ApoE/NOS3 Knockout Mice as a Novel Cardiovascular Disease Model of Hypertension and Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of ApoE and NOS3 Double Gene Knockout Mice
2.2. Measurements of Systolic Blood Pressure
2.3. Ophthalmoscope
2.4. Hematoxylin-Eosin (H&E) Staining
2.5. Serum Lipid Profiling
2.6. Quantification of Atherosclerosis
2.7. Statistical Analysis
3. Results
3.1. ApoE/NOS3−/− Mice with a Hypertensive Phenotype
3.2. ApoE/NOS3−/− Mice Have a Dyslipidemia Phenotype
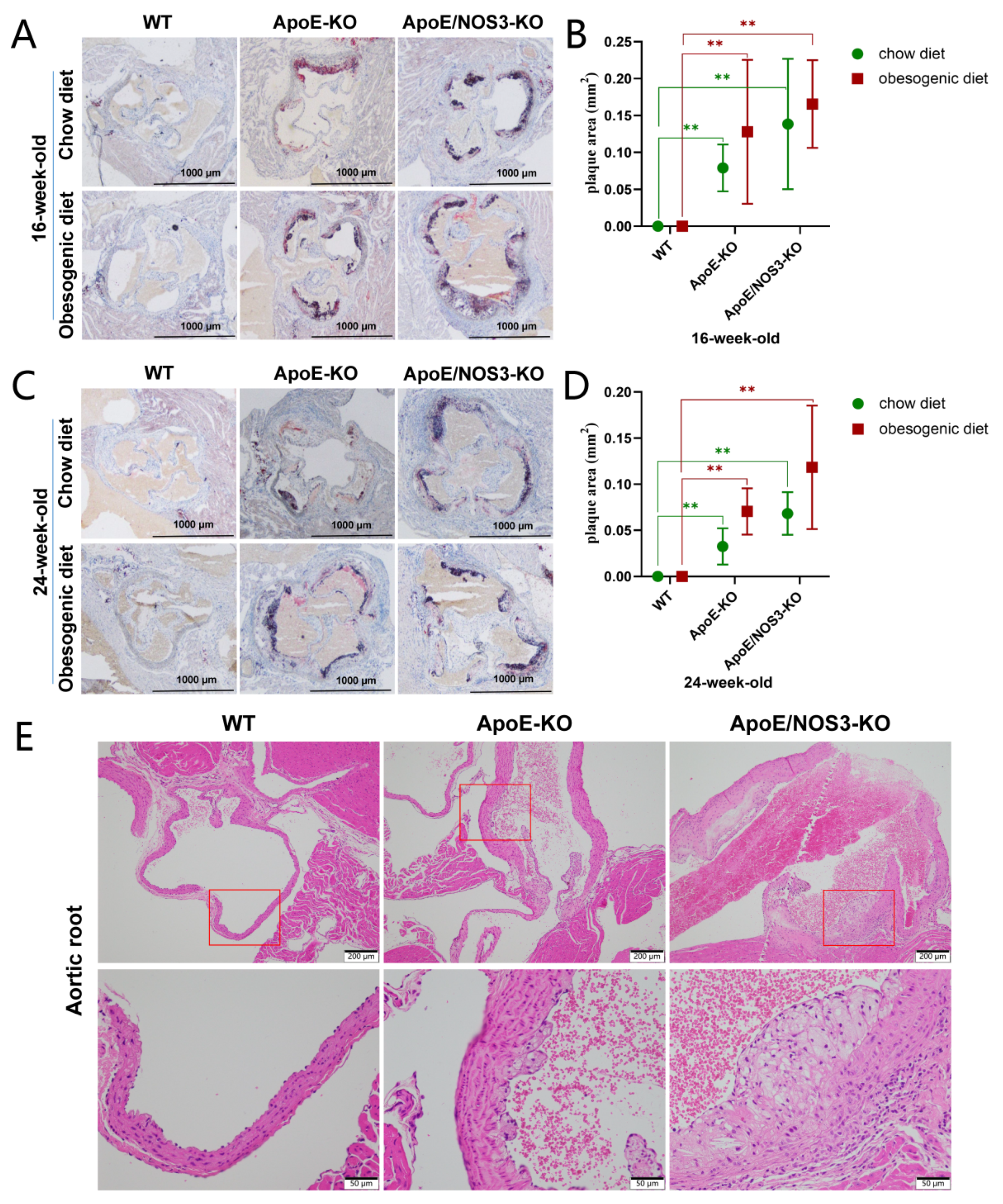
3.3. ApoE/NOS3−/− Mice with a Typical Atherosclerotic Phenotype
3.4. ApoE/NOS3−/− Mice Can Reproduce Offspring from Homozygous Interbreeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Kamato, D.; Little, P.J.; Nakagawa, S.; Pelisek, J.; Jin, Z.G. Targeting epigenetics and non-coding RNAs in atherosclerosis: From mechanisms to therapeutics. Pharmacol. Ther. 2019, 196, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, W.; Li, X.; Zhou, H. Inflammation: A Novel Therapeutic Target/Direction in Atherosclerosis. Curr. Pharm. Des. 2017, 23, 1216–1227. [Google Scholar] [CrossRef]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Al-Sharea, A.; Lee, M.K.S.; Whillas, A.; Michell, D.L.; Shihata, W.A.; Nicholls, A.J.; Cooney, O.D.; Kraakman, M.J.; Veiga, C.B.; Jefferis, A.M.; et al. Chronic sympathetic driven hypertension promotes atherosclerosis by enhancing hematopoiesis. Haematologica 2019, 104, 456–467. [Google Scholar] [CrossRef] [Green Version]
- Solberg, L.A.; Strong, J.P. Risk factors and atherosclerotic lesions. A review of autopsy studies. Arteriosclerosis 1983, 3, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Ning, B.; Chen, Y.; Waqar, A.B.; Yan, H.; Shiomi, M.; Zhang, J.; Chen, Y.E.; Wang, Y.; Itabe, H.; Liang, J.; et al. Hypertension Enhances Advanced Atherosclerosis and Induces Cardiac Death in Watanabe Heritable Hyperlipidemic Rabbits. Am. J. Pathol. 2018, 188, 2936–2947. [Google Scholar] [CrossRef]
