HPRT1 Most Suitable Reference Gene for Accurate Normalization of mRNA Expression in Canine Dermal Tissues with Radiation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Animals
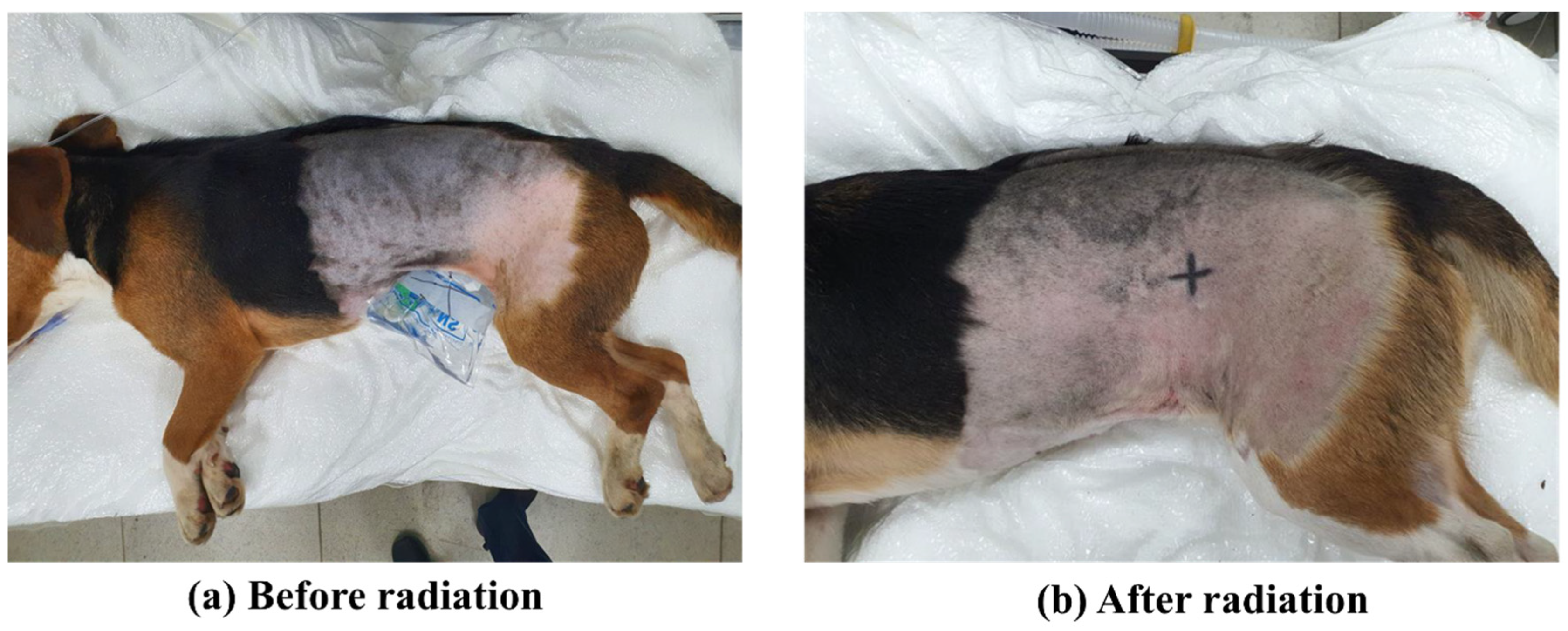
2.2. Expose Irradiation and Collect Sample
2.3. Candidate Reference Gene and Primer
2.4. RNA Isolation, cDNA Synthesis, and qRT-PCR
2.5. Determination of Stable Reference Gene Expression
2.6. The Use of Different Reference Gene in the Normalization of Gene of Interest
2.7. Statistical Analysis
3. Results
3.1. Exposure to Radiation in Healthy Canine Skin
3.2. Evaluation of Amplicon Size, Ct Values, and Primer Efficiency of Selected Reference Genes
3.3. Analysis of the Most Stable Reference Gene by geNorm
3.4. Analysis of the Most Stable Reference Gene by Normfinder
3.5. Analysis of the Most Stable Reference Gene by Bestkeeper
3.6. Use of Most Stable Reference Genes for Normalization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTB | β-actin |
| CV | Coefficient variance |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GOI | Gene of interest |
| GUSB | β-glucuronidase |
| HPRT1 | Hypoxanthine phosphoribosyl transferase 1 |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RPL4 | Ribosomal protein 4 |
| RPS5 | Ribosomal protein S5 |
| SD | Standard deviation |
| TBP | TATA box binding protein |
| VBA | Visual Basic application |
| YWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide |
References
- Bloomfield, R. Stereotactic radiation therapy in veterinary medicine. Can. Veter. J. 2015, 56, 95. [Google Scholar]
- Gieger, T.L.; Nolan, M.W. Linac-based stereotactic radiation therapy for canine non-lymphomatous nasal tumours: 29 cases (2013–2016). Veter. Comp. Oncol. 2018, 16, E68–E75. [Google Scholar] [CrossRef] [Green Version]
- Griffin, L.R.; Nolan, M.W.; Selmic, L.E.; Randall, E.; Custis, J.; LaRue, S. Stereotactic radiation therapy for treatment of canine intracranial meningiomas. Veter. Comp. Oncol. 2016, 14, e158–e170. [Google Scholar] [CrossRef] [PubMed]
- Swift, K.E.; LaRue, S.M. Outcome of 9 dogs treated with stereotactic radiation therapy for primary or metastatic vertebral osteosarcoma. Veter. Comp. Oncol. 2018, 16, E152–E158. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.N.; Simmons, B.J.; Wolfson, A.H.; Nouri, K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol. Ther. 2016, 6, 185–206. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Asaad, N.; Held, K.D. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene 2005, 24, 2096–2103. [Google Scholar] [CrossRef] [Green Version]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Dulić, V.; Kaufmann, W.K.; Wilson, S.J.; Tisty, T.D.; Lees, E.; Harper, J.W.; Elledge, S.J.; Reed, S.I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 1994, 76, 1013–1023. [Google Scholar] [CrossRef]
