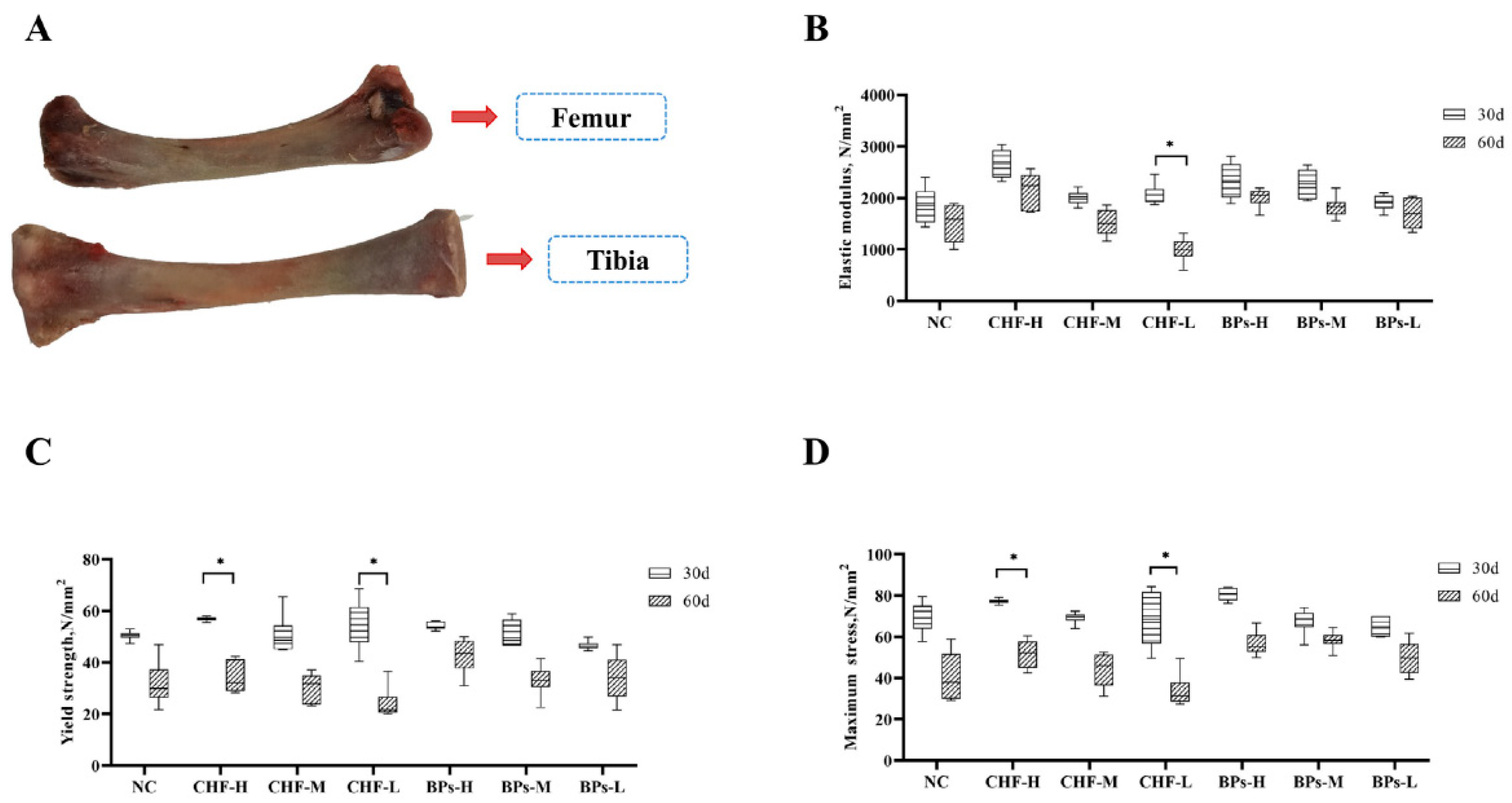
3.2. Comparative Analysis of Bone Strength Indexes
3.2.1. Comparative Analysis of Modulus of Elasticity
3.2.2. Comparative Analysis of Yield Strength
3.2.3. Comparison and Analysis of Maximum Stress
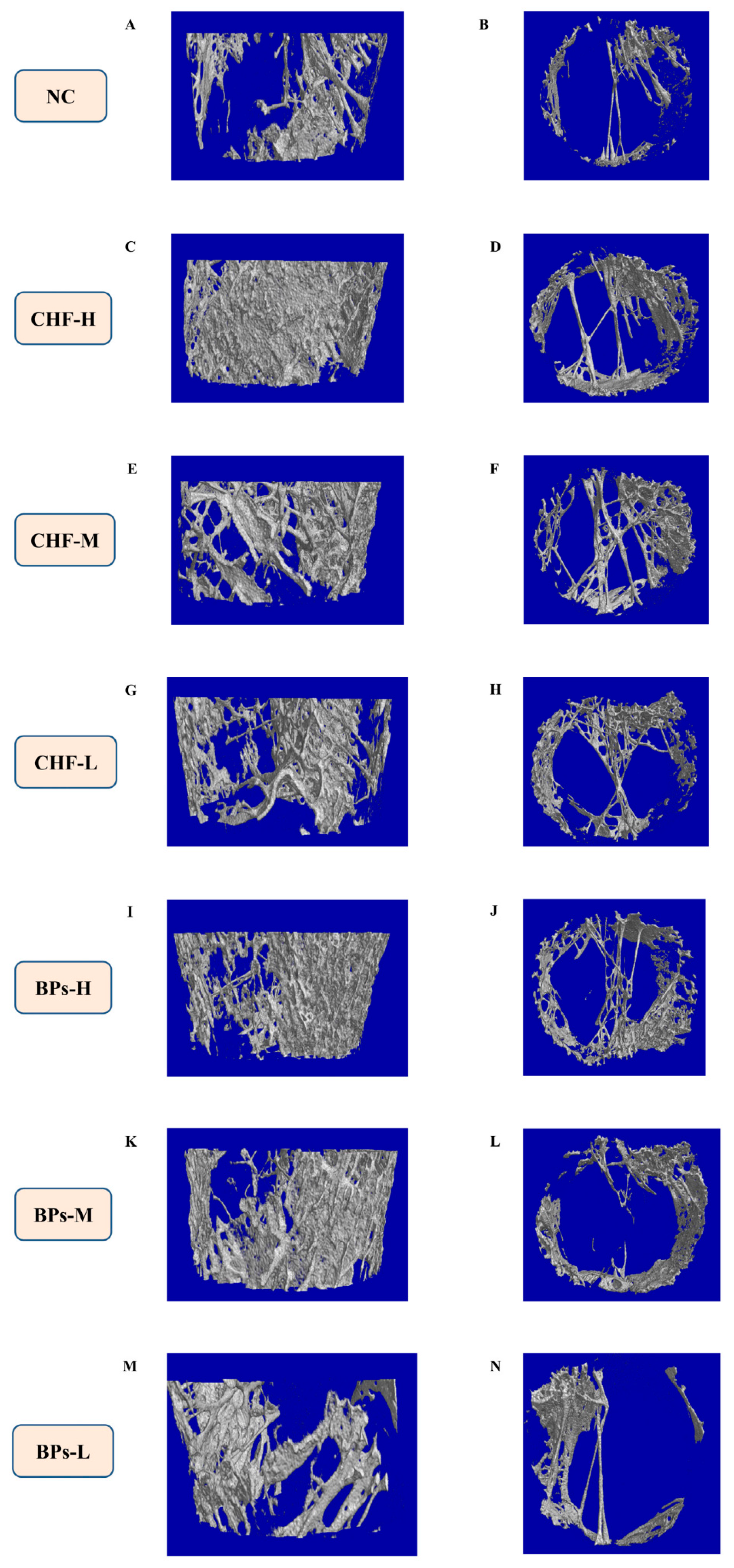
3.3. Comparative Analysis of the Microstructure of Bone Tissue
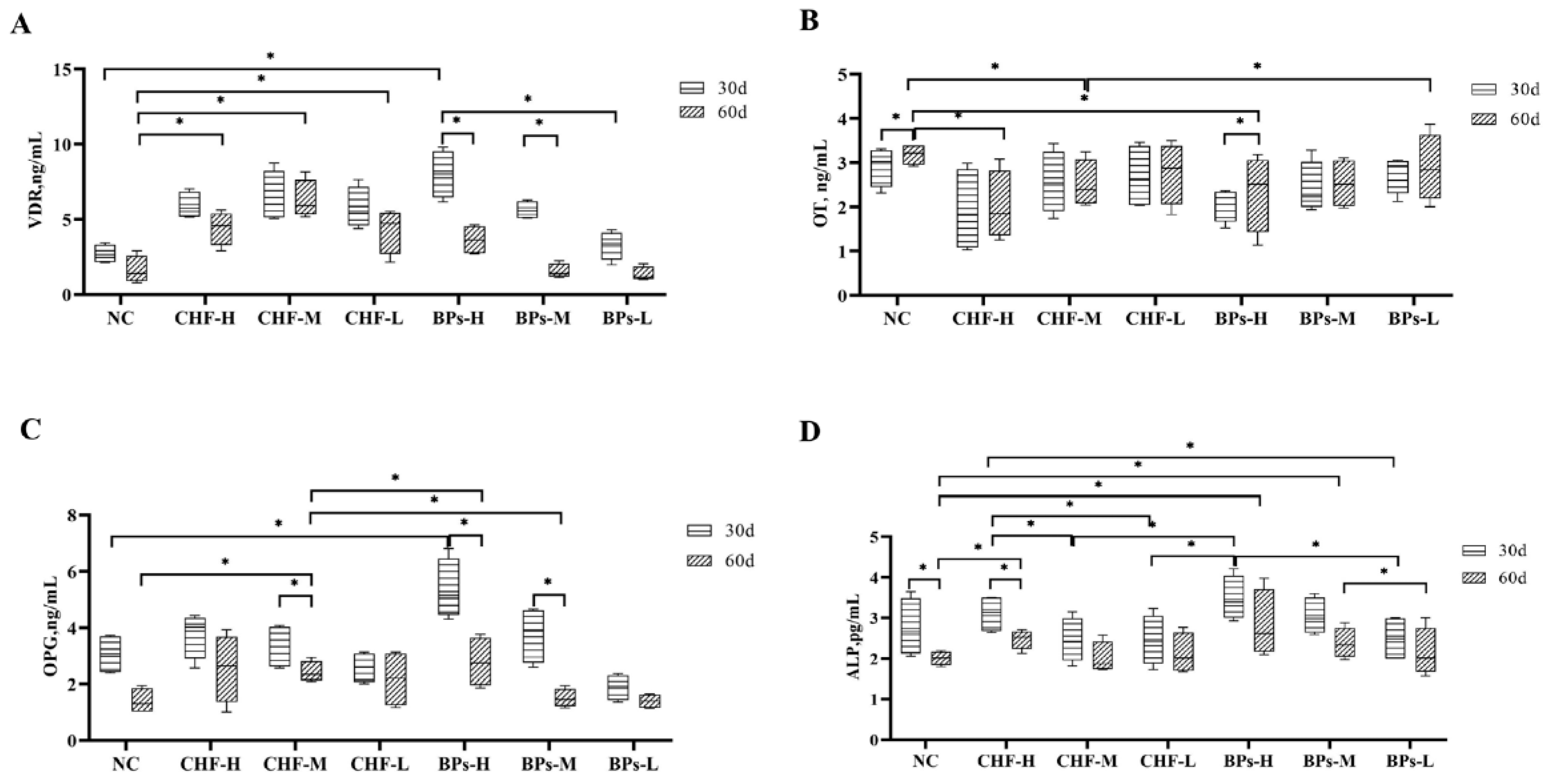
3.4. Comparative Analysis of Blood Biochemical Indicators
3.4.1. Comparative Analysis of VDR Content
3.4.2. Comparative Analysis of OT Content
3.4.3. Comparative Analysis of OPG Content
3.4.4. Comparative Analysis of ALP Content
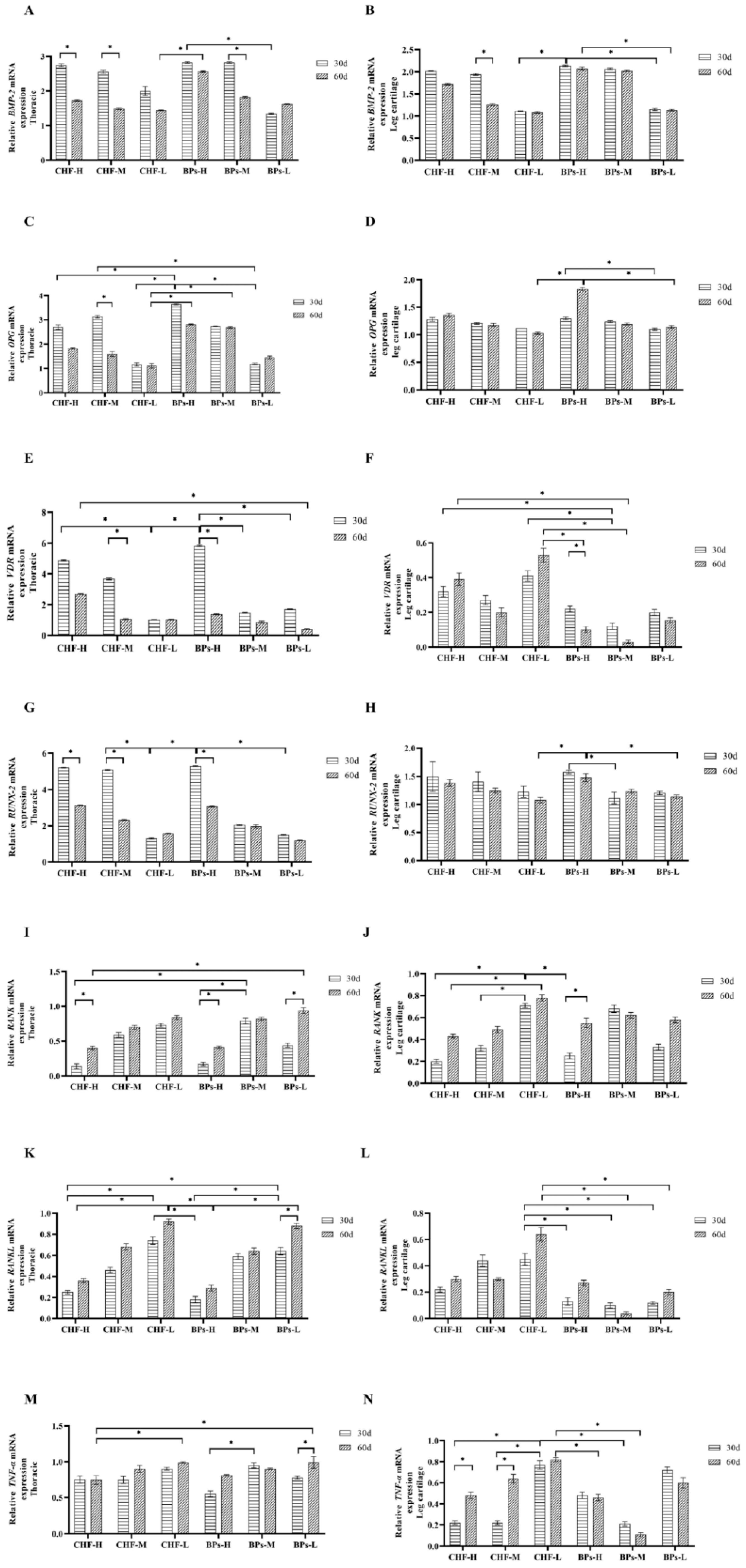
3.5. Comparative Analysis of Gene Expression Levels Related to Bone Metabolism
3.5.1. Comparative Analysis of Relative Expression Levels of BMP-2
3.5.2. Comparative Analysis of Relative Expression Levels of OPG
3.5.3. Comparative Analysis of Relative Expression Levels of VDR
3.5.4. Comparative Analysis of the Relative Expression of Runx-2
3.5.5. Comparative Analysis of Relative Expression Levels of RANK
3.5.6. Comparative Analysis of Relative Expression Levels of RANKL
3.5.7. Comparative Analysis of Relative Expression Levels of TNF-α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burr, D.B. Fifty years of bisphosphonates: What are their mechanical effects on bone? Bone 2020, 138, 115518. [Google Scholar] [CrossRef]
- Wang, S.J.; Yue, W.; Rahman, K.; Xin, H.L.; Zhang, Q.Y.; Qin, L.P.; Zhang, H. Mechanism of Treatment of Kidney Deficiency and Osteoporosis is Similar by Traditional Chinese Medicine. Curr. Pharm. Des. 2016, 22, 312–320. [Google Scholar] [CrossRef]