- De França, E.; Alves, J.G.; Hutz, M.H. Apolipoprotein E polymorphism and its association with serum lipid levels in Brazilian children. Hum. Biol. 2004, 76, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita, J.A.; Zhang, S.H.; Hagaman, J.R.; Oliver, P.M.; Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1992, 89, 4471–4475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, S.; Herz, J.; Maeda, N.; Goldstein, J.L.; Brown, M.S. The two-receptor model of lipoprotein clearance: Tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 4431–4435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Yang, Y.; Xing, R.; Cui, X.; Xiao, Y.; Xie, L.; You, P.; Wang, T.; Zeng, L.; Peng, W.; et al. Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis 2018, 271, 26–35. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [Green Version]
- Suri, M.F.; Qureshi, A.I. Prehypertension as a risk factor for cardiovascular diseases. J. Cardiovasc. Nurs. 2006, 21, 478–482; quiz 483–484. [Google Scholar] [CrossRef]
- Rafflenbeul, W. Hypertension treatment and prevention of new atherosclerotic plaque formation. Drugs 1994, 48 (Suppl. 1), 11–15. [Google Scholar] [CrossRef]
- Iring, A.; Jin, Y.J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [Green Version]
- Raij, L. Nitric oxide in hypertension: Relationship with renal injury and left ventricular hypertrophy. Hypertension 1998, 31 Pt 2, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Gkaliagkousi, E.; Douma, S.; Zamboulis, C.; Ferro, A. Nitric oxide dysfunction in vascular endothelium and platelets: Role in essential hypertension. J. Hypertens. 2009, 27, 2310–2320. [Google Scholar] [CrossRef]
- Spinelli, G.; Ciliberto, G. Functional activity and chromatin configuration of SV40 enhancer injected in Xenopus laevis oocytes. Nucleic Acids Res. 1985, 13, 8065–8081. [Google Scholar] [CrossRef] [PubMed]