- Coleman, M.A.; Yin, E.; Peterson, L.E.; Nelson, D.; Sorensen, K.; Tucker, J.D.; Wyrobek, A.J. Low-dose irradiation alters the transcript profiles of human lymphoblastoid cells including genes associated with cytogenetic radioadaptive response. Radiat. Res. 2005, 164, 369–382. [Google Scholar] [CrossRef]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Warrington, J.A.; Nair, A.; Mahadevappa, M.; Tsyganskaya, M. Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol. Genom. 2000, 2, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thellin, O.; Zorzi, W.; Lakaye, B.; De Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef]
- Ropenga, A.; Chapel, A.; Vandamme, M.; Griffiths, N.M. Use of reference gene expression in rat distal colon after radiation exposure: A caveat. Radiat. Res. 2004, 161, 597–602. [Google Scholar] [CrossRef]
- Brinkhof, B.; Spee, B.; Rothuizen, J.; Penning, J.C. Development and evaluation of canine reference genes for accurate quantification of gene expression. Anal. Biochem. 2006, 356, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Schlotter, Y.M.; Veenhof, E.Z.; Brinkhof, B.; Rutten, V.P.M.G.; Spee, B.; Willemse, T.; Penning, L.C. A GeNorm algorithm-based selection of reference genes for quantitative real-time PCR in skin biopsies of healthy dogs and dogs with atopic dermatitis. Veter. Immunol. Immunopathol. 2009, 15, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Iyer, G.; Wang, A.R.; Brennan, S.R.; Bourgeois, S.; Armstrong, E.; Shah, P.; Harari, P.M. Identification of stable housekeeping genes in response to ionizing radiation in cancer research. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, D.A.; Morley, M.; Shin, E.; Spielman, R.S.; Cheung, V.G. Genetic analysis of radiation-induced changes in human gene expression. Nature 2009, 459, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Jeon, R.H.; Jang, S.J.; Park, J.S.; Lee, S.C.; Baregundi Subbarao, R.; Lee, S.L.; Park, B.W.; King, W.A.; Rho, G.J. Selection of reference genes for quantitative gene expression in porcine mesenchymal stem cells derived from various sources along with differentiation into multilineages. Stem Cells Int. 2015, 2015, 235192. [Google Scholar] [CrossRef] [PubMed]