- Xie, Q.F.; Xie, J.H.; Dong, T.T.; Su, J.Y.; Cai, D.K.; Chen, J.P.; Liu, L.F.; Li, Y.C.; Lai, X.P.; Tsim, K.W.; et al. Effect of a derived herbal recipe from an ancient Chinese formula, Danggui Buxue Tang, on ovariectomized rats. J. Ethnopharmacol. 2012, 144, 567–575. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, E.Y.; Lee, B.; Min, J.H.; Song, D.U.; Lim, J.M.; Eom, J.W.; Yeom, M.; Jung, H.S. The effects of Lycii Radicis Cortex on RANKL-induced osteoclast differentiation and activation in RAW 264.7 cells. Int. J. Mol. Med. 2016, 37, 649–658. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, R.; Deng, H.; Wang, G.; Zhao, J.; Yang, H.; Ning, N.; Ma, J.; Zhou, J. Yam-Containing Serum Promotes Proliferation and Chondrogenic Differentiation of Rabbit Bone Marrow Mesenchymal Stem Cells and Synthesis of Glycosaminoglycan. Pharmacology 2020, 105, 377–385. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Shen, Q. Salvianolic acid B promotes bone formation by increasing activity of alkaline phosphatase in a rat tibia fracture model: A pilot study. BMC Complement. Altern. Med. 2014, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, H.; Kim, M.H.; Yang, W.M. Inhibition of RANKL-stimulated osteoclast differentiation by Schisandra chinensis through down-regulation of NFATc1 and c-fos expression. BMC Complement. Altern. Med. 2018, 18, 270. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Leung, W.N.; Li, G.; Kong, S.K.; Lu, X.; Wong, Y.M.; Chan, C.W. Osthole Enhances Osteogenesis in Osteoblasts by Elevating Transcription Factor Osterix via cAMP/CREB Signaling In Vitro and In Vivo. Nutrients 2017, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Huang, J.; Guo, H.; Song, X.; Li, J.; Shi, Y.; Xie, X. A randomized controlled study for Yuanhu Zhitong dropping pills in the treatment of knee osteoarthritis. Medicine 2020, 99, e20666. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Jung, S.K.; Chang, Y.H.; Kwak, H.S. Highly bioavailable nanocalcium from oyster shell for preventing osteoporosis in rats. Int. J. Food Sci. Nutr. 2017, 68, 931–940. [Google Scholar] [CrossRef]
