Anterior Umbilication of Lens in a Family with Congenital Cataracts Associated with a Missense Mutation of MIP Gene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessments
2.3. Genetic Variant Screening
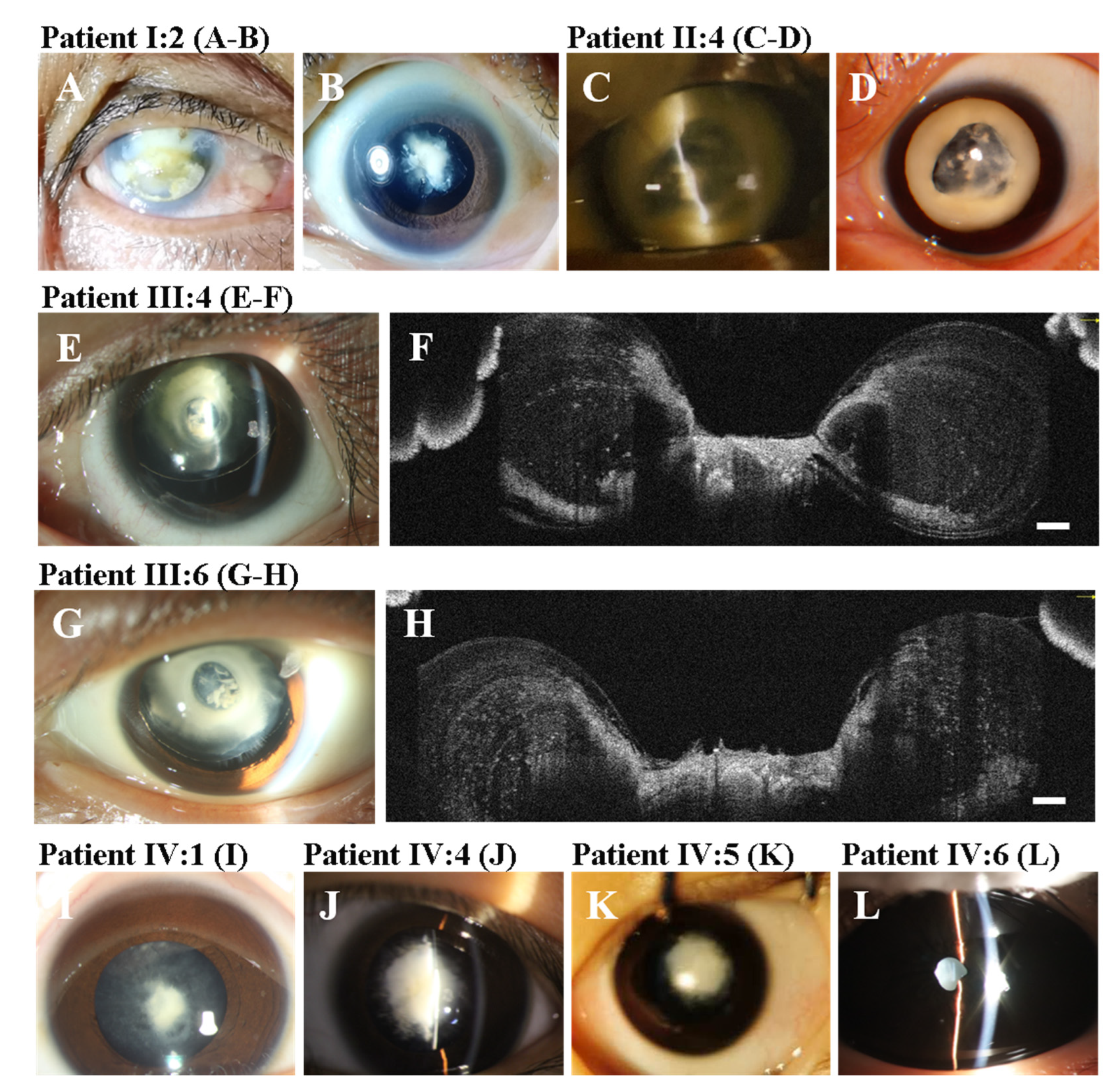
3. Results
3.1. Clinical Findings
3.2. Molecular Findings and Bioinformatics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheeladevi, S.; Lawrenson, J.G.; Fielder, A.R.; Suttle, C.M. Global prevalence of childhood cataract: A systematic review. Eye 2016, 30, 1160–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, L.; Mikkelsen, A.; Nürnberg, P.; Nürnberg, G.; Anjum, I.; Eiberg, H.; Rosenberg, T. Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataract. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.D.; Liu, N.; Shi, H.R.; Dong, J.M.; Zhao, Z.H.; Liu, J.; Li-Ling, J.; Yang, Y.X. A novel 3-base pair deletion of the CRYAA gene identified in a large Chinese pedigree featuring autosomal dominant congenital perinuclear cataract. Genet. Mol. Res. 2015, 14, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Yan, Y.; Yao, K. Molecular genetics of congenital cataracts. Exp. Eye Res. 2020, 191, 107872. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.K.; Biswas, S.K.; Brako, L.; Shiels, A.; Gu, S.; Jiang, J.X. Aquaporin-0 targets interlocking domains to control the integrity and transparency of the eye lens. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1202–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Xu, J.; Gu, Y.; Du, C. The relationship between major intrinsic protein genes and cataract. Int. Ophthalmol. 2021, 41, 375–387. [Google Scholar] [CrossRef]
- Gu, F.; Zhai, H.; Li, D.; Zhao, L.; Li, C.; Huang, S.; Ma, X. A novel mutation in major intrinsic protein of the lens gene (MIP) underlies autosomal dominant cataract in a Chinese family. Mol. Vis. 2007, 13, 1651–1656. [Google Scholar]
- Zhou, Z.; Li, L.; Lu, L.; Min, L. Identification of a missense mutation in MIP gene via mutation analysis of a Guangxi Zhuang ethnic pedigree with congenital nuclear cataracts. Exp. Ther. Med. 2018, 16, 3256–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, A.S.; Grigg, J.R.; Ho, G.; Prokudin, I.; Farnsworth, E.; Holman, K.; Cheng, A.; Billson, F.A.; Martin, F.; Fraser, C.; et al. Sporadic and Familial Congenital Cataracts: Mutational Spectrum and New Diagnoses Using Next-Generation Sequencing. Hum. Mutat. 2016, 37, 371–384. [Google Scholar] [CrossRef] [Green Version]
