Development of CRISPR-Mediated Nucleic Acid Detection Technologies and Their Applications in the Livestock Industry
Abstract
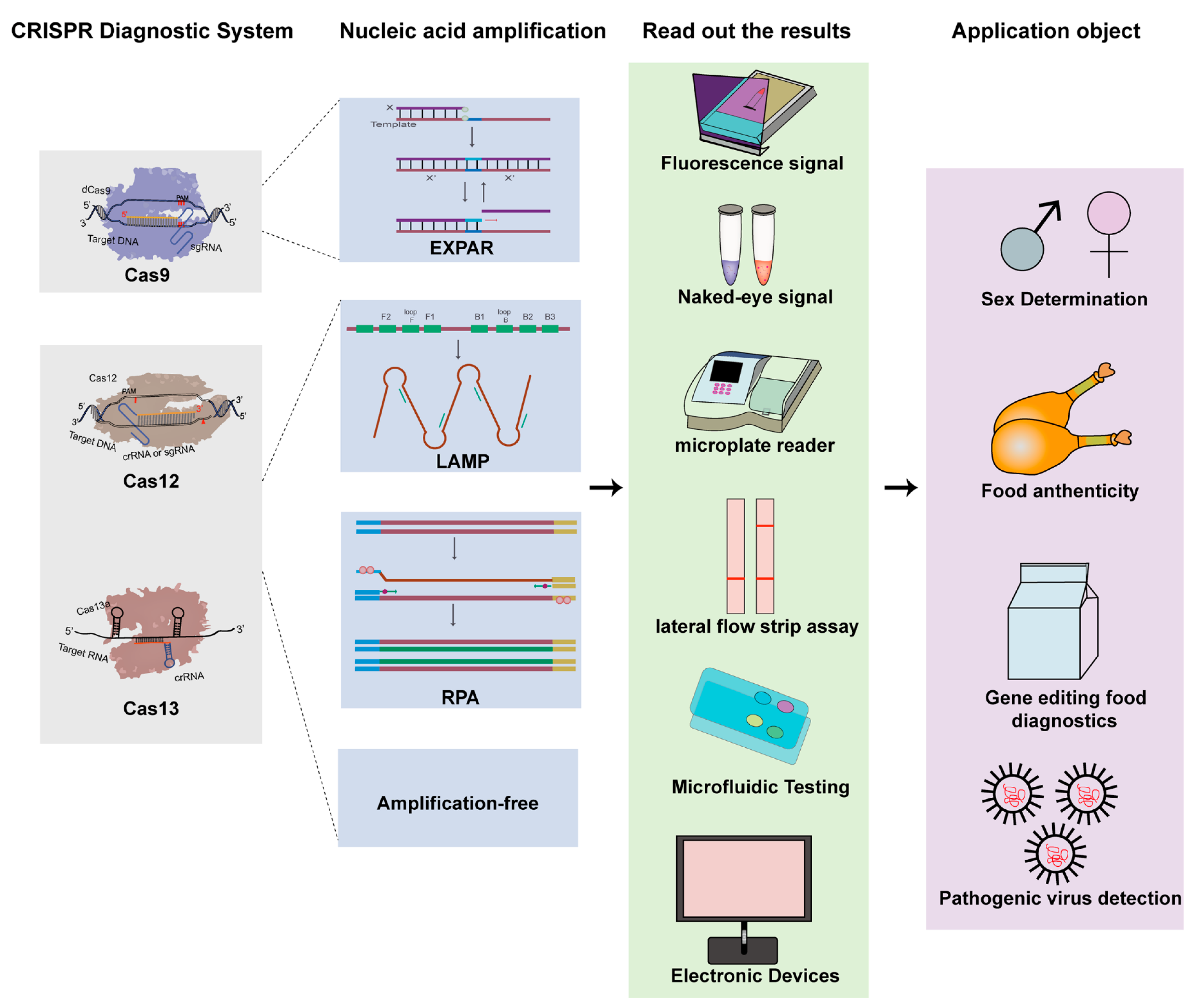
:1. Introduction
2. Development of CRISPR/Cas-Based Nucleic Acid Detection Systems
2.1. Cas Type II Based Diagnostic Platforms
2.2. Cas Type V and VI Based Diagnostic Platforms
2.3. Software for Designing CRISPR/Cas-Based Nucleic Acid Assays
2.4. Readout Methods
3. Current Applications of CRISPR/Cas-Based Nucleic Acid Detection Technologies in Livestock
3.1. CRISPR/Cas-Based Detection of Pathogenic Viruses in Livestock
3.2. CRISPR-Based Detection of Pathogenic Bacteria and Parasites in Livestock
3.3. CRISPR Assays for Sex Determination and Meat/Milk Products
4. Conclusions and Future Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, K.; Ye, C.; Chang, X.-B.; Jiang, C.-G.; Wang, S.-J.; Cai, X.-H.; Tong, G.-Z.; Tian, Z.-J.; Shi, M.; An, T.-Q. Importation and Recombination Are Responsible for the Latest Emergence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus in China. J. Virol. 2015, 89, 10712–10716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Byrum, B.; Zhang, Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014, 20, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Koenen, F.; De Clercq, K.; Lefebvre, J.; Strobbe, R. Reproductive failure in sows following experimental infection with a Belgian EMCV isolate. Vet. Microbiol. 1994, 39, 111–116. [Google Scholar] [CrossRef]
- Zhou, B. Classical Swine Fever in China-An Update Minireview. Front. Vet. Sci. 2019, 6, 187. [Google Scholar] [CrossRef] [Green Version]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef] [Green Version]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef]
- Davies, F.G. Lumpy skin disease, an African capripox virus disease of cattle. Br. Vet. J. 1991, 147, 489–503. [Google Scholar] [CrossRef]
- Uzzau, S.; Brown, D.J.; Wallis, T.; Rubino, S.; Leori, G.; Bernard, S.; Casadesús, J.; Platt, D.J.; Olsen, J.E. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 2000, 125, 229–255. [Google Scholar] [CrossRef]
- Xiao, L.; Fayer, R.; Ryan, U.; Upton, S.J. Cryptosporidium taxonomy: Recent advances and implications for public health. Clin. Microbiol. Rev. 2004, 17, 72–97. [Google Scholar] [CrossRef]
- La Rosa, G.; Fratini, M.; Della Libera, S.; Iaconelli, M.; Muscillo, M. Emerging and potentially emerging viruses in water environments. Ann. Ist. Super. Sanita 2012, 48, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Nichol, S.T.; Arikawa, J.; Kawaoka, Y. Emerging viral diseases. Proc. Natl. Acad. Sci. USA 2000, 97, 12411–12412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournié, G.; Kearsley-Fleet, L.; Otte, J.; Pfeiffer, D.U. Spatiotemporal trends in the discovery of new swine infectious agents. Vet. Res. 2015, 46, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Tognon, M.; Martini, F. Simultaneous Detection and Viral DNA Load Quantification of Different Human Papillomavirus Types in Clinical Specimens by the High Analytical Droplet Digital PCR Method. Front. Microbiol. 2020, 11, 591452. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Tests and Collection Kits Authorized by the FDA: Infographic. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/covid-19-tests-and-collection-kits-authorized-fda-infographic (accessed on 18 October 2022).