- Yin, M.Y.; Liu, S.X.; Zhang, T.j.; Chen, C.Q. Overview of extracting technique of compound Chinese materia medica. Chin. Tradit. Herb. Drugs 2015, 46, 3279–3283. [Google Scholar]
- Bose, P.C.; Sen, T.C. Ultrasonic extraction of alkaloids from rauvolfia serpentina roots. Indian J. Pharm. 1961, 23, 222–223. [Google Scholar]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental Programming of Fetal Growth and Development. Veter. Clin. North Am. Food Anim. Pract. 2019, 35, 229–247. [Google Scholar]
- Brickett, K.E.; Dahiya, J.P.; Classen, H.L.; Annett, C.B.; Gomis, S. The impact of nutrient density, feed form, and photoperiod on the walking ability and skeletal quality of broiler chickens. Poult. Sci. 2007, 86, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar] [PubMed]
- Jensen, V.F.H.; Mølck, A.M.; Dalgaard, M.; McGuigan, F.E.; Akesson, K.E. Changes in bone mass associated with obesity and weight loss in humans: Applicability of animal models. Bone 2021, 145, 115781. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Fuggle, N.R.; Cooper, C.; Harvey, N.C. Epigenetic regulation of bone mass. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101612. [Google Scholar] [CrossRef]
- Viero, A.; Biehler-Gomez, L.; Messina, C.; Cappella, A.; Giannoukos, K.; Viel, G.; Tagliaro, F.; Cattaneo, C. Utility of micro-CT for dating post-cranial fractures of known post-traumatic ages through 3D measurements of the trabecular inner morphology. Sci. Rep. 2022, 12, 10543. [Google Scholar] [CrossRef]
- Boyde, A. Scanning Electron Microscopy of Bone. Methods Mol. Biol. 2019, 1914, 571–616. [Google Scholar]
- Chai, S.; Wan, L.; Wang, J.L.; Huang, J.C.; Huang, H.X. Gushukang inhibits osteocyte apoptosis and enhances BMP-2/Smads signaling pathway in ovariectomized rats. Phytomedicine 2019, 64, 153063. