Responses of Humoral and Cellular Immune Mediators in BALB/c Mice to LipX (PE11) as Seed Tuberculosis Vaccine Candidates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Plasmid and Protein Recombinants
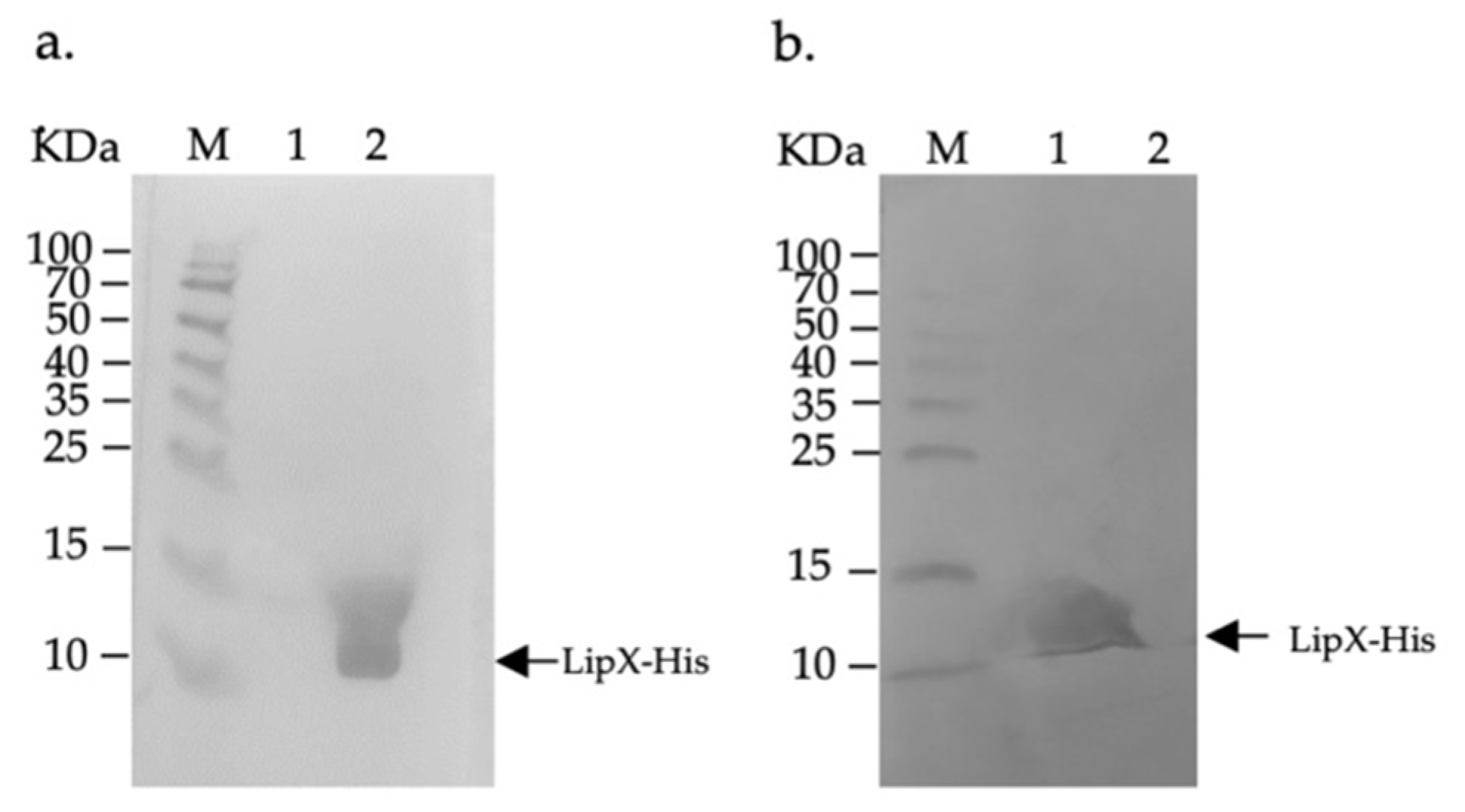
2.3. LipX-His Protein Isolation
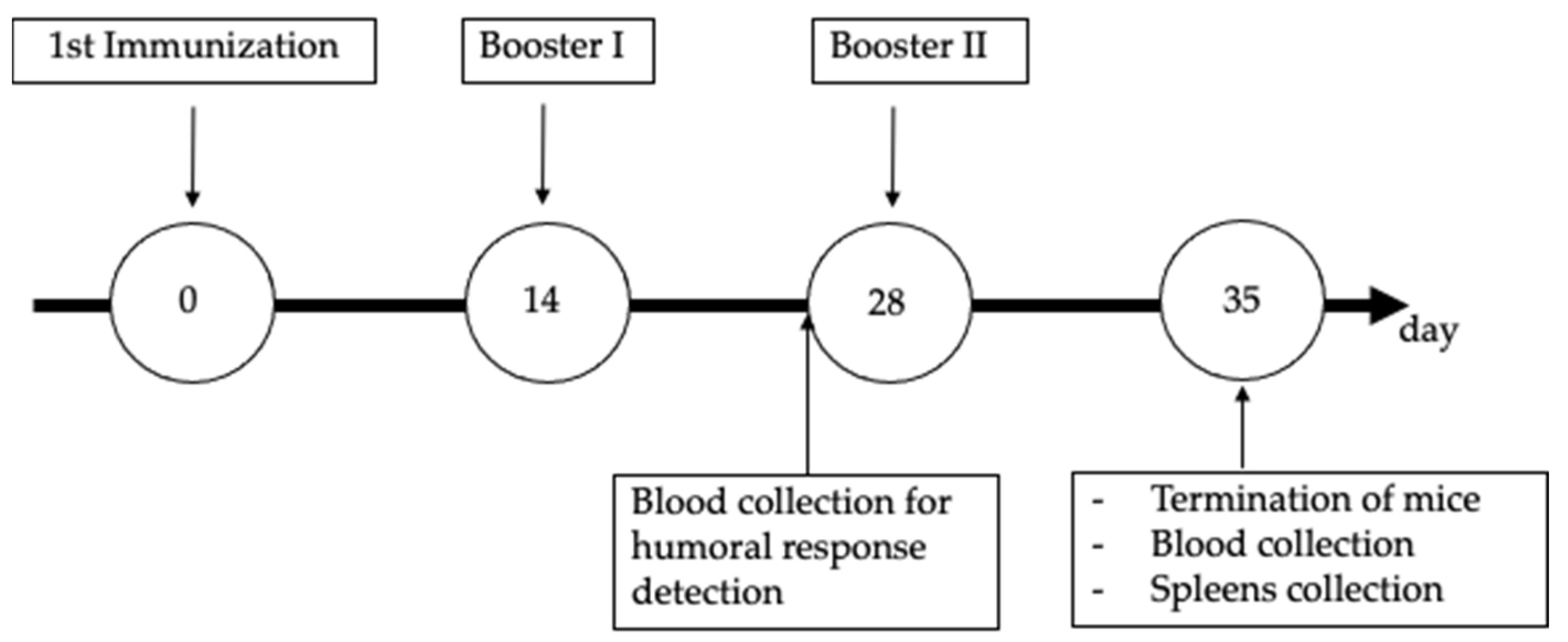
2.4. Sample Size and Animal Care
2.5. Procedure for Mouse Immunization and Blood Collection
2.6. Examination of Humoral Immune Response Using Western Blotting
2.7. Cellular Immune Mediator Response Assay
2.8. Statistical Analysis
2.9. Limitations of the Study
3. Results
3.1. LipX Isolation and Observation of Humoral Immune Response by Western Blotting
3.2. Analysis of the Cellular Immune Mediator Response Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Plasmid Name | Vector | Insert | Functions | Source | Reference |
|---|---|---|---|---|---|
| pET28a-lipX | pET28a | Full length of lipX gene | Expression of LipX protein in E.coli cells | Department of Microbiology, Medical Faculty Universitas Indonesia | This work (unpublished data). |
| pCDNA3.1-lipX | pCDNA3.1 | Full length of lipX gene | Expression of LipX protein in eukaryotic cells | Department of Microbiology, Medical Faculty Universitas Indonesia | [32] |
Appendix B
References
- Marimani, M.; Ahmad, A.; Duse, A. The role of epigenetics, bacterial and host factors in progression of Mycobacterium tuberculosis infection. Tuberculosis 2018, 113, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Yanti, B.; Harapan, H.; Mertaniasih, N.M. The role of Mycobacterium tuberculosis lineages on lung tissue damage and TNF-α level among tuberculosis patients Indonesia. Clin. Epidemiol. Glob. Health 2019, 7, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Rosser, A.; Stover, C.; Pareek, M.; Mukamolova, G.V.; Rosser, A.; Stover, C.; Mukamolova, G.V. Resuscitation-promoting factors are important determinants of the pathophysiology in Mycobacterium tuberculosis infection. Crit. Rev. Microbiol. 2017, 43, 621–630. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Fine, P.E.M. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 349, 1339–1345. [Google Scholar] [CrossRef]
- Colditz, G.A.; Brewer, T.F.; Angeles, L.; Berkey, C.S.; Wilson, M.E.; Francisco, S. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Ahn, S.K.; Ng, M.; Li, M.; Liu, J. Loss of lipid virulence factors reduces the efficacy of the BCG vaccine. Sci. Rep. 2016, 6, 29076. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.; Tim, M. DNA vaccination: An immunological perspective. Immunologia 2004, 23, 41–55. [Google Scholar]