- Leong, X.F.; Ng, C.Y.; Jaarin, K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. Biomed Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoljevic, D.; Veiga, C.B.; Michell, D.L.; Shihata, W.A.; Al-Sharea, A.; Head, G.A.; Murphy, A.J.; Kraakman, M.J.; Lee, M.K.S. A spontaneously hypertensive diet-induced atherosclerosis-prone mouse model of metabolic syndrome. Biomed. Pharmacother. 2021, 139, 111668. [Google Scholar] [CrossRef]
- Gonzalez-Guerra, A.; Roche-Molina, M.; García-Quintáns, N.; Sánchez-Ramos, C.; Martín-Pérez, D.; Lytvyn, M.; de Nicolás-Hernández, J.; Rivera-Torres, J.; Arroyo, D.F.; Sanz-Rosa, D.; et al. Sustained Elevated Blood Pressure Accelerates Atherosclerosis Development in a Preclinical Model of Disease. Int. J. Mol. Sci. 2021, 22, 8448. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z. ApoE and Neurodegenerative Diseases in Aging. Adv. Exp. Med. Biol. 2018, 1086, 77–92. [Google Scholar]
- Rebeck, G.W. The role of APOE on lipid homeostasis and inflammation in normal brains. J. Lipid Res. 2017, 58, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Wang, Y.X. Cardiovascular functional phenotypes and pharmacological responses in apolipoprotein E deficient mice. Neurobiol. Aging 2005, 26, 309–316. [Google Scholar] [CrossRef]
- Agúndez, J.A.G.; García-Martín, E.; Rodríguez, C.; Benito-León, J.; Millán-Pascual, J.; Díaz-Sánchez, M.; Calleja, P.; Turpín-Fenoll, L.; Alonso-Navarro, H.; García-Albea, E.; et al. Endothelial nitric oxide synthase (NOS3) rs2070744 polymorphism and risk for multiple sclerosis. J. Neural. Transm. 2020, 127, 1167–1175. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Ghulmiyyah, L.M.; Tamayo, E.; Clark, S.M.; Hankins, G.D.; Anderson, G.D.; Saade, G.R.; Longo, M. Effect of a previous pregnancy on vascular function in endothelial nitric oxide synthase 3 knockout mice. Am. J. Obstet. Gynecol. 2007, 197, e1–e5. [Google Scholar] [CrossRef]
- Gao, Y.; Stuart, D.; Takahishi, T.; Kohan, D.E. Nephron-Specific Disruption of Nitric Oxide Synthase 3 Causes Hypertension and Impaired Salt Excretion. J. Am. Heart Assoc. 2018, 7, e009236. [Google Scholar] [CrossRef] [Green Version]