- Falzone, L.; Gattuso, G.; Tsatsakis, A.; Spandidos, D.A.; Libra, M. Current and innovative methods for the diagnosis of COVID-19 infection (Review). Int. J. Mol. Med. 2021, 47, 100. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Mostafa, A.; Berger, J.; Aydin, M.Y.; Sun, F.; de Ramirez, S.A.S.; Valera, E.; Cunningham, B.T.; King, W.P.; Bashir, R. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 22727–22735. [Google Scholar] [CrossRef]
- Subsoontorn, P.; Lohitnavy, M.; Kongkaew, C. The diagnostic accuracy of isothermal nucleic acid point-of-care tests for human coronaviruses: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 22349. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Caselli, E.; Gallenga, C.E.; Guarino, M.; de Giorgio, R.; Mazziotta, C.; Tramarin, M.L.; Badiale, G.; et al. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms 2022, 10, 1193. [Google Scholar] [CrossRef]
- Fitri, L.E.; Widaningrum, T.; Endharti, A.T.; Prabowo, M.H.; Winaris, N.; Nugraha, R.Y.B. Malaria diagnostic update: From conventional to advanced method. J. Clin. Lab. Anal. 2022, 36, e24314. [Google Scholar] [CrossRef]
- Kostyusheva, A.; Brezgin, S.; Babin, Y.; Vasilyeva, I.; Glebe, D.; Kostyushev, D.; Chulanov, V. CRISPR-Cas systems for diagnosing infectious diseases. Methods 2022, 203, 431–446. [Google Scholar] [CrossRef]
- Huang, W.E.; Lim, B.; Hsu, C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoli, L.M.; Spoto, G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors 2013, 3, 18–43. [Google Scholar] [CrossRef] [Green Version]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef]
- Chang, Y.; Deng, Y.; Li, T.; Wang, J.; Wang, T.; Tan, F.; Li, X.; Tian, K. Visual detection of porcine reproductive and respiratory syndrome virus using CRISPR-Cas13a. Transbound. Emerg. Dis. 2020, 67, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Thekisoe, O.; Kuboki, N.; Nambota, A.; Fujisaki, K.; Sugimoto, C.; Igarashi, I.; Yasuda, J.; Inoue, N. Species-specific loop-mediated isothermal amplification (LAMP) for diagnosis of trypanosomosis. Acta Trop. 2007, 102, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Yamano, T.; Nishimasu, H.; Zetsche, B.; Hirano, H.; Slaymaker, I.M.; Li, Y.; Fedorova, I.; Nakane, T.; Makarova, K.S.; Koonin, E.V.; et al. Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 2016, 165, 949–962. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.T.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guk, K.; Keem, J.O.; Hwang, S.G.; Kim, H.; Kang, T.; Lim, E.-K.; Jung, J. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosens. Bioelectron. 2017, 95, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, L.; Wei, W.; Wang, Y.; Wang, B.; Lin, P.; Liu, W.; Yixuan, Y.; Li, X.; Liu, D.; et al. Paired Design of dCas9 as a Systematic Platform for the Detection of Featured Nucleic Acid Sequences in Pathogenic Strains. ACS Synth. Biol. 2017, 6, 211–216. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, X.; Wang, H.; Xing, D. Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 Triggered Isothermal Amplification for Site-Specific Nucleic Acid Detection. Anal. Chem. 2018, 90, 2193–2200. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Xu, X.; Long, F.; Wang, J. CRISPR-typing PCR (ctPCR), a new Cas9-based DNA detection method. Sci. Rep. 2018, 8, 14126. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Hu, L.; Ying, L.; Zhao, Z.; Chu, P.K.; Yu, X.-F. A CRISPR–Cas9-triggered strand displacement amplification method for ultrasensitive DNA detection. Nat. Commun. 2018, 9, 5012. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, Q.; Xu, X.; Xia, Q.; Long, F.; Li, W.; Shui, Y.; Xia, X.; Wang, J. Detection of target DNA with a novel Cas9/sgRNAs-associated reverse PCR (CARP) technique. Anal. Bioanal. Chem. 2018, 410, 2889–2900. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, Q.; Wang, Q.; Xia, X.; Wang, J. Detecting and typing target DNA with a novel CRISPR-typing PCR (ctPCR) technique. Anal. Biochem. 2018, 561–562, 37–46. [Google Scholar] [CrossRef]
- Quan, J.; Langelier, C.; Kuchta, A.; Batson, J.; Teyssier, N.; Lyden, A.; Caldera, S.; McGeever, A.; Dimitrov, B.; King, R.; et al. FLASH: A next-generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. Nucleic Acids Res. 2019, 47, e83. [Google Scholar] [CrossRef]
- Hajian, R.; Balderston, S.; Tran, T.; DeBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.-Y.; Nokes, J.; Athaiya, M.; et al. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Cheng, Q.-X.; Wang, J.-M.; Li, X.-Y.; Zhang, Z.-L.; Gao, S.; Cao, R.-B.; Zhao, G.-P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, F.; Guo, L.; Cui, T.; Wang, X.-G.; Xu, K.; Gao, Q.; Zhou, Q.; Li, W. CDetection: CRISPR-Cas12b-based DNA detection with sub-attomolar sensitivity and single-base specificity. Genome Biol. 2019, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Somoza, R.; Wang, L.; Welter, J.F.; Li, Y.; Caplan, A.I.; Liu, C.C. Exploring the Trans-Cleavage Activity of CRISPR-Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor. Angew. Chem. Int. Ed. 2019, 58, 17399–17405. [Google Scholar] [CrossRef]
- English, M.A.; Soenksen, L.R.; Gayet, R.V.; de Puig, H.; Angenent-Mari, N.M.; Mao, A.S.; Nguyen, P.Q.; Collins, J.J. Programmable CRISPR-responsive smart materials. Science 2019, 365, 780–785. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Wu, N.; Wu, J.; Wang, G.; Zhao, G.-P.; Wang, J. HOLMESv2: A CRISPR-Cas12b-Assisted Platform for Nucleic Acid Detection and DNA Methylation Quantitation. ACS Synth. Biol. 2019, 8, 2228–2237. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, M.; Liu, Y.; Ma, P.; Dang, L.; Meng, Q.; Wan, W.; Ma, X.; Liu, J.; Yang, G.; et al. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci. Bull. 2020, 65, 1436–1439. [Google Scholar] [CrossRef]
- Guo, L.; Sun, X.; Wang, X.; Liang, C.; Jiang, H.; Gao, Q.; Dai, M.; Qu, B.; Fang, S.; Mao, Y.; et al. SARS-CoV-2 detection with CRISPR diagnostics. Cell. Discov. 2020, 6, 34. [Google Scholar] [CrossRef]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N.; Woolley, A.E.; Segel, M.; Barretto, R.P.J.; Ranu, A.; Macrae, R.K.; Faure, G.; et al. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020, 383, 1492–1494. [Google Scholar] [CrossRef]