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martín, S.; López-Peña, M.; Muñoz, F.; Permuy, M.; González-Cantalapiedra, A. Bisphosphonates as disease-modifying drugs in osteoarthritis preclinical studies: A systematic review from 2000 to 2020. Arthritis Res. Ther. 2021, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhong, Q.; Wang, J.; Wang, M.; Fang, F.; Xia, Z.; Zhong, R.; Huang, H.; Ke, Z.; Wei, Y.; et al. Corrigendum: Beneficial Effects and Toxicity Studies of Xian-ling-gu-bao on Bone Metabolism in Ovariectomized Rats. Front. Pharmacol. 2020, 11, 570876. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, J.; Zhang, J.; Qin, R.; Wang, R.; Qiu, Y.; Mao, Z.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis. Aging Cell. 2020, 19, 13095. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Horibe, K.; Mizoguchi, T.; Yamamoto, Y.; Nakamura, T.; Hosoya, A.; Kato, S.; Suda, T.; Takahashi, N. VDR in Osteoblast-Lineage Cells Primarily Mediates Vitamin D Treatment-Induced Increase in Bone Mass by Suppressing Bone Resorption. J. Bone Miner. Res. 2017, 32, 1297–1308. [Google Scholar]
- Naik, S.; Sahu, S.; Bandyopadhyay, D.; Tripathy, S. Serum levels of osteoprotegerin, RANK-L & vitamin D in different stages of osteoarthritis of the knee. Indian J. Med. Res. 2021, 154, 491–496. [Google Scholar] [PubMed]
- Wang, S.; Xu, S.; Shi, Z.; Wu, J.; Lei, S.; Wang, Y. Progress of research on the relationship between calcitonin gene-related peptide and RANK/RANKL/OPG system in the bone reconstruction. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 4, 511–515. [Google Scholar]
- Misof, B.M.; Blouin, S.; Roschger, P.; Werzowa, J.; Klaushofer, K.; Lehmann, G. Bone matrix mineralization and osteocyte lacunae characteristics in patients with chronic kidney disease—Mineral bone disorder (CKD-MBD). J. Musculoskelet. Neuronal Interact. 2019, 19, 196–206. [Google Scholar]