- Taşbulak, O.; Aktemur, T.; Şahin, A.A.; Demir, A.R.; Güler, A.; Topel, C.; Turkvatan, A.; Pusuroglu, H.; Erturk, M. Association of dipping pattern of blood pressure and atherosclerotic burden of coronary arteries in hypertensive patients. Kardiologiia 2022, 62, 56–64. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Nait, F.; Capobianco, F.; Sechi, L.A. Plasma lipoprotein(a) levels and atherosclerotic renal artery stenosis in hypertensive patients. Kidney Blood Press. Res. 2015, 40, 166–175. [Google Scholar] [CrossRef]
- Liang, C.; Oest, M.E.; Jones, J.C.; Prater, M.R. Gestational high saturated fat diet alters C57BL/6 mouse perinatal skeletal formation. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 362–369. [Google Scholar] [CrossRef]
- Li, Q.N.; Li, L.; Hou, G.; Wang, Z.B.; Hou, Y.; Liu, Z.H.; Schatten, H.; Sun, Q.Y. Glucocorticoid exposure affects female fertility by exerting its effect on the uterus but not on the oocyte: Lessons from a hypercortisolism mouse model. Hum. Reprod. 2018, 33, 2285–2294. [Google Scholar] [CrossRef]
- Bernardo, A.F.; Cortez, E.; Neves, F.A.; Vieira, A.K.; Soares Vde, M.; Rodrigues-Cunha, A.C.; Andrade, D.C.; Thole, A.A.; Gabriel-Costa, D.; Brum, P.C.; et al. Overnutrition during lactation leads to impairment in insulin signaling, up-regulation of GLUT1 and increased mitochondrial carbohydrate oxidation in heart of weaned mice. J. Nutr. Biochem. 2016, 29, 124–132. [Google Scholar] [CrossRef]
- Zuo, E.; Cai, Y.J.; Li, K.; Wei, Y.; Wang, B.A.; Sun, Y.; Liu, Z.; Liu, J.; Hu, X.; Wei, W.; et al. One-step generation of complete gene knockout mice and monkeys by CRISPR/Cas9-mediated gene editing with multiple sgRNAs. Cell Res. 2017, 27, 933–945. [Google Scholar] [CrossRef]
- Gao, Y.; Stuart, D.; Pollock, J.S.; Takahishi, T.; Kohan, D.E. Collecting duct-specific knockout of nitric oxide synthase 3 impairs water excretion in a sex-dependent manner. Am. J. Physiol. Renal Physiol. 2016, 311, F1074–F1083. [Google Scholar] [CrossRef]