- Javadiyan, S.; Lucas, S.E.; Wangmo, D.; Ngy, M.; Edussuriya, K.; Craig, J.E.; Rudkin, A.; Casson, R.; Selva, D.; Sharma, S.; et al. Identification of novel mutations causing pediatric cataract in Bhutan, Cambodia, and Sri Lanka. Mol. Genet. Genom. Med. 2018, 6, 555–564. [Google Scholar] [CrossRef]
- Rechsteiner, D.; Issler, L.; Koller, S.; Lang, E.; Bähr, L.; Feil, S.; Rüegger, C.M.; Kottke, R.; Toelle, S.P.; Zweifel, N.; et al. Genetic Analysis in a Swiss Cohort of Bilateral Congenital Cataract. JAMA Ophthalmol. 2021, 139, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, X.; Li, S.; Jia, X.; Wang, P.; Guo, X.; Jiao, X.; Zhang, Q.; Hejtmancik, J.F. Detection of variants in 15 genes in 87 unrelated Chinese patients with Leber congenital amaurosis. PLoS ONE 2011, 6, e19458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, S.E.; Patch, A.M.; Ellard, S. Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet. Test Mol. Biomark. 2010, 14, 533–537. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Houdayer, C.; Dehainault, C.; Mattler, C.; Michaux, D.; Caux-Moncoutier, V.; Pagès-Berhouet, S.; d’Enghien, C.D.; Laugé, A.; Castera, L.; Gauthier-Villars, M.; et al. Evaluation of in silico splice tools for decision-making in molecular diagnosis. Hum. Mutat. 2008, 29, 975–982. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gu, S.; Quan, Y.; Varadaraj, K.; Jiang, J.X. Development of a potent embryonic chick lens model for studying congenital cataracts in vivo. Commun. Biol. 2021, 4, 325. [Google Scholar] [CrossRef]
- Jensen, M.; Dror, R.O.; Xu, H.; Borhani, D.W.; Arkin, I.T.; Eastwood, M.P.; Shaw, D.E. Dynamic control of slow water transport by aquaporin 0: Implications for hydration and junction stability in the eye lens. Proc. Natl. Acad. Sci. USA 2008, 105, 14430–14435. [Google Scholar] [CrossRef] [Green Version]
- Chepelinsky, A.B. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb. Exp. Pharmacol. 2009, 190, 265–297. [Google Scholar]
- Kumari, S.S.; Gandhi, J.; Mustehsan, M.H.; Eren, S.; Varadaraj, K. Functional characterization of an AQP0 missense mutation, R33C, that causes dominant congenital lens cataract, reveals impaired cell-to-cell adhesion. Exp. Eye Res. 2013, 116, 371–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herwig, M.C.; Müller, A.M.; Klarmann-Schulz, U.; Holz, F.G.; Loeffler, K.U. Lens artifacts in human fetal eyes—The challenge of interpreting the histomorphology of human fetal lenses. Graefes. Arch. Clin. Exp. Ophthalmol. 2014, 252, 155–162. [Google Scholar] [CrossRef] [PubMed]