- Bennett, B.J.; Scatena, M.; Kirk, E.A.; Rattazzi, M.; Varon, R.M.; Averill, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Osteoprotegerin inactivation accelerates advanced atherosclerot-ic lesion progression and calcification in older ApoE-/-mice. Arterioscler Thromb. Vasc. Biol. 2006, 26, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, A.; Balawender, K. Structural and Metabolic Changes in Bone. Animals 2022, 12, 1946. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 5, 144855. [Google Scholar] [CrossRef]
- Nguyen, V.; Meyers, C.A.; Yan, N.; Agarwal, S.; Levi, B.; James, A.W. BMP-2-induced bone for-mation and neural inflammation. J. Orthop. 2017, 14, 252–256. [Google Scholar] [CrossRef]
- Raval, P.; Hsu, H.H.; Schneider, D.J.; Sarras, M.P.; Masuhara, K.; Bonewald, L.F.; Anderson, H.C. Expression of bone morphogenetic proteins by osteoinductive and non-osteoinductive human osteosarcoma cells. J. Dent. Res. 1996, 75, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Chugh, R.M.; Park, H.S.; Esfandyari, S.; Elsharoud, A.; Ulin, M.; Al-Hendy, A. Mesenchymal Stem Cell-Conditioned Media Regulate Steroidogenesis and Inhibit Androgen Secretion in a PCOS Cell Model via BMP-2. Int. J. Mol. Sci. 2021, 22, 9184. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Liang, Z.; Zhou, M.; Chen, C. Comparative Clinical Effectiveness and Safety of Bone Morphogenetic Protein Versus Autologous Iliac Crest Bone Graft in Lumbar Fusion: A Metaanalysis and Systematic Review. Spine 2020, 45, E729–E741. [Google Scholar] [CrossRef] [PubMed]
- Primadhi, R.A.; Gunawan, H.; Rachmayati, S.; Rasyid, H.N. Autologous osteophyte grafting for ankle arthrodesis. SICOT J. 2022, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Y.; Liu, Z.; Wang, P. OPG is Required for the Postnatal Maintenance of Condylar Cartilage. Calcif. Tissue Int. 2019, 104, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, J.; Chen, D.; Weng, Y.; Du, H.; Sun, Y.; Zhang, Q. Osteoprotegerin deficiency leads to deformation of the articular cartilage in femoral head. J. Mol. Histol. 2016, 47, 475–483. [Google Scholar] [CrossRef]