- Abebe, F.; Bjune, G. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille Calmette-Guérin (BCG) vaccines: Is there a link? Clin. Exp. Immunol. 2006, 145, 389–397. [Google Scholar] [CrossRef]
- Madacki, J.; Fiol, G.M.; Brosch, R. Update on the virulence factors of the obligate pathogen Mycobacterium tuberculosis and related tuberculosis-causing mycobacteria. Infect. Genet. Evol. 2019, 72, 67–77. [Google Scholar] [CrossRef]
- Lowrie, D.B. DNA vaccines for therapy of tuberculosis: Where are we now? Vaccine 2006, 24, 1983–1989. [Google Scholar] [CrossRef]
- Morris, S.; Kelley, C.; Howard, A.; Li, Z.; Collins, F. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 2000, 18, 2155–2163. [Google Scholar] [CrossRef]
- Lee, J.; Kumar, S.A.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, S.; Teimourpour, R.; Meshkat, Z. Designing and construction of a DNA vaccine encoding Tb10.4 gene of Mycobacterium tuberculosis. Irian J. Pathol. 2016, 11, 112–119. [Google Scholar]
- Khoshnood, S.; Heidary, M.; Haeili, M.; Drancourt, M.; Darban-sarokhalil, D.; Javad, M.; Lohrasbi, V. Novel vaccine candidates against Mycobacterium tuberculosis. Int. J. Biol. Macromol. 2018, 120, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Peng, H.; Fang, Y.; Feng, J.; Yang, D.S. The construction of the eukaryotic plasmid pcDNA3.1/Azurin and increase apoptosis of U2OS cells transfected with it. Cell. Mol. Biol. Lett. 2007, 12, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Pei, C.; Sun, X.; Zhang, C.; Li, L.; Kong, X. Plasmid pcDNA3.1-s11 constructed based on the S11 segment of grass carp reovirus as DNA vaccine provides immune protection. Vaccine 2018, 36, 3613–3621. [Google Scholar] [CrossRef]
- Liu, S.; Shi, L.; Cheng, Y.; Fan, G.; Ren, H.; Yuan, Y. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol. Res. 2009, 105, 267. [Google Scholar] [CrossRef]
- Naaz, S.; Kazim, S.N. Designing and reconstruction of pcDNA3.0 mammalian expression vector with its multiple cloning sites by directional cloning method. Cloning Transgenes 2018, 7, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.J. The enigmatic PE/PPE multigene family of Mycobacteria and tuberculosis vaccination. Infect. Immun. 2017, 85, e00969-16. [Google Scholar] [CrossRef] [Green Version]
- Delogu, G.; Cole, S.T.; Brosch, R. The PE and PPE protein families of Mycobacterium tuberculosis; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 133–144. [Google Scholar]
- Hasnain, S.E.; Ehtesham, N.Z.; Grover, S. Mycobacterium tuberculosis Molecular Infection Biology, Pathogenesis, Diagnostics and New Interventions; Springer: Singapore, 2019; pp. 123–167. [Google Scholar]