- Jeon, R.H.; Lee, W.J.; Son, Y.B.; Bharti, D.; Shivakumar, S.B.; Lee, S.L.; Rho, G.J. PPIA, HPRT1, and YWHAZ genes are suitable for normalization of mRNA expression in long-term expanded human mesenchymal stem cells. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragni, E.; Vigano, M.; Rebulla, P.; Giordano, R.; Lazzari, L. What is beyond aq RT-PCR study on mesenchymal stem cell differentiation properties: How to choose the most reliable housekeeping genes. J. Cell. Mol. Med. 2013, 17, 168–180. [Google Scholar] [CrossRef]
- Hildyard, J.C.; Taylor-Brown, F.; Massey, C.; Wells, D.J.; Piercy, R.J. Determination of qPCR reference genes suitable for normalizing gene expression in a canine model of Duchenne muscular dystrophy. J. Neuromuscul. Dis. 2018, 5, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.J.; Jeon, R.H.; Kim, H.D.; Hwang, J.C.; Lee, H.J.; Bae, S.G.; Lee, S.L.; Rho, G.J.; Kim, S.J.; Lee, W.J. TATA box binding protein and ribosomal protein 4 are suitable reference genes for normalization during quantitative polymerase chain reaction study in bovine mesenchymal stem cells. Asian-Aust. J. Anim. Sci. 2020, 33, 2021. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Tayerjiang, J.L.T.; Zhao, X.B.; Wang, H.; Wang, Q.; Yuan, H. Study of optimal scheme of spinal image-guided radiotherapy based on expression of caspase-3 in spinal cord neurons by orthogonal design. Genet. Mol. Res. 2015, 14, 3223–3233. [Google Scholar] [CrossRef]
- Sculley, D.G.; Dawson, P.A.; Emmerson, B.T.; Gordon, R.B. A review of the molecular basis of hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Hum. Genet. 1992, 90, 195–207. [Google Scholar] [CrossRef]
- Chan, R.J.; Webster, J.; Chung, B.; Marquart, L.; Ahmed, M.; Garantziotis, S. Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014, 14, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Judge, S.J.; Yanagisawa, M.; Sturgill, I.R.; Bateni, S.B.; Gingrich, A.A.; Foltz, J.A.; Lee, D.A.; Modiano, J.F.; Monjazeb, A.M.; Culp, W.T.; et al. Blood and tissue biomarker analysis in dogs with osteosarcoma treated with palliative radiation and intra-tumoral autologous natural killer cell transfer. PLoS ONE 2020, 15, e0224775. [Google Scholar] [CrossRef] [Green Version]
- Marino, E.R.; Boreges, A.A.; Perez, A.B.; Perez, J.A. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008, 8, 131. [Google Scholar]
- Maroufi, A.; Bockstaele, E.V.; Loose, M.D. Validation of reference genes for gene expression analysis in chicory (Cichorium intybus) using quantitative real-time PCR. BMC Mol. Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Walz, J.Z. Radiation Therapy Side Effects: Skin. Available online: https://www.mspca.org/angell_services/radiation-therapy-side-effects-skin/ (accessed on 8 August 2022).