- Nakamura, M.; Udagawa, N.; Matsuura, S.; Mogi, M.; Nakamura, H.; Horiuchi, H.; Saito, N.; Hiraoka, B.Y.; Kobayashi, Y.; Takaoka, K.; et al. Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology 2003, 144, 5441–5449. [Google Scholar] [CrossRef]
- Makris, K.; Bhattoa, H.P.; Cavalier, E.; Phinney, K.; Sempos, C.T.; Ulmer, C.Z.; Vasikaran, S.D.; Vesper, H.; Heijboer, A.C. Recommendations on the measurement and the clinical use of vitamin D metabolites and vitamin D binding protein—A position paper from the IFCC Committee on bone metabolism. Clin. Chim Acta 2021, 517, 171–197. [Google Scholar] [CrossRef]
- Tsuprykov, O.; Elitok, S.; Buse, C.; Chu, C.; Krämer, B.K.; Hocher, B. Opposite correlation of 25-hydroxy-vitamin D- and 1,25-dihydroxy-vitamin D-metabolites with gestational age, bone- and lipid-biomarkers in pregnant women. Sci. Rep. 2021, 11, 1923. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Xu, H.; Ning, Y.; Fang, W.; Liao, W.; Zou, J.; Yang, Y.; Shao, N. Effects of altered CXCL12/CXCR4 axis on BMP2/Smad/Runx2/Osterix axis and osteogenic gene expressions during osteogenic differentiation of MSCs. Am. J. Transl. Res. 2017, 9, 1680–1693. [Google Scholar]
- Hou, J.; Su, H.; Kuang, X.; Qin, W.; Liu, K.; Pan, K.; Zhang, B.; Yang, S.; Yang, S.; Peng, X.; et al. Knowledge Domains and Emerging Trends of Osteoblasts-Osteoclasts in Bone Disease From 2002 to 2021: A Bibliometrics Analysis and Visualization Study. Front. Endocrinol. 2022, 13, 922070. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Ruilian, Z.; Ying, G.; Hongmei, S.; Lihua, G. Exploration of the Effect of Icariin on Nude Mice with Lung Cancer Bone Metastasis via the OPG/RANKL/RANK System. Comput. Math. Methods Med. 2022, 28, 2011625. [Google Scholar] [CrossRef] [PubMed]