- Phelan, J.E.; Coll, F.; Bergval, I.; Anthony, R.M.; Warren, R.; Sampson, S.L.; Clark, T.G. Recombination in pe/ppe genes contributes to genetic variation in Mycobacterium tuberculosis lineages. Genomics 2016, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, S.; Singh, A.K.; Pant, G.; Mitra, K.; Sashidhara, K.V.; Krishnan, M.Y. Down-regulation of PE11, a cell wall associated esterase, enhances the biofilm growth of Mycobacterium tuberculosis and reduces cell wall virulence lipid levels. Microbiology 2017, 163, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rao, R.N.; Ram, J.; Reddy, C.; Prasad, R.B.N. PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulence. Sci. Rep. 2016, 6, 21624. [Google Scholar] [CrossRef]
- Narayana, Y.; Joshi, B.; Katoch, V.M.; Mishra, K.C.; Balaji, K.N. Differential B-cell responses are induced by Mycobacterium tuberculosis PE antigens Rv1169c, Rv0978c, and Rv1818c. Clin. Vaccine Immunol. 2007, 14, 1334–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Basic Immunology, 5th ed.; Elsevier Inc.: North York, ON, Canada, 2015; pp. 69–73. [Google Scholar]
- Ismail, N.; Olano, J.P.; Feng, H.; Walker, D.H. Current status of immune mechanisms of killing of intracellular microorganism. FEMS Microbiol. Lett. 2002, 207, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K. The pathology of Mycobacterium tuberculosis infection. Vet. Pathol. 2012, 42, 423–439. [Google Scholar] [CrossRef] [Green Version]
- Kiros, T.G.; Levast, B.; Auray, G.; Strom, S.; van Kessel, J.; Gerdts, V. The importance of animal models in the development of vaccines. Innov. Vaccinol. 2012, 29, 251–264. [Google Scholar]
- Garcia-Pelayo, M.C.; Bachy, V.S.; Kaveh, D.A.; Hogarth, P.J. BALB/c mice display more enhanced BCG vaccine induced Th1 and Th17 response than C57BL/6 mice but have equivalent protection. Tuberculosis 2015, 95, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Azmi, L.; Rukmana, A.; Sjatha, F. Cloning of the pe11 (LipX, Rv1169c) gene of a Mycobacterium tuberculosis Beijing Strain into the pcDNA3.1 Plasmid Vector. Makara J. Sci. 2021, 25, 35–42. [Google Scholar]
- Zhang, W.; Jiang, H.; Bai, Y.-L.; Kang, J.; Xu, Z.-K.; Wang, L.-M. Construction and immunogenicity of the DNA vaccine of Mycobacterium Tuberculosis dormancy antigen rv1733c. Scand. J. Immunol. 2014, 79, 292–298. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Q.; Fu, R. Differential immunogenicity and protective efficacy of DNA vaccines expressing proteins of Mycobacterium tuberculosis in a mouse model. Microbiol. Res. 2009, 164, 374–382. [Google Scholar] [CrossRef]