- Lu, S.; Li, F.; Chen, Q.; Wu, J.; Duan, J.; Lei, X.; Zhang, Y.; Zhao, D.; Bu, Z.; Yin, H. Rapid detection of African swine fever virus using Cas12a-based portable paper diagnostics. Cell Discov. 2020, 6, 18. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, C.; Tian, T.; Wang, X.; Sun, J.; Xiong, E.; Zhou, X. Single-Step, Salt-Aging-Free, and Thiol-Free Freezing Construction of AuNP-Based Bioprobes for Advancing CRISPR-Based Diagnostics. J. Am. Chem. Soc. 2020, 142, 7506–7513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yan, Y.; Que, H.; Yang, T.; Cheng, X.; Ding, S.; Zhang, X.; Cheng, W. CRISPR/Cas12a-Mediated Interfacial Cleaving of Hairpin DNA Reporter for Electrochemical Nucleic Acid Sensing. ACS Sens. 2020, 5, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shi, Z.; Qian, J.; Bi, K.; Fang, M.; Xu, Z. A CRISPR-Cas12a-derived biosensor enabling portable personal glucose meter readout for quantitative detection of SARS-CoV-2. Biotechnol. Bioeng. 2021, 118, 1587–1596. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, L.; Liu, C.; Ye, S.; Chen, W.; Li, D.; Huang, W. One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a. J. Transl. Med. 2021, 19, 74. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z.; et al. opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-CoV-2 detection. Biosens. Bioelectron. 2021, 172, 112766. [Google Scholar] [CrossRef]
- Choi, J.H.; Lim, J.; Shin, M.; Paek, S.; Choi, J. CRISPR-Cas12a-Based Nucleic Acid Amplification-Free DNA Biosensor via Au Nanoparticle-Assisted Metal-Enhanced Fluorescence and Colorimetric Analysis. Nano Lett. 2021, 21, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Zhang, Q.; Xia, X.; Li, C.; Ho, W.K.H.; Yan, J.; Huang, Y.; Wu, H.; Wang, P.; Yi, C.; et al. A CRISPR-Cas12a integrated SERS nanoplatform with chimeric DNA/RNA hairpin guide for ultrasensitive nucleic acid detection. Theranostics 2022, 12, 5914–5930. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Gurijala, J.; Rananaware, S.R.; Pizzano, B.L.; Stone, B.T.; Jain, P.K. CRISPR-ENHANCE: An enhanced nucleic acid detection platform using Cas12a. Methods 2022, 203, 116–124. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Li, T.; Fan, T.; Meng, C.; Li, C.; Kang, J.; Chai, L.; Hao, Y.; Tang, Y.; et al. A CRISPR/Cas12a-empowered surface plasmon resonance platform for rapid and specific diagnosis of the Omicron variant of SARS-CoV-2. Natl. Sci. Rev. 2022, 9, nwac104. [Google Scholar] [CrossRef]
- Xie, S.; Tao, D.; Fu, Y.; Xu, B.; Tang, Y.; Steinaa, L.; Hemmink, J.D.; Pan, W.; Huang, X.; Nie, X.; et al. Rapid Visual CRISPR Assay: A Naked-Eye Colorimetric Detection Method for Nucleic Acids Based on CRISPR/Cas12a and a Convolutional Neural Network. ACS Synth. Biol. 2022, 11, 383–396. [Google Scholar] [CrossRef]
- Lu, S.; Tong, X.; Han, Y.; Zhang, K.; Zhang, Y.; Chen, Q.; Duan, J.; Lei, X.; Huang, M.; Qiu, Y.; et al. Fast and sensitive detection of SARS-CoV-2 RNA using suboptimal protospacer adjacent motifs for Cas12a. Nat. Biomed. Eng. 2022, 6, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chan, C.; Springs, S.L.; Lee, Y.H.; Lu, T.K.; Yu, H. A warm-start digital CRISPR/Cas-based method for the quantitative detection of nucleic acids. Anal. Chim. Acta 2022, 1196, 339494. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F.; Doudna, J.A. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhrvold, C.; Freije, C.A.; Gootenberg, J.S.; Abudayyeh, O.O.; Metsky, H.C.; Durbin, A.F.; Kellner, M.J.; Tan, A.L.; Paul, L.M.; Parham, L.A.; et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.-S.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection with Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef]
- Arizti-Sanz, J.; Freije, C.A.; Stanton, A.C.; Petros, B.A.; Boehm, C.K.; Siddiqui, S.; Shaw, B.M.; Adams, G.; Kosoko-Thoroddsen, T.-S.F.; Kemball, M.E.; et al. Streamlined inactivation, amplification, and Cas13-based detection of SARS-CoV-2. Nat. Commun. 2020, 11, 5921. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, T.; Zhang, S.; Johnston, M.; Zheng, X.; Shan, Y.; Liu, T.; Huang, Z.; Qian, F.; Xie, Z.; et al. A CRISPR/Cas13a-powered catalytic electrochemical biosensor for successive and highly sensitive RNA diagnostics. Biosens. Bioelectron. 2021, 178, 113027. [Google Scholar] [CrossRef]
- Fozouni, P.; Son, S.; Derby, M.D.d.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 2021, 184, 323–333.e9. [Google Scholar] [CrossRef]
- Tian, T.; Shu, B.; Jiang, Y.; Ye, M.; Liu, L.; Guo, Z.; Han, Z.; Wang, Z.; Zhou, X. An Ultralocalized Cas13a Assay Enables Universal and Nucleic Acid Amplification-Free Single-Molecule RNA Diagnostics. ACS Nano 2021, 15, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Wu, G.; Weng, Z.; Song, Y.; Zhang, Y.; Vanegas, J.A.; Avery, L.; Gao, D.Z.; Sun, H.; et al. Amplification-Free Detection of SARS-CoV-2 and Respiratory Syncytial Virus Using CRISPR Cas13a and Graphene Field-Effect Transistors. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203826. [Google Scholar] [PubMed]
- Samanta, D.; Ebrahimi, S.B.; Ramani, N.; Mirkin, C.A. Enhancing CRISPR-Cas-Mediated Detection of Nucleic Acid and Non-nucleic Acid Targets Using Enzyme-Labeled Reporters. J. Am. Chem. Soc. 2022, 144, 16310–16315. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Tian, T.; Jiang, Y.; Xiong, E.; Zhu, D.; Zhou, X. A CRISPR/Cas9 eraser strategy for contamination-free PCR end-point detection. Biotechnol. Bioeng. 2021, 118, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Liu, J.; Nie, X.; Xu, B.; Tran-Thi, T.-N.; Niu, L.; Liu, X.; Ruan, J.; Lan, X.; Peng, G.; et al. Application of CRISPR-Cas12a Enhanced Fluorescence Assay Coupled with Nucleic Acid Amplification for the Sensitive Detection of African Swine Fever Virus. ACS Synth. Biol. 2020, 9, 2339–2350. [Google Scholar] [CrossRef]
- Yang, B.; Shi, Z.; Ma, Y.; Wang, L.; Cao, L.; Luo, J.; Wan, Y.; Song, R.; Yan, Y.; Yuan, K.; et al. LAMP assay coupled with CRISPR/Cas12a system for portable detection of African swine fever virus. Transbound. Emerg. Dis. 2022, 69, e216–e223. [Google Scholar] [CrossRef]
- Wei, N.; Zheng, B.; Niu, J.; Chen, T.; Ye, J.; Si, Y.; Cao, S. Rapid Detection of Genotype II African Swine Fever Virus Using CRISPR Cas13a-Based Lateral Flow Strip. Viruses 2022, 14, 179. [Google Scholar] [CrossRef]
- Zeng, M.; Ke, Y.; Zhuang, Z.; Qin, C.; Li, L.Y.; Sheng, G.; Li, Z.; Meng, H.; Ding, X. Harnessing Multiplex crRNA in the CRISPR/Cas12a System Enables an Amplification-Free DNA Diagnostic Platform for ASFV Detection. Anal. Chem. 2022, 94, 10805–10812. [Google Scholar] [CrossRef]
- Liu, S.; Tao, D.; Liao, Y.; Yang, Y.; Sun, S.; Zhao, Y.; Yang, P.; Tang, Y.; Chen, B.; Liu, Y.; et al. Highly Sensitive CRISPR/Cas12a-Based Fluorescence Detection of Porcine Reproductive and Respiratory Syndrome Virus. ACS Synth. Biol. 2021, 10, 2499–2507. [Google Scholar] [CrossRef]