- Deligiorgi, M.V.; Panayiotidis, M.I.; Griniatsos, J.; Trafalis, D.T. Harnessing the versatile role of OPG in bone oncology: Counterbalancing RANKL and TRAIL signaling and beyond. Clin. Exp. Metastasis 2020, 37, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.K.; Wong, C.H.; Cui, C.; Li, M.M.; Wong, R.M.Y.; Cheung, W.H. Modulating macrophage polarization for the enhancement of fracture healing, a systematic review. J. Orthop. Translat. 2022, 36, 83–90. [Google Scholar] [CrossRef]
- Zhou, H.; Dong, Y.; Alhaskawi, A.; Lai, J.; Wang, Z.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.; Lu, H. The Roles of TNF Signaling Pathways in Metabolism of Bone Tumors. Front. Pharmacol. 2022, 13, 907629. [Google Scholar] [CrossRef]
- Han, Y.; Pei, D.; Li, W.; Luo, B.; Jiang, Q. Epigallocatechin gallate attenuates tumor necrosis factor (TNF)-α-induced inhibition of osteoblastic differentiation by up-regulating lncRNA TUG1 in os-teoporosis. Bioengineered 2022, 13, 8950–8961. [Google Scholar] [CrossRef]
- Ingwersen, L.C.; Frank, M.; Naujokat, H.; Loger, K.; Bader, R.; Jonitz-Heincke, A. BMP-2 Long-Term Stimulation of Human Pre-Osteoblasts Induces Osteogenic Differentiation and Promotes Transdifferentiation and Bone Remodeling Processes. Int. J. Mol. Sci. 2022, 23, 3077. [Google Scholar] [CrossRef] [PubMed]

| Compositions of Diets % | Chick Feed | Flocks of Adult Feed |
|---|---|---|
| Corn | 64.70 | 60.90 |
| Soy protein | 30.2 | 25.1 |
| Wheat bran | 0.00 | 10.00 |
| Soybean oil | 1.10 | 0.00 |
| Calcium hydrogen phosphate | 1.50 | 1.50 |
| Stone meal | 0.70 | 0.60 |
| Middling flour | 0.41 | 0.46 |
| Met | 0.08 | 0.07 |
| Salt | 0.35 | 0.35 |
| Minerals and vitamins | 1.00 | 1.00 |
| Total | 100 | 100 |
| Nutrients levels | ||
| Metabolism energy (Kcal·kg−1) | 2900 | 2860 |
| Crude protein | 20.00 | 19.50 |
| Calcium | 0.90 | 0.80 |
| Phosphorus | 0.37 | 0.37 |
| Lys | 0.98 | 0.75 |
| Met | 0.39 | 0.32 |
| Age (Day) | Vaccine | Way |
|---|---|---|
| 1 | Marek’s disease | Subcutaneous injection |