| Patients | I:2 | II:4 | III:4 | III:6 | IV:1 | IV:4 | IV:5 | IV:6 |
|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | F | F | F | F |
| Age | 71 ys | 49 ys | 27 ys | 25 ys | 5 ys 8 ms | 5 ys | 3 ys 2 ms | 1 y 2 ms |
| BCVA * (logMAR **, od/os) | NLP/FC | 1.00/FC | 1.00/HM | 1.00/HM | HM/HM | HM/HM | HM/HM | NA |
| Variant site | c.97C > T | c.97C > T | c.97C > T | c.97C > T | c.97C > T | c.97C > T | c.97C > T | c.97C > T |
| Amino acid change | p.R33C | p.R33C | p.R33C | p.R33C | p.R33C | p.R33C | p.R33C | p.R33C |
| Cataract morphology | Nuclear opacity | Total opacity | Nuclear opacity | Nuclear opacity | Nuclear opacity | Nuclear opacity | Nuclear opacity | Nuclear opacity |
| Anterior umbilication of lens | − | + | + | + | − | − | − | − |
| Subluxation of lens | − | − | + | + | − | − | − | − |
| Cataract surgery | od | od | od | od | ou | ou | ou | ou |
| Date of surgery | 1966-06 | 2013-06 | 2013-06 | 2013-06 | 2020-10 | 2020-12 | 2021-01 | 2021-01 |
| Pre-op axial length (mm, od/os) | NA | 32.35/NA | 26.85/NA | 26.84/NA | 25.33/25.03 | 23.54/23.52 | 21.75/21.34 | 17.22/17.17 |
| Latest axial length (mm, od/os) | NA | 32.74/NA | 31.52/31.96 | 31.08/31.20 | NA | NA | NA | NA |
| Nystagmus | + | + | + | + | + | + | + | + |
| Complications | Leukoma od | PCO od | PCO, RRD od | PCO, RRD od | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Wang, X.; Wang, Q.; Zhang, X.; Wang, D.; Huang, W.; Dongye, M.; Feng, X.; Zheng, D.; Lin, H. Anterior Umbilication of Lens in a Family with Congenital Cataracts Associated with a Missense Mutation of MIP Gene. Genes 2022, 13, 1987. https://doi.org/10.3390/genes13111987
Cheng Z, Wang X, Wang Q, Zhang X, Wang D, Huang W, Dongye M, Feng X, Zheng D, Lin H. Anterior Umbilication of Lens in a Family with Congenital Cataracts Associated with a Missense Mutation of MIP Gene. Genes. 2022; 13(11):1987. https://doi.org/10.3390/genes13111987
Chicago/Turabian StyleCheng, Zhixing, Xun Wang, Qiwei Wang, Xulin Zhang, Dongni Wang, Weiming Huang, Meimei Dongye, Xiaocheng Feng, Danying Zheng, and Haotian Lin. 2022. "Anterior Umbilication of Lens in a Family with Congenital Cataracts Associated with a Missense Mutation of MIP Gene" Genes 13, no. 11: 1987. https://doi.org/10.3390/genes13111987