- Yang, K.; Liang, Y.; Li, Y.; Liu, Q.; Zhang, W.; Yin, D.; Song, X.; Shao, Y.; Tu, J.; Qi, K. Reverse transcription–enzymatic recombinase amplification coupled with CRISPR-Cas12a for rapid detection and differentiation of PEDV wild-type strains and attenuated vaccine strains. Anal. Bioanal. Chem. 2021, 413, 7521–7529. [Google Scholar] [CrossRef]
- Qian, B.; Liao, K.; Zeng, D.; Peng, W.; Wu, X.; Li, J.; Bo, Z.; Hu, Y.; Nan, W.; Wen, Y.; et al. Clustered Regularly Interspaced Short Palindromic Repeat/Cas12a Mediated Multiplexable and Portable Detection Platform for GII Genotype Porcine Epidemic Diarrhoea Virus Rapid Diagnosis. Front. Microbiol. 2022, 13, 920801. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tao, D.; Chen, X.; Shen, L.; Zhu, L.; Xu, B.; Liu, H.; Zhao, S.; Li, X.; Liu, X.; et al. Detection of Four Porcine Enteric Coronaviruses Using CRISPR-Cas12a Combined with Multiplex Reverse Transcriptase Loop-Mediated Isothermal Amplification Assay. Viruses 2022, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, L.; Liu, Q.; Cao, Y.; Yang, K.; Song, X.; Shao, Y.; Tu, J.; Qi, K. Enzymatic recombinase amplification coupled with CRISPR-Cas12a for ultrasensitive, rapid, and specific Porcine circovirus 3 detection. Mol. Cell. Probes 2021, 60, 101772. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Cao, Y.; Liu, Q.; Yang, K.; Song, X.; Shao, Y.; Qi, K.; Tu, J. Rapid and Visual Detection of Porcine Parvovirus Using an ERA-CRISPR/Cas12a System Combined with Lateral Flow Dipstick Assay. Front. Cell. Infect. Microbiol. 2022, 12, 879887. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gong, P.; Zhang, Y.; Wang, Y.; Tao, D.; Fu, L.; Khazalwa, E.M.; Liu, H.; Zhao, S.; Zhang, X.; et al. A one-tube rapid visual CRISPR assay for the field detection of Japanese encephalitis virus. Virus Res. 2022, 319, 198869. [Google Scholar] [CrossRef]
- Wei, N.; Xiong, J.; Ma, J.; Ye, J.; Si, Y.; Cao, S. Development of efficient, sensitive, and specific detection method for Encephalomyocarditis virus based on CRISPR/Cas13a. J. Virol. Methods 2022, 309, 114592. [Google Scholar] [CrossRef]
- Jiang, C.; Tao, D.; Geng, Y.; Yang, H.; Xu, B.; Chen, Y.; Hu, C.; Chen, H.; Xie, S.; Guo, A. Sensitive and Specific Detection of Lumpy Skin Disease Virus in Cattle by CRISPR-Cas12a Fluorescent Assay Coupled with Recombinase Polymerase Amplification. Genes 2022, 13, 734. [Google Scholar] [CrossRef]
- Yao, R.; Xu, Y.; Wang, L.; Wang, D.; Ren, L.; Ren, C.; Li, C.; Li, X.; Ni, W.; He, Y.; et al. CRISPR-Cas13a-Based Detection for Bovine Viral Diarrhea Virus. Front. Vet. Sci. 2021, 8, 603919. [Google Scholar] [CrossRef]
- Chen, X.; Nie, F.; Xiong, Y.; Lin, L.; Shi, M.; Yang, J.; Wang, Y.; Wang, G.; Li, Y.; Huo, D.; et al. Ultra-sensitive and point-of-care detection of Capripoxvirus (CaPV) based on loop-mediated amplification (LAMP) and trans-cleavage activity of CRISPR/Cpf1. Anal. Chim. Acta 2022, 1191, 339330. [Google Scholar] [CrossRef]
- Lei, R.; Li, L.; Wu, P.; Fei, X.; Zhang, Y.; Wang, J.; Zhang, D.; Zhang, Q.; Yang, N.; Wang, X. RPA/CRISPR/Cas12a-Based On-Site and Rapid Nucleic Acid Detection of Toxoplasma gondii in the Environment. ACS Synth. Biol. 2022, 11, 1772–1781. [Google Scholar] [CrossRef]
- Ma, Q.-N.; Wang, M.; Zheng, L.-B.; Lin, Z.-Q.; Ehsan, M.; Xiao, X.-X.; Zhu, X.-Q. RAA-Cas12a-Tg: A Nucleic Acid Detection System for Toxoplasma gondii Based on CRISPR-Cas12a Combined with Recombinase-Aided Amplification (RAA). Microorganisms 2021, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Xue, Q.; Zhu, Z.; Zou, M.; Fang, F. A novel rapid visual detection assay for Toxoplasma gondii combining recombinase-aided amplification and lateral flow dipstick coupled with CRISPR-Cas13a fluorescence (RAA-Cas13a-LFD). Parasite 2022, 29, 21. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, K.; Wang, Y.; Li, D.; Cui, Z.; Huang, J.; Zhang, S.; Li, X.; Zhang, L. CRISPR/Cas12a-based on-site diagnostics of Cryptosporidium parvum IId-subtype-family from human and cattle fecal samples. Parasites Vectors 2021, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, J.; Li, Y.; Kang, L.; Yuan, B.; Li, S.; Chao, J.; Wang, L.; Wang, J.; Su, S.; et al. A general RPA-CRISPR/Cas12a sensing platform for Brucella spp. detection in blood and milk samples. Sens. Actuators B Chem. 2022, 364, 131864. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, Y.; Liu, W.; Sun, Y.; Ding, X. A One-Pot Toolbox Based on Cas12a/crRNA Enables Rapid Foodborne Pathogen Detection at Attomolar Level. ACS Sens. 2020, 5, 1427–1435. [Google Scholar] [CrossRef]
- Fang, T.; Shen, J.; Xue, J.; Jiang, Y.; Guo, D.; Yang, J.; Kong, X.; Xu, X.; Wang, X. Sensitive and rapid detection of Escherichia coli O157:H7 from beef samples based on recombinase aided amplification assisted CRISPR/Cas12a system. J. AOAC Int. 2022, qsac101. [Google Scholar] [CrossRef]
- Zhi, S.; Shen, J.; Li, X.; Jiang, Y.; Xue, J.; Fang, T.; Xu, J.; Wang, X.; Cao, Y.; Yang, D.; et al. Development of Recombinase-Aided Amplification (RAA)-Exo-Probe and RAA-CRISPR/Cas12a Assays for Rapid Detection of Campylobacter jejuni in Food Samples. J. Agric. Food Chem. 2022, 70, 9557–9566. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, T.; Liu, C.; Xu, Q.; Fang, S.; Wu, Y.; Wu, M.; Liu, Q. An ultrasensitive and contamination-free on-site nucleic acid detection platform for Listeria monocytogenes based on the CRISPR-Cas12a system combined with recombinase polymerase amplification. LWT 2021, 152, 112166. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, H.; Hu, P.; Wang, Y.; Wang, H.; Li, Y.; Feng, K.; Wang, C.; Cao, Q.; Guo, Y.; et al. Ultra-Sensitive and Rapid Detection of Pathogenic Yersinia enterocolitica Based on the CRISPR/Cas12a Nucleic Acid Identification Platform. Foods 2022, 11, 2160. [Google Scholar] [CrossRef]
- Tao, D.; Liu, J.; Li, Q.; Jiang, Y.; Xu, B.; Khazalwa, E.M.; Gong, P.; Xu, J.; Ma, Y.; Ruan, J.; et al. A Simple, Affordable, and Rapid Visual CRISPR-Based Field Test for Sex Determination of Earlier Porcine Embryos and Pork Products. Mol. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Li, H.-T.; Zhang, T.; Dong, Y.; Deng, S.; Lv, Y.; He, Q.; Deng, R. CRISPR-Cas system meets DNA barcoding: Development of a universal nucleic acid test for food authentication. Sens. Actuators B Chem. 2022, 353, 131138. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Yao, C.; Xie, P.; Li, X.; Xu, Z.; Xian, Y.; Lei, H.; Shen, X. Alkaline lysis-recombinase polymerase amplification combined with CRISPR/Cas12a assay for the ultrafast visual identification of pork in meat products. Food Chem. 2022, 383, 132318. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Xiao, X.; Lan, X.; Xu, B.; Wang, Y.; Khazalwa, E.M.; Pan, W.; Ruan, J.; Jiang, Y.; Liu, X.; et al. An Inexpensive CRISPR-Based Point-of-Care Test for the Identification of Meat Species and Meat Products. Genes 2022, 13, 912. [Google Scholar] [CrossRef]