- Li, L.; Garikepati, R.M.; Tsukerman, S.; Tiwari, S.; Ecelbarger, C.M. Salt sensitivity of nitric oxide generation and blood pressure in mice with targeted knockout of the insulin receptor from the renal tubule. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R505–R512. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Barton, M.; Husmann, M.; Meyer, M.R. Accelerated Vascular Aging as a Paradigm for Hypertensive Vascular Disease: Prevention and Therapy. Can. J. Cardiol. 2016, 32, 680–686.e4. [Google Scholar] [CrossRef]
- Wikstrand, J. New concepts in the treatment of elderly hypertensive patients. Am. Heart J. 1988, 116 Pt 2, 296–300. [Google Scholar] [CrossRef]
- Hurtubise, J.; McLellan, K.; Durr, K.; Onasanya, O.; Nwabuko, D.; Ndisang, J.F. The Different Facets of Dyslipidemia and Hypertension in Atherosclerosis. Curr. Atheroscler. Rep. 2016, 18, 82. [Google Scholar] [CrossRef]
- Hu, H.; Garcia-Barrio, M.; Jiang, Z.S.; Chen, Y.E.; Chang, L. Roles of Perivascular Adipose Tissue in Hypertension and Atherosclerosis. Antioxid. Redox Signal. 2021, 34, 736–749. [Google Scholar] [CrossRef]
- Forde, A.T.; Lewis, T.T.; Kershaw, K.N.; Bellamy, S.L.; Diez Roux, A.V. Perceived Discrimination and Hypertension Risk Among Participants in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2021, 10, e019541. [Google Scholar] [CrossRef]
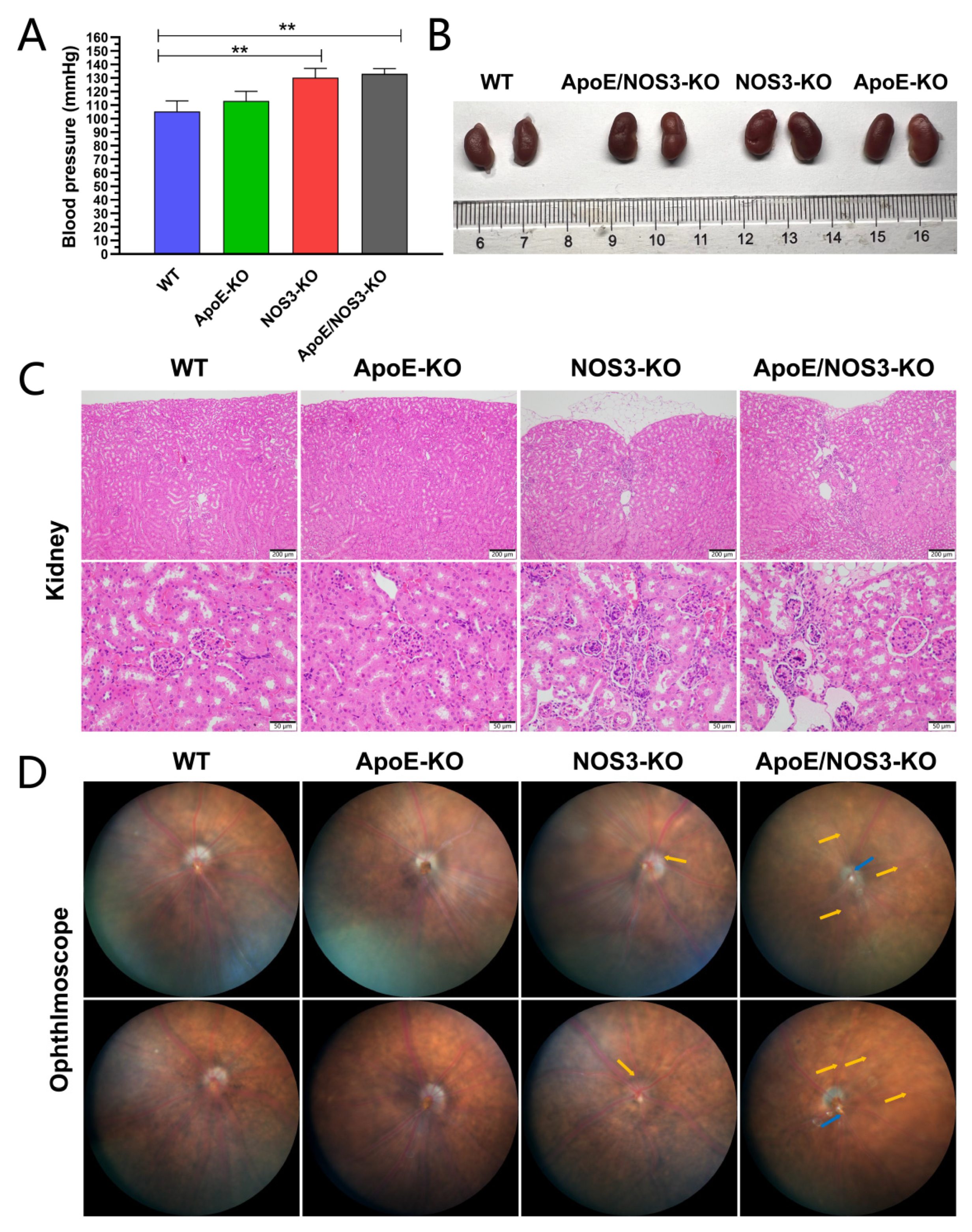

| WT | ApoE−/− | NOS3−/− | ApoE/NOS3−/− | |
|---|---|---|---|---|
| Blood pressure (mmHg) | 105.25 ± 7.74 | 113.00 ± 7.15 | 130.25 ± 6.71 ** | 133.00 ± 3.85 ** |
| Items (mmol/L) | 16 Weeks Old | 24 Weeks Old | ||||
|---|---|---|---|---|---|---|
| WT | ApoE−/− | ApoE/NOS3−/− | WT | ApoE−/− | ApoE/NOS3−/− | |
| TC | 2.49 ± 0.42 | 12.86 ± 1.92 ** | 13.45 ± 1.52 ** | 2.35 ± 0.31 | 15.78 ± 2.99 ** | 17.35 ± 1.07 ** |
| TG | 0.56 ± 0.12 | 0.66 ± 0.09 | 0.69 ± 0.16 | 0.77 ± 0.35 | 0.92 ± 0.28 | 0.86 ± 0.26 |
| HDL | 1.29 ± 0.29 | 1.21 ± 0.19 | 1.49 ± 0.31 | 1.31 ± 0.27 | 1.85 ± 0.31 ** | 1.69 ± 0.26 * |