- Goulart, M.R.; Hlavaty, S.I.; Chang, Y.M.; Polton, G.; Stell, A.; Perry, J.; Wu, Y.; Sharma, E.; Broxholme, J.; Lee, A.C.; et al. Phenotypic and transcriptomic characterization of canine myeloid-derived suppressor cells. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, S.H.; Clements, D.N.; McEwan, N.A.; Nuttall, T.; Carter, S.D. Reference genes for canine skin when using quantitative real-time PCR. Veter. Immunol. Immunopathol. 2008, 126, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Ayers, D.; Clements, D.N.; Salway, F.; Day, P.J. Expression stability of commonly used reference genes in canine articular connective tissues. BMC Veter. Res. 2007, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Voss, P.; Hajimiragha, H.; Engels, M.; Ruhwiedel, C.; Calles, C.; Schroeder, P.; Grune, T. Irradiation of GAPDH: A model for environmentally induced protein damage. Biol. Chem. 2007, 388, 583–592. [Google Scholar] [CrossRef]
- Park, S.J.; Huh, J.W.; Kim, Y.H.; Lee, S.R.; Kim, S.H.; Kim, S.U.; Kim, H.S.; Kim, M.K.; Chang, K.T. Selection of internal reference genes for normalization of quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis in the canine brain and other organs. Mol. Biotechnol. 2013, 54, 47–57. [Google Scholar] [CrossRef]
- Pisamai, S.; Rungsipipat, A.; Kalpravidh, C.; Suriyaphol, G. Selection of reference genes for real-time polymerase chain reaction in canine oral tumor and cancer. Thai J. Vet. Med. 2016, 46, 295–304. [Google Scholar]
- Nanashima, N.; Ito, K.; Ishikawa, T.; Nakano, M.; Nakamura, T. Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia. Int. J. Mol. Med. 2012, 30, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Patel, G.K.; Wilson, C.H.; Harding, K.G.; Finlay, A.Y.; Bowden, P.E. Numerous keratinocyte subtypes involved in wound re-epithelialization. J. Investig. Dermatol. 2006, 126, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Eriksson, J.E. Intermediate filaments and the regulation of cell motility during regeneration and wound healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef] [PubMed]
- von Neubeck, C.; Shankaran, H.; Geniza, M.J.; Kauer, P.M.; Robinson, R.J.; Chrisler, W.B.; Sowa, M.B. Integrated experimental and computational approach to understand the effects of heavy ion radiation on skin homeostasis. Integr. Biol. 2013, 5, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- von Neubeck, C.; Geniza, M.J.; Kauer, P.M.; Robinson, R.J.; Chrisler, W.B.; Sowa, M.B. The effect of low dose ionizing radiation on homeostasis and functional integrity in an organotypic human skin model. Mutat. Res. Mol. Mech. Mutagen. 2015, 775, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, H.; Odake, Y.; Takada, K.; Funasaka, Y.; Ichihashi, M. Involvement of changes in stratum corneum keratin in wrinkle formation by chronic ultraviolet irradiation in hairless mice. Exp. Dermatol. 2003, 12, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Rees, J.L. Wavelength-specific upregulation of keratin mRNA expression in response to ultra-violet radiation. J. Investig. Dermatol. 1994, 102, 433–439. [Google Scholar] [CrossRef]