- Pan, R.; Liu, J.; Wang, P.; Wu, D.; Chen, J.; Wu, Y.; Li, G. Ultrasensitive CRISPR/Cas12a-Driven SERS Biosensor for On-Site Nucleic Acid Detection and Its Application to Milk Authenticity Testing. J. Agric. Food Chem. 2022, 70, 4484–4491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, E.; Tian, T.; Cheng, M.; Lin, W.; Sun, J.; Zhou, X. CASLFA: CRISPR/Cas9-mediated lateral flow nucleic acid assay. bioRxiv 2019. [Google Scholar] [CrossRef]
- Srivastava, S.; Upadhyay, D.J.; Srivastava, A. Next-Generation Molecular Diagnostics Development by CRISPR/Cas Tool: Rapid Detection and Surveillance of Viral Disease Outbreaks. Front. Mol. Biosci. 2020, 7, 582499. [Google Scholar] [CrossRef]
- Welch, N.L.; Zhu, M.; Hua, C.; Weller, J.; Mirhashemi, M.E.; Nguyen, T.G.; Mantena, S.; Bauer, M.R.; Shaw, B.M.; Ackerman, C.M.; et al. Multiplexed CRISPR-based microfluidic platform for clinical testing of respiratory viruses and identification of SARS-CoV-2 variants. Nat. Med. 2022, 28, 1083–1094. [Google Scholar] [CrossRef]
- Thakku, S.G.; Ackerman, C.M.; Myhrvold, C.; Bhattacharyya, R.P.; Livny, J.; Ma, P.; Gomez, G.I.; Sabeti, P.C.; Blainey, P.C.; Hung, D.T. Multiplexed detection of bacterial nucleic acids using Cas13 in droplet microarrays. PNAS Nexus 2022, 1, pgac021. [Google Scholar] [CrossRef]
- Concordet, J.-P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef] [Green Version]
- Labun, K.; Montague, T.G.; Gagnon, J.A.; Thyme, S.B.; Valen, E. CHOPCHOP v2: A web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016, 44, W272–W276. [Google Scholar] [CrossRef]
- Pliatsika, V.; Rigoutsos, I. “Off-Spotter”: Very fast and exhaustive enumeration of genomic lookalikes for designing CRISPR/Cas guide RNAs. Biol. Direct 2015, 10, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, G.-H.; Kim, J.-S.; Bae, S. Web-Based CRISPR Toolkits: Cas-OFFinder, Cas-Designer, and Cas-Analyzer. Methods Mol. Biol. 2021, 2162, 23–33. [Google Scholar] [PubMed]
- Liu, H.; Wei, Z.; Dominguez, A.; Li, Y.; Wang, X.; Qi, L.S. CRISPR-ERA: A comprehensive design tool for CRISPR-mediated gene editing, repression and activation. Bioinformatics 2015, 31, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Heigwer, F.; Kerr, G.; Boutros, M. E-CRISP: Fast CRISPR target site identification. Nat. Methods 2014, 11, 122–123. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, X.; Qu, W.; Li, G.; Li, X.; Miao, Y.-L.; Changzhi, Z.; Liu, X.; Li, Z.; Ma, Y.; et al. CRISPR-offinder: A CRISPR guide RNA design and off-target searching tool for user-defined protospacer adjacent motif. Int. J. Biol. Sci. 2017, 13, 1470–1478. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Richmond, E.; Liang, C. CRISPR-RT: A web application for designing CRISPR-C2c2 crRNA with improved target specificity. Bioinformatics 2018, 34, 117–119. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Liang, C. CRISPR-DT: Designing gRNAs for the CRISPR-Cpf1 system with improved target efficiency and specificity. Bioinformatics 2019, 35, 2783–2789. [Google Scholar] [CrossRef]
- McKenna, A.; Shendure, J. FlashFry: A fast and flexible tool for large-scale CRISPR target design. BMC Biol. 2018, 16, 74. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, Z.; Li, C.; Hao, Y.; Tang, Y.; Yuan, Y.; Chai, L.; Fan, T.; Yu, J.; Ma, X.; et al. CRISPR-Cas12a-Empowered Electrochemical Biosensor for Rapid and Ultrasensitive Detection of SARS-CoV-2 Delta Variant. Nano-Micro Lett. 2022, 14, 159. [Google Scholar] [CrossRef]
- Hu, T.; Ke, X.; Ou, Y.; Lin, Y. CRISPR/Cas12a-Triggered Chemiluminescence Enhancement Biosensor for Sensitive Detection of Nucleic Acids by Introducing a Tyramide Signal Amplification Strategy. Anal. Chem. 2022, 94, 8506–8513. [Google Scholar] [CrossRef]
- Ke, X.; Ou, Y.; Lin, Y.; Hu, T. Enhanced chemiluminescence imaging sensor for ultrasensitive detection of nucleic acids based on HCR-CRISPR/Cas12a. Biosens. Bioelectron. 2022, 212, 114428. [Google Scholar] [CrossRef] [PubMed]
- Garigliany, M.; Desmecht, D.; Tignon, M.; Cassart, D.; Lesenfant, C.; Paternostre, J.; Volpe, R.; Cay, A.B.; van den Berg, T.; Linden, A. Phylogeographic Analysis of African Swine Fever Virus, Western Europe, 2018. Emerg. Infect. Dis. 2019, 25, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, K.; Sun, W.; Zhao, J.; Yin, Y.; Si, H.; Qu, S.; Lu, W. Development a multiplex RT-PCR assay for simultaneous detection of African swine fever virus, classical swine fever virus and atypical porcine pestivirus. J. Virol. Methods 2021, 287, 114006. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Chu, J.-Q.; Hu, X.-M.; Kim, M.-C.; Park, C.-S.; Jun, M.-H. Development and validation of a recombinant nucleocapsid protein-based ELISA for detection of the antibody to porcine reproductive and respiratory syndrome virus. J. Microbiol. 2009, 47, 582–588. [Google Scholar] [CrossRef]
- Chai, Z.; Ma, W.; Fu, F.; Lang, Y.; Wang, W.; Tong, G.; Liu, Q.; Cai, X.; Li, X. A SYBR Green-based real-time RT-PCR assay for simple and rapid detection and differentiation of highly pathogenic and classical type 2 porcine reproductive and respiratory syndrome virus circulating in China. Arch. Virol. 2013, 158, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Spear, A.; Faaberg, K.S. Development of a genome copy specific RT-qPCR assay for divergent strains of type 2 porcine reproductive and respiratory syndrome virus. J. Virol. Methods 2015, 218, 1–6. [Google Scholar] [CrossRef]
- Lin, C.-N.; Lin, W.-H.; Hung, L.-N.; Wang, S.-Y.; Chiou, M.-T. Comparison of viremia of type II porcine reproductive and respiratory syndrome virus in naturally infected pigs by zip nucleic acid probe-based real-time PCR. BMC Vet. Res. 2013, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-W.; Dickerman, A.W.; Piñeyro, P.; Li, L.; Fang, L.; Kiehne, R.; Opriessnig, T.; Meng, X.-J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio 2013, 4, e00737-13. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Li, W.; van Kuppeveld, F.J.M.; He, Q.; Rottier, P.J.M.; Bosch, B. Cellular entry of the porcine epidemic diarrhea virus. Virus. Res. 2016, 226, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, G.; Wang, J.; Man, K.; Yang, Q. Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine 2017, 35, 7033–7041. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, G.; Stasko, J.; Thomas, J.T.; Stensland, W.R.; Pillatzki, A.E.; Gauger, P.C.; Schwartz, K.J.; Madson, D.; Yoon, K.-J.; et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014, 52, 234–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlasova, A.N.; Marthaler, D.; Wang, Q.; Culhane, M.R.; Rossow, K.D.; Rovira, A.; Collins, J.; Saif, L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg. Infect. Dis. 2014, 20, 1620–1628. [Google Scholar] [CrossRef] [Green Version]