| 3 | Newcastle | Oral vaccination |
| 12 | Gumboro | Subcutaneous injection |
| 20 | Newcastle | Oral vaccination |
| 42 | Fowl cholera | Subcutaneous injection |
| Group | Medicines | Dosage |
|---|---|---|
| NC | NA | NA |
| CHF-H | CHF | 0.6% |
| CHF-M | 0.4% | |
| CHF-L | 0.2% | |
| BPs-H | BPS | 0.6% |
| BPs-M | 0.4% | |
| BPs-L | 0.2% |
| Item | NC | CHF-H | CHF-M | CHF-L | BPs-H | BPs-M | BPs-L |
|---|---|---|---|---|---|---|---|
| 30d | 941.91 abc ±70.02 | 1012.42 ab ±140.81 | 1058.02 a ±122.14 | 982.25 abc ±84.91 | 936.82 abc ±9.15 | 895.55 bc ±60.81 | 852.91 c ±14.79 |
| 60d | 2603.56 a ±105.94 | 2620.85 a ±282.32 | 2612.83 a ±180.23 | 2612.14 a ±178.73 | 2358.85 b ±97.68 | 2364.74 b ±113.95 | 2251.21 b ±247.58 |
| Survival rate | 93.50% | 98.00% | 97.40% | 96.00% | 86.40% | 88.00% | 89.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liang, S.; Zi, X.; Yan, S.; Liu, M.; Li, M.; Zhao, Y.; Dou, T.; Ge, C.; Wang, K.; et al. Influence of Chinese Herbal Formula on Bone Characteristics of Cobb Broiler Chickens. Genes 2022, 13, 1865. https://doi.org/10.3390/genes13101865
Liu Y, Liang S, Zi X, Yan S, Liu M, Li M, Zhao Y, Dou T, Ge C, Wang K, et al. Influence of Chinese Herbal Formula on Bone Characteristics of Cobb Broiler Chickens. Genes. 2022; 13(10):1865. https://doi.org/10.3390/genes13101865
Chicago/Turabian StyleLiu, Yong, Shuangmin Liang, Xiannian Zi, Shixiong Yan, Mengqian Liu, Mengyuan Li, Yanhao Zhao, Tengfei Dou, Changrong Ge, Kun Wang, and et al. 2022. "Influence of Chinese Herbal Formula on Bone Characteristics of Cobb Broiler Chickens" Genes 13, no. 10: 1865. https://doi.org/10.3390/genes13101865
APA StyleLiu, Y., Liang, S., Zi, X., Yan, S., Liu, M., Li, M., Zhao, Y., Dou, T., Ge, C., Wang, K., & Jia, J. (2022). Influence of Chinese Herbal Formula on Bone Characteristics of Cobb Broiler Chickens. Genes, 13(10), 1865. https://doi.org/10.3390/genes13101865