| Information of Primers | Standard Curve Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Gene Name (Symbol) | Sequence | Base Pair | Accession | R2 | M | B | E |
| β−actin (ACTB) | F : GCACTCTTCCAACCTTCTTTCC | 179 | AF021873.2 | 0.993 | −3.489 | 34.252 | 1.02 |
| R : GCTGTGATTTCCTTCTGCATCC | |||||||
| Glyceraldehyde-3−phosphate dehydrogenase (GAPDH) | F : GGAGAAAGCTGCCAAATATGACG | 118 | NM_001003142.2 | 0.991 | −3.436 | 35.514 | 0.98 |
| R : ACTGTTGAAGTCACAGGAGACC | |||||||
| Tyrosine 3−monooxygenase/tryptophan 5−monooxygenase activation protein, zeta polypeptide (YWHAZ) | F : GTGAAGAGTCATACAAAGACAGCA | 110 | XM_014118550.1 | 0.992 | −3.511 | 37.145 | 1.01 |
| R : CCCTCCTTCTCCTGCTTCAG | |||||||
| β−glucuronidase (GUSB) | F : ATCTGTAGTCATGTGGTCTGTAGC | 149 | AF019759.1 | 0.996 | −3.332 | 33.257 | 0.99 |
| R : GGTCTGCTTCATAGTTGGAATTGG | |||||||
| Hypoxanthine phosphoribosyl transferase 1 (HPRT1) | F : GACTGAAGAGCTACTGTAATGACC | 168 | NM_001003357.2 | 0.996 | −3.412 | 36.915 | 0.98 |
| R : TCTTTGGATTATGCTCCTTGACC | |||||||
| Ribosomal protein 4 (RPL4) | F : AATGAGAAACCGTCGTCGTATCC | 141 | NM_001252409.1 | 0.992 | −3.355 | 39.041 | 1.01 |
| R : GGAGCAAGTTTCAGAATGTTCAGC | |||||||
| Ribosomal protein S5 (RPS5) | F : TGAAGGAGAAGTATGCCAAGTACC | 188 | XM_533568.5 | 0.995 | −3.435 | 39.145 | 0.97 |
| R : GAGCAGATGGATGATCTCGAAGG | |||||||
| TATA box binding protein (TBP) | F : ATCTGGTATCCCTTACGCTTCG | 137 | XM_849432.4 | 0.995 | −3.498 | 36.972 | 1.03 |
| R : GCAAGAGAGTCTGGTTTGTTTCC | |||||||
| Keratin 10 | F : CTCGTGACTACAGCAAATACTACC | 105 | NM_001013425.1 | 0.997 | −3.501 | 33.783 | 1.01 |
| R : TGGCATTGTCGATCTGAAGC | |||||||
| GAPDH | YWHAZ | TBP | RPS5 | RPL4 | ACTB | HPRT1 | GUSB | |
|---|---|---|---|---|---|---|---|---|
| SD [±CT] | 1.198596 | 0.993889 | 0.95946 | 0.950324 | 0.93821 | 0.935185 | 0.901281 | 0.654506 |
| CV [% CT] | 7.732737 | 5.277597 | 3.791541 | 5.959286 | 3.811451 | 4.742051 | 4.112048 | 2.958517 |
| ACTB | GAPDH | YWHAZ | GUSB | HPRT1 | RPL4 | RPS5 | TBP | |
|---|---|---|---|---|---|---|---|---|
| geNorm | 4 | 8 | 5 | 6 | 3 | 1 | 7 | 1 |
| Normfinder | 4 | 8 | 5 | 6 | 1 | 3 | 7 | 2 |
| Bestkeeper | 3 | 8 | 7 | 1 | 2 | 4 | 5 | 6 |
| Total Rank | 4 | 8 | 6 | 5 | 1 | 2 | 7 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Choe, Y.-H.; Han, J.-H.; Hwang, G.; Choi, M.-Y.; Thakur, G.; Jo, C.-H.; Oh, S.-J.; Lee, W.-J.; Rho, G.-J.; et al. HPRT1 Most Suitable Reference Gene for Accurate Normalization of mRNA Expression in Canine Dermal Tissues with Radiation Therapy. Genes 2022, 13, 1928. https://doi.org/10.3390/genes13111928
Lee S-Y, Choe Y-H, Han J-H, Hwang G, Choi M-Y, Thakur G, Jo C-H, Oh S-J, Lee W-J, Rho G-J, et al. HPRT1 Most Suitable Reference Gene for Accurate Normalization of mRNA Expression in Canine Dermal Tissues with Radiation Therapy. Genes. 2022; 13(11):1928. https://doi.org/10.3390/genes13111928
Chicago/Turabian StyleLee, Sang-Yun, Yong-Ho Choe, Jang-Ho Han, Gunha Hwang, Moon-Yeong Choi, Gitika Thakur, Chan-Hee Jo, Seong-Ju Oh, Won-Jae Lee, Gyu-Jin Rho, and et al. 2022. "HPRT1 Most Suitable Reference Gene for Accurate Normalization of mRNA Expression in Canine Dermal Tissues with Radiation Therapy" Genes 13, no. 11: 1928. https://doi.org/10.3390/genes13111928