- Godfroid, J.; Scholz, H.; Barbier, T.; Nicolas, C.; Wattiau, P.; Fretin, D.; Whatmore, A.; Cloeckaert, A.; Blasco, J.; Moriyon, I.; et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Vet. Med. 2011, 102, 118–131. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E. The one hundred year journey of the genus Brucella (Meyer and Shaw 1920). FEMS Microbiol. Rev. 2021, 45, fuaa045. [Google Scholar] [CrossRef]
- Głowacka, P.; Żakowska, D.; Naylor, K.; Niemcewicz, M.; Bielawska-Drózd, A. Brucella—Virulence Factors, Pathogenesis and Treatment. Pol. J. Microbiol. 2018, 67, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 2015, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Nissapatorn, V. Toxoplasma gondii and HIV: A never-ending story. Lancet HIV 2017, 4, e146–e147. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, Z.; Li, H.-L.; Zheng, H.; He, S.; Lin, R.-Q.; Zhu, X.-Q. Toxoplasma gondii infection in humans in China. Parasites Vectors 2011, 4, 165. [Google Scholar] [CrossRef]
- Nayeri, T.; Sarvi, S.; Moosazadeh, M.; Daryani, A. Global prevalence of Toxoplasma gondii infection in the aborted fetuses and ruminants that had an abortion: A systematic review and meta-analysis. Vet. Parasitol. 2021, 290, 109370. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cervantes, R.; Córdova-Izquierdo, A. Sexing sperm of domestic animals. Trop. Anim. Health Prod. 2012, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schinckel, A.P.; Mahan, D.C.; Wiseman, T.G.; Einstein, M.E. Growth of protein, moisture, lipid, and ash of two genetic lines of barrows and gilts from twenty to one hundred twenty-five kilograms of body weight1. J. Anim. Sci. 2008, 86, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Osada, M.; Iwabuchi, H.; Aoki, T.; Sasaki, K.; Ushijima, H.; Ozawa, T. Economic evaluation of artificial insemination of sex-sorted semen on a Brown Swiss dairy farm—A case study. Anim. Sci. J. 2019, 90, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Carabús, A.; Sainz, R.D.; Oltjen, J.W.; Gispert, M.; Font-I-Furnols, M. Growth of total fat and lean and of primal cuts is affected by the sex type. Animal 2017, 11, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Choi, T.J.; Cho, K.H.; Cho, E.S.; Lee, J.J.; Chung, H.J.; Baek, S.Y.; Jeong, Y.D. Effects of Sex and Breed on Meat Quality and Sensory Properties in Three-way Crossbred Pigs Sired by Duroc or by a Synthetic Breed Based on a Korean Native Breed. Korean J. Food Sci. Anim. Resour. 2018, 38, 544–553. [Google Scholar]
- Gunawan, A.; Sahadevan, S.; Neuhoff, C.; Große-Brinkhaus, C.; Gad, A.; Frieden, L.; Tesfaye, D.; Tholen, E.; Looft, C.; Uddin, M.J.; et al. RNA Deep Sequencing Reveals Novel Candidate Genes and Polymorphisms in Boar Testis and Liver Tissues with Divergent Androstenone Levels. PLoS ONE 2013, 8, e63259. [Google Scholar] [CrossRef]
- Barnett, J.; Begen, F.; Howes, S.; Regan, A.; McConnon, A.; Marcu, A.; Rowntree, S.; Verbeke, W. Consumers’ confidence, reflections and response strategies following the horsemeat incident. Food Control 2016, 59, 721–730. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Calleja, I.M.; Gonzalez, I.; Fajardo, V.; Hernández, P.E.; García, T.; Martín, R. Application of an indirect ELISA and a PCR technique for detection of cows’ milk in sheep’s and goats’ milk cheeses. Int. Dairy J. 2007, 17, 87–93. [Google Scholar] [CrossRef]
- Jian, S.-H.; Yeh, P.-J.; Wang, C.-H.; Chen, H.-C.; Chen, S.-F. Analysis of heterocyclic amines in meat products by liquid chromatography—Tandem mass spectrometry. J. Food Drug Anal. 2019, 27, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.X.; Cui, J.Q.; Park, H.; Chan, K.W.; Leung, T.; Tang, B.Z.; Yao, S. Isothermal Background-Free Nucleic Acid Quantification by a One-Pot Cas13a Assay Using Droplet Microfluidics. Anal. Chem. 2022, 94, 5883–5892. [Google Scholar] [CrossRef] [PubMed]
| Nuclease | Platform Name | Target | Amplification Method | Readout Method | Mechanism | Model Organism | Year | Refs. |
|---|---|---|---|---|---|---|---|---|
| Cas9 | NASBACC | RNA | NASBA | Colorimetry | PAM identification and cleavage to trigger Toehead switch | Zika Virus | 2016 | [33] |
| DNA-FISH | DNA | - | Fluorescence | dCas9/sgRNA complex serves as targeting material, SYBR Green I works as fluorescent probe | MRSA | 2017 | [34] | |
| PC REPORTER | DNA | PCR | Luminescence | dCas9 linked to the N-terminal and C-terminal halves of firefly luciferase are programmed with sgRNA complementary to the up- and down-stream fragments of target DNA sequence to induce luminescence after colocalization | Mycobacterium tuberculosis | 2017 | [35] | |
| CAS-EXPAR | DNA, RNA | EXPAR | Fluorescence | Cas9 generates nicks and NEase cycling generates ssDNA internal primers | Listeria monocytogenes | 2018 | [36] | |
| ctPCR | DNA | PCR | Electrophoresis/qPCR | Amplification of target DNA by PCR1 with a pair of universal primers, treatment of PCR1 products via a procedure of Cas9 cleavage, A tailing and T adaptor ligation, and amplification of the treated DNA by PCR2 using universal specific primers | HPV 16/18 | 2018 | [37] | |
| CRISDA | DNA | SDA | Fluorescence | Cas9 forms notches at the boundary, target gene is amplified by external primers | SNPs | 2018 | [38] | |
| CARP | DNA | PCR | Electrophoresis/qPCR | Cleavage of target DNA with Cas9 targeted by a pair of sgRNAs, ligation of the cleaved DNA using DNA ligase, PCR amplification of the ligated DNA | HPV 16/18 | 2018 | [39] | |
| ctPCR3.0 | DNA | qPCR | qPCR | Amplification of Cas9/sgRNA-cleaved DNA sequences by qPCR | HPV 16/18 | 2018 | [40] | |
| FLASH-NGS | DNA | PCR | NGS | The cDNA/gDNA is blocked by phosphatase processing and digested by Cas9 with a set of gRNA targeting the gene of interest. Ligation of sequencing adaptors, amplification, sequencing follows. | Antimicrobial resistance genes | 2019 | [41] | |
| CRISPR-Chip | DNA | - | Potentiometry | CRISPR-Chip biosensor utilizes the gene targeting ability of catalytically deactivated sgRNA-CRISPR/Cas9 and anchored to a transistor to produce a label-free test device. The output signal is monitored by a simple handheld reader. | SNPs | 2019 | [42] | |