| LDL | 0.27 ± 0.07 | 1.88 ± 0.42 ** | 2.45 ± 0.59 ** | 0.36 ± 0.06 | 4.21 ± 1.35 ** | 5.90 ± 0.44 ** |
| Items (mmol/L) | 16 Weeks Old | 24 Weeks Old | ||||
|---|---|---|---|---|---|---|
| WT | ApoE−/− | ApoE/NOS3−/− | WT | ApoE−/− | ApoE/NOS3−/− | |
| TC | 3.44 ± 0.60 | 19.10 ± 2.14 ** | 27.54 ± 2.88 ** | 3.39 ± 0.27 | 21.32 ± 2.97 ** | 28.46 ± 4.73 ** |
| TG | 0.56 ± 0.08 | 0.65 ± 0.08 | 1.50 ± 0.82 ** | 0.65 ± 0.15 | 0.85 ± 0.20 * | 1.31 ± 0.51 ** |
| HDL | 1.63 ± 0.41 | 2.08 ± 0.19 * | 2.64 ± 0.47 ** | 1.83 ± 0.18 | 2.11 ± 0.21 * | 3.33 ± 0.45 ** |
| LDL | 0.39 ± 0.09 | 5.65 ± 1.36 ** | 9.45 ± 1.93 ** | 0.41 ± 0.04 | 7.17 ± 1.39 ** | 13.84 ± 4.14 ** |
| Strain | Average Litter Size (n = 8) | Average Weaned Number (n = 8) | ||||||
|---|---|---|---|---|---|---|---|---|
| Parity 1 | Parity 2 | Parity 3 | Parity 4 | Parity 1 | Parity 2 | Parity 3 | Parity 4 | |
| A | 5.25 ± 2.53 | 6.17 ± 2.26 | 5.75 ± 2.73 | 5.92 ± 2.27 | 5.08 ± 2.85 | 5.08 ± 2.44 | 5.50 ± 2.58 | 4.92 ± 2.45 |
| N | 5.20 ± 1.23 | 5.10 ± 1.91 | 5.50 ± 2.84 | 4.20 ± 1.20 | 4.80 ± 1.55 | 4.70 ± 1.49 | 5.10 ± 2.51 | 3.50 ± 1.08 |
| AN | 4.71 ± 2.39 | 4.88 ± 2.47 | 4.64 ± 2.21 | 4.53 ± 2.20 | 4.36 ± 2.75 | 3.71 ± 2.90 | 3.23 ± 2.71 | 3.53 ± 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Chen, B.; Zeng, F.; Wang, G.; Wu, X.; Liu, Y.; Li, G.; Yan, J.; Zhang, S. ApoE/NOS3 Knockout Mice as a Novel Cardiovascular Disease Model of Hypertension and Atherosclerosis. Genes 2022, 13, 1998. https://doi.org/10.3390/genes13111998
Liu K, Chen B, Zeng F, Wang G, Wu X, Liu Y, Li G, Yan J, Zhang S. ApoE/NOS3 Knockout Mice as a Novel Cardiovascular Disease Model of Hypertension and Atherosclerosis. Genes. 2022; 13(11):1998. https://doi.org/10.3390/genes13111998
Chicago/Turabian StyleLiu, Ke, Bangzhu Chen, Fanwen Zeng, Gang Wang, Xin Wu, Yueshu Liu, Guiling Li, Jiarong Yan, and Shouquan Zhang. 2022. "ApoE/NOS3 Knockout Mice as a Novel Cardiovascular Disease Model of Hypertension and Atherosclerosis" Genes 13, no. 11: 1998. https://doi.org/10.3390/genes13111998