- Ahmad, S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin. Dev. Immunol. 2011, 2011, 814943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A.M.; Roberts, A.D.; Rhoades, E.R.; Callahan, J.E.; Getzy, D.M.; Orme, I.M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 1995, 84, 423–432. [Google Scholar] [PubMed]
- Garra, A.O.; Redford, P.S.; Mcnab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The Immune Response in Tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Sharma, D.; Sarkar, D. Pathophysiology of tuberculosis: An update review. Pharma Tutor 2018, 6, 15–21. [Google Scholar] [CrossRef]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-α and IFN-γ Activation on the Dynamics of iNOS Gene Expression in LPS Stimulated Macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulton, S.A.; Cross, J.V.; Toossi, Z.T.; Boom, W.H. Regulation of interleukin-12 by interleukin-10, transforming growth factor-β, tumor necrosis factor-α, and interferon-γ in human monocytes infected with Mycobacterium tuberculosis H37Ra. J. Infect. Dis. 1998, 178, 1105–1114. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Young, R.A. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect. Immun. 1999, 67, 3087–3095. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, B.M.; Kannan, N.; Vemu, L.; Raghunand, T.R. The Mycobacterium tuberculosis PE Proteins Rv0285 and Rv1386 Modulate Innate Immunity and Mediate Bacillary Survival in Macrophages. PLoS ONE 2012, 7, e51686. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Zeng, J.; Xiang, X.; Li, P.; Xie, J. PE11 (Rv1169c) selectively alters fatty acid components of Mycobacterium smegmatis and host cell interleukin-6 level accompanied with cell death. Front Microbiol. 2015, 23, 613. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukmana, A.; Supardi, L.A.; Sjatha, F.; Nurfadilah, M. Responses of Humoral and Cellular Immune Mediators in BALB/c Mice to LipX (PE11) as Seed Tuberculosis Vaccine Candidates. Genes 2022, 13, 1954. https://doi.org/10.3390/genes13111954
Rukmana A, Supardi LA, Sjatha F, Nurfadilah M. Responses of Humoral and Cellular Immune Mediators in BALB/c Mice to LipX (PE11) as Seed Tuberculosis Vaccine Candidates. Genes. 2022; 13(11):1954. https://doi.org/10.3390/genes13111954
Chicago/Turabian StyleRukmana, Andriansjah, Lulut Azmi Supardi, Fithriyah Sjatha, and Mifa Nurfadilah. 2022. "Responses of Humoral and Cellular Immune Mediators in BALB/c Mice to LipX (PE11) as Seed Tuberculosis Vaccine Candidates" Genes 13, no. 11: 1954. https://doi.org/10.3390/genes13111954