| Cas 12 | DETECTR | DNA | RPA | Fluorescence | Combining activation of Cas12a ssDNase with isothermal amplification | HPV 16/18 | 2018 | [31] |
| HOLMES | DNA, RNA | PCR | Fluorescence | Cas12a/crRNA binds to target DNA, which trans-cleaves non-target ssDNA, illuminating fluorescent signal. | JEV | 2018 | [43] | |
| CDetection | DNA | RPA | Fluorescence | Combining optimized tuned gRNA enables distinguishing differences at single base level | HPV16/18 | 2019 | [44] | |
| E-CRISPR | DNA, protein | Amplification free | Electrochemical | Cas12a converts target identification activity into detectable electrochemical signal via an interrogating electrode which is constructed from non-specific ssDNA | DNA: HPV16, PB19; Protein: TGFβ1 | 2019 | [45] | |
| CRISPR-responsive hydrogel | DNA/RNA | RPA/RT-RPA | μPAD readout | Upon activation with input defined by gRNA, Cas12a cleaves DNA in the gel, translating biological information into material property changes | Ebola | 2019 | [46] | |
| HOLMESv2 | DNA, RNA | LAMP | Fluorescence | Upgrade of HOLMES/ integration of LAMP and Cas12b trans-cleavage into a single step | JEV | 2019 | [47] | |
| CRISPR-Cas12a-NER | RNA | RT-RAA | Fluorescent signals by Naked eye under blue light | When a target nucleic acid is present in the detection system, the quenched green fluorescent molecule-labeled ssDNA reporter is cleaved by Cas12a, resulting in green fluorescence visible to the naked eye | SARS-CoV-2 | 2020 | [48] | |
| CASdetec | RNA | RT-RAA | Fluorescence from fluorescence reader or under blue light | Integrating sample processing protocols and nucleic acid amplification approaches with CDetection | SARS-CoV-2 | 2020 | [49] | |
| STOP | RNA | RT–LAMP | Fluorescence, lateral flow assay | Combining simplified viral RNA isolation with isothermal amplification and CRISPR mediated testing | SARS-CoV-2 | 2020 | [50] | |
| Cas-gold | DNA | RPA | Gold nanoparticle-based LFS test | Integration of Cas12a-based assay and gold nanoparticle based LFS | ASFV | 2020 | [51] | |
| Poly (A)- AuNPs | DNA | RPA | Naked eye | AuNP-based bioprobes with freezing-based labeling approach | ASFV | 2020 | [52] | |
| Electrochemical DNA biosensing | DNA | Amplification free | Differential pulse voltammetry | Binding to target DNA activates Cas12a ssDNase activity; the low surface coverage and non-compact morphological structure of the immobilized hpDNA electrochemical reporters provide exploitable substrates for efficient cleavage of Cas12a, resulting in a high-sensitive electrochemical DNA biosensor | HPV16/18 | 2020 | [53] | |
| PGMs-CRISPR | RNA | RT-RAA | Glucose meter readout | Samples are rapidly pretreated and amplified by RT-RAA; the viral signal is converted to glucose signal by integrating CRISPR/Cas12a system and a glucose production reaction, allowing quantitative readout by a personal glucose meter | SARS-CoV-2 | 2021 | [54] | |
| OR-DETECTR | RNA | RT-RPA | Fluorescence, Lateral flow assay | Single-tube assay platform based on RT-RPA and DNA endonuclease targeted CRISPR trans-reporter technology | SARS-CoV-2 | 2021 | [55] | |
| opvCRISPR | RNA | RT-LAMP | Fluorescent detection by naked eye under blue light | Integrating RT-LAMP, Cas12a cleavage in single reaction system | SARS-CoV-2 | 2021 | [56] | |
| MEF biosensor | DNA | Amplification free | Fluorescence | Metal-enhanced fluorescence through the use of DNA-functionalized Au-nanoparticles, and embedded DNA/RNA hairpin director for ultra-sensitive nucleic acid detection | DNA | 2021, 2022 | [57,58] | |
| CRISPR-ENHANCE | RNA | Amplification free | Fluorescence, lateral flow assay | Significantly high sensitivity was achieved using engineered crRNAs and optimized conditions, enabling nucleic acid detection at femtomolar levels even without target pre-amplification | SARS-CoV-2 | 2022 | [59] | |
| MOPCS | RNA | Amplification free | Surface plasmon resonance signal | Coupling optical sensing “surface plasmon resonance” with CRISPR “gene scissors” for high sensitivity and specificity | SARS-CoV-2 | 2022 | [60] | |
| RAVI-CRISPR | DNA/RNA | LAMP/RT-LAMP | Naked-eye colorimetric detection | A field deployable detection platform based on ROX-labeled reporter, isothermal amplification and CRISPR/Cas12a system; a convolutional neural network algorithm developed for standardizing and automating the analytical colorimetric evaluation of images and implemented into MagicEye cell phone software | SARS-CoV-2, ASFV | 2022 | [61] | |
| sPAMC | DNA/RNA | RPA | Fluorescence | Cas12a’s reduced binding affinity to suboptimal PAM substrates is critical for its diminished cis-cleavage activity, thereby facilitating an equilibrium shift to isothermal amplification, resulting in stronger fluorescence | SARS-CoV-2, HCMV | 2022 | [62] | |
| WS-RADICA | DNA/RNA | Evaluation of two digital chips for DNA/RNA quantification | SARS-CoV-2, human adenovirus, herpes simplex virus | 2022 | [63] | |||
| Cas 14 | Cas14-DETECTR | DNA | RPA | Fluorescence | Cas14 protein can cleave ssDNA in a targeted manner without restrictive sequence requirements. Non-specific cleavage of ssDNA molecules is triggered by targeted recognition of Cas14, which activity allows high-fidelity SNP genotyping | 2018 | [64] | |
| Cas13 | SHERLOCK | DNA/RNA | RPA | Fluorescence | crRNA/Cas13 targets ssRNA and splits fluorescent ssRNA probe | ZIKV, DENV, KPC, NDM-1 | 2017 | [65] |
| SHERLOCKv2 | DNA/RNA | RPA | Lateral flow assay | Upgrade of SHERLOCK; high quantitation, high sensitivity | ZIKV, DENV | 2018 | [66] | |
| HUDSON + SHERLOCK | DNA/RNA | RPA | Fluorescence | Pairing HUDSON and SHERLOCK enables instrument-free detection of viruses directly from body fluids | ZIKV, DENV, WNV, YFV | 2018 | [67] | |
| CARMEN | DNA/RNA | PCR/RPA | Fluorescence | Over 4500 nucleic acids in one array by SHERLOCK methodology | HCV, HIV, ZIKV, DENV, influenza, SARS | 2020 | [68] | |
| SHINE | RNA | RPA | Smartphone (in-tube fluorescence readout or lateral flow strip) | Modified HUDSON quickly deactivates viruses in samples such as saliva and nasopharyngeal swabs in 10 min, and target RNA detection results are visualized by in-tube fluorescent readout and interpreted by a mobile app | SARS-CoV-2 | 2020 | [69] | |
| Electrochemical CRISPR/CHDC system | RNA | - | Electrochemical readout | Dual signal enhancement strategy (CRISPR/Cas13a system plus catalytic hairpin DNA circuit) embedded in a re-usable electrochemical biosensor to rapidly and accurately detect target RNAs | NSCLC-related RNAs | 2021 | [70] | |
| OR-SHERLOCK | RNA | RPA | Fluorescence/Lateral flow assay | Single-tube assay platform based on RT-RPA and CRISPR/Cas12a | SARS-CoV-2 | 2021 | [55] | |
| Multiple enhanced CRISPR-Cas13 assay | RNA | Amplification free | Fluorescence measurement by mobile phone camera with additional optics | Non-amplification CRISPR/Cas13a test for direct measurement from nasal swab RNA, readable with a cell phone microscope | SARS-CoV-2 | 2021 | [71] | |
| Ultralocalized Cas13a Assay | RNA | Amplification free | Fluorescent microscopy (digital droplet readout) | Enclosing RNA-triggered Cas13a catalytic system in cell-like sized reactors by droplet microfluidics to simultaneously increase local concentrations of targets and reporters | SARS-CoV-2 | 2021 | [72] | |
| gFETs | RNA | Amplification free | Fluorescence | By utilizing Cas13a’s transcleavage mechanism and ultra-sensitive Graphene field effect transistors | SARS-CoV-2, RSV | 2022 | [73] | |
| CRISPR-Cas13a/HRP assay | DNA/RNA | Amplification free | Naked-eye colorimetric detection | Coupling target induced Cas13 activity with subsequent release into solution of the enzymatic reporter HRP | SARS-CoV-2 | 2022 | [74] |
| Species | Detection Target | Assay Name | Target Region | Nucleic Acid Amplification | CRISPR Protein | Readout | LOD | Testing Time | One Tube vs. Two Tubes | Year | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Detection of pathogenic viruses | |||||||||||
| Pig | ASFV | CRISPR/Cas9 eraser-based PAM-implanted PCR visual end-point detection | p72 | PAM-implanted PCR | Cas9 eraser | LFA | One | 2021 | [75] | ||
| Pig | ASFV | RAVI-CRISPR | p72 | LAMP | Cas12a | Naked-eye colorimetric readout | 7 total copies | 35 min | One | 2020, 2022 | [61,76,77] |
| Pig | ASFV | CRISPR/Cas13a-LFD | p72 | RAA | Cas13a | LFS visual readout | 101 copies/µL | <1 h | One | 2022 | [78] |
| Pig | ASFV | multiplex-crRNA CRISPR/Cas12a system | B646L | Amplification-free | Cas12a | LightCycler 96 | 1 pM | 2022 | [79] | ||
| Pig | PRRSV | Highly sensitive CRISPR/Cas12a-Based Fluorescence detection | nsp2 | RT-RPA | Cas12a | Fluorescent readout | 1 copy | 25 min | One | 2021 | [80] |
| Pig | PRRSV | enhanced Cas13a lateral flow detection | M | RPA | Cas13a | Lateral flow, fluorescence | 172 copies/μL | 2020 | [25] | ||
| Pig | PEDV | RT-ERA-CRISPR/Cas12a detection | ORF3 | RT-ERA | Cas12a | Visual detection under LED blue light | 2 copies | 30 min | 2021 | [81] | |
| Pig | PEDV | RT-RAA- CRISPR/Cas12a assay | S | RT-RAA | Cas12a | Fluorescence, visual, UV light, or flow strip detection | 100 copies | 1.5 h | 2022 | [82] | |
| Pig | PEDV, TGEV, PDCoV, SADS-CoV | RT-LAMP-CRISPR/Cas12a | ORF3, N, N, N | Multiplex RT-LAMP | Cas12a | Naked-eye colorimetric detection | 1 copy | 25 min | 2022 | [83] | |
| Pig | PCV3 | ERA-CRISPR/Cas12a assay | ERA | Cas12a | Under UV/LED-blue light | 7 copies | <1 h | 2021 | [84] | ||
| Pig | PPV | ERA-CRISPR/Cas12a system | VP2 | ERA | Cas12a | Lateral flow detection | 3.75 × 102 copies/μL | 2022 | [85] | ||
| Pig | JEV | RAVI-CRISPR | C | RT-LAMP | Cas12a | Naked-eye colorimetric readout | 8.97 total copies | 1 h | One | 2022 | [86] |
| Pig | EMCV | RAA-CRISPR/Cas13a assay | RAA | Cas13a | LFS | 101 copies/µL | 1 h | 2022 | [87] | ||
| Cattle | LSDV | RPA-Cas12a-fluorescence assay | orf068 | RPA | Cas12a | Fluorescent signal | 100 TCID50/mL | 15 min | Two | 2022 | [88] |
| Cattle | BVDV | LwCas13a-based detection system | reported BVDV sequence in 5′UTR conserved region | - | Cas13a | Fluorescence | 103 pM | - | 2021 | [89] | |
| Cattle | CaPV | LAMP-CRISPR/Cpf1 fluorescence detection | LAMP | Cas12a | Fluorometer, lateral flow test | 1.47 × 10−3 TCID50 | 50 min | 2022 | [90] | ||
| Detection of pathogenic bacteria and parasites | |||||||||||
| Pig, Cattle, etc. | Toxoplasma gondii | RPA-CRISPR/Cas12a assay | B1 | RPA | Cas12a | Fluorometer or LFS | 3.3 copies/μL | - | One | 2022 | [91] |
| Pig, Cattle, etc. | Toxoplasma gondii | RAA-Cas12a assay | RE | RAA | Cas12a | Fluorescence detection | 1 fM | ~1 h | 2021 | [92] | |
| Pig, Cattle, etc. | Toxoplasma gondii | RAA-Cas13a-LFD assay | B1 | RAA | Cas13 | LFD | 1 × 10−6 ng/μL | <2 h | 2022 | [93] | |
| Pig, Cattle, etc. | Cryptosporidium parvum IId-subtype-family | ReCTC-based diagnoses | GP60 | RPA | Cas12a | LFS biosensor | single copy | 2021 | [94] | ||
| Pig, Cattle, etc. | Brucellosis | Dual- biosensors based on RPA-CRISPR/Cas12a | RPA | Cas12a | Fluorescent biosensor, electrochemical biosensor | 2 copies | 2022 | [95] | |||
| Pig, Cattle, etc. | Escherichia coli, Streptococcus aureus | RPA-CRISPR/Cas12a | rfbE, nuc | RPA | Cas12a | Fluorescence | 1 CFU/mL | <50 min | One | 2020 | [96] |
| Pig, Cattle, etc. | Escherichia coli | RAA-CRISPR/Cas12a | wzy | RAA | Cas12a | Fluorescence | 5.4 × 102 CFU/mL | 30 min | 2022 | [97] | |
| Pig, Cattle, etc. | Campylobacter jejuni | RAA-CRIPSR/Cas12a | hipO | RAA | Cas12a | Fluorescence | 5 copies | 15–30 min | 2022 | [98] | |
| Pig, Cattle, etc. | Listeria monocytogenes | RPA-CRISPR/Cas12a | RPA | Cas12a | Fluorescence | 10 CFU/mL | 2021 | [99] | |||
| Pig, Cattle, etc. | Yersinia enterocolitica | RPA-CRISPR/Cas12a | ail | RPA | Cas12a | Fluorescence | 1.7 CFU/mL | <45 min | 2022 | [100] | |
| Other applications | |||||||||||
| Pig | Sex determination | RAVI-CRISPR | SRY, ZFX | LAMP | Cas12a | Fluorescence | 2 copies | ~45 min to 1 h | 2022 | [101] | |
| Pig | Pig-derived component | CAPCOD | PCR | Cas12 | 0.1% (w/w) | 2022 | [102] | ||||
| Pig | Pig-derived component | RPA-CRISPR/Cas12a assay | RPA | Cas12a | Visual identification | 0.1–0.001% (w/w) | <30 min | 2022 | [103] | ||
| Pig, Chicken, Duck | Meat species | RAVI-CRISPR | porcine NADH4, chicken ND2, duck D-loop | LAMP | Cas12a | Naked-eye colorimetric detection | 1.0 pg gDNA | 40 min | 2022 | [104] | |
| Cattle | Milk authenticity | CRISPR/Cas12a-Driven SERS Biosensor | cytb | LAMP | Cas12a | Spectrometer | 224 aM | 2022 | [105] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X. Development of CRISPR-Mediated Nucleic Acid Detection Technologies and Their Applications in the Livestock Industry. Genes 2022, 13, 2007. https://doi.org/10.3390/genes13112007
Zhang X. Development of CRISPR-Mediated Nucleic Acid Detection Technologies and Their Applications in the Livestock Industry. Genes. 2022; 13(11):2007. https://doi.org/10.3390/genes13112007
Chicago/Turabian StyleZhang, Xuying. 2022. "Development of CRISPR-Mediated Nucleic Acid Detection Technologies and Their Applications in the Livestock Industry" Genes 13, no. 11: 2007. https://doi.org/10.3390/genes13112007




