Multi-Omic Profiles in Infants at Risk for Food Reactions
Abstract
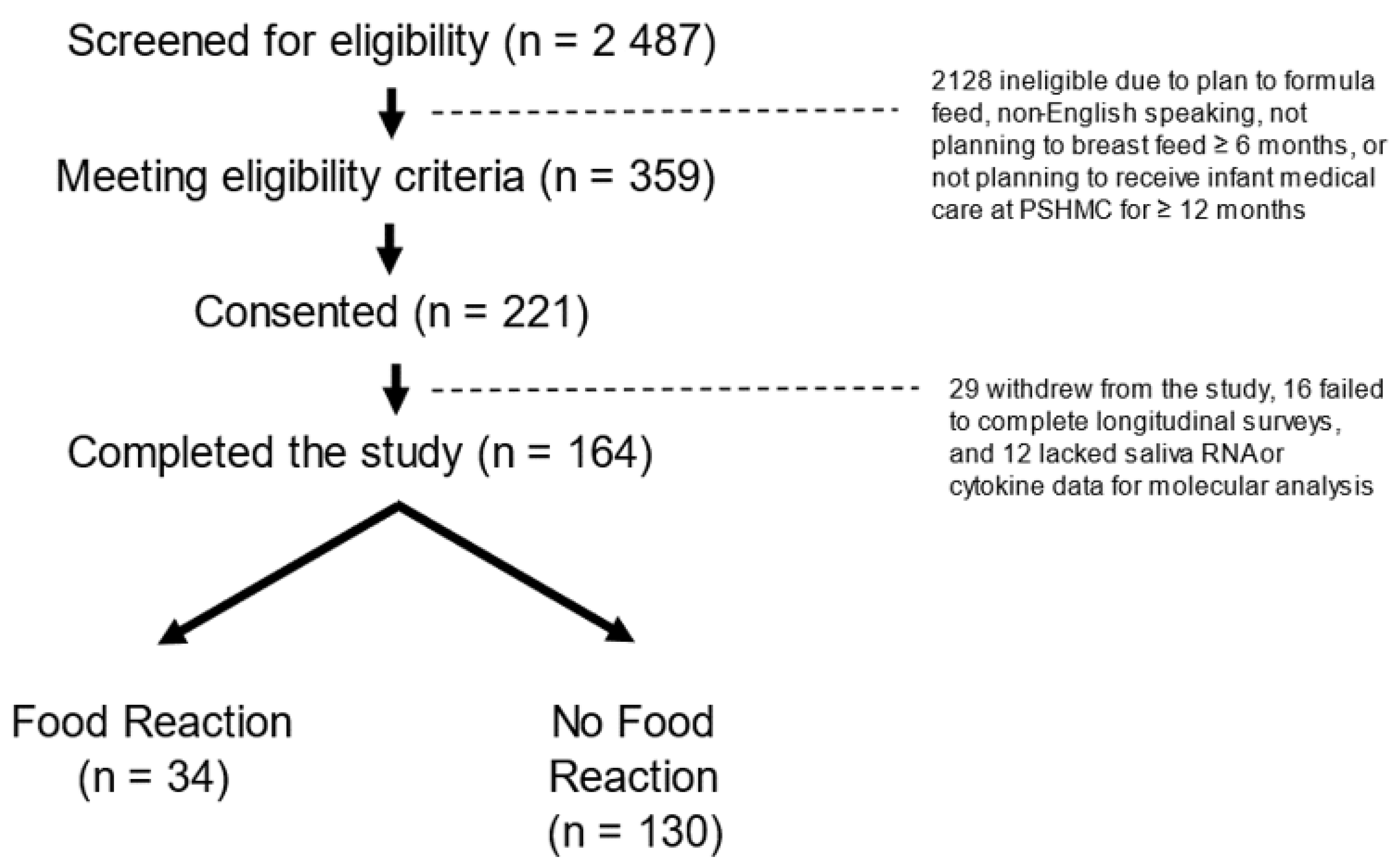
:1. Introduction
2. Methods
2.1. Participants
2.2. Measures
2.3. Sample Collection and Processing
2.4. Cytokine Analysis
2.5. RNA Analysis
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Food Reaction Characteristics
3.3. Saliva Molecular Profiles
3.4. Relationships between Molecular Factors Implicated in Food Reactions
3.5. Predicting Food Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Wang, X. Early life precursors, epigenetics, and the development of food allergy. Semin. Immunopathol. 2012, 34, 655–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedé, S.; Blázquez, A.B.; Chiang, D.; Tordesillas, L.; Berin, M.C. The rise of food allergy: Environmental factors and emerging treatments. EBioMedicine 2016, 7, 27–34. [Google Scholar]
- Fujimura, T.; Lum, S.Z.C.; Nagata, Y.; Kawamoto, S.; Oyoshi, M.K. Influences of Maternal Factors Over Offspring Allergies and the Application for Food Allergy. Front. Immunol. 2019, 10, 1933. [Google Scholar] [CrossRef]
- Bager, P.; Wohlfahrt, J.; Westergaard, T. Caesarean delivery and risk of atopy and allergic disease: Meta-analyses. Clin. Exp. Allergy 2008, 38, 634–642. [Google Scholar] [CrossRef]
- Johnson, C.C.; Ownby, D.R. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl. Res. 2017, 179, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.S.; Abrams, E.M.; Hildebrand, K.J.; Watson, W. Early introduction of foods prevents food allergy. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 57. [Google Scholar]
- Feeney, M.; Du Toit, G.; Roberts, G.; Sayre, P.H.; Lawson, K.; Bahnson, H.T.; Sever, M.L.; Radulovic, S.; Plaut, M.; Lack, G.; et al. Immune Tolerance Network LEAP Study Team. Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition. J. Allergy Clin. Immunol. 2016, 138, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Linacero, R.; Cuadrado, C. New Research in Food Allergen Detection. Foods 2022, 11, 1520. [Google Scholar] [CrossRef]
- Turcanu, V.; A Brough, H.; Du Toit, G.; Foong, R.-X.; Marrs, T.; Santos, A.; Lack, G. Immune mechanisms of food allergy and its prevention by early intervention. Curr. Opin. Immunol. 2017, 48, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India 2010, 27, 66–71. [Google Scholar] [CrossRef]
- Poole, A.; Song, Y.; Brown, H.; Hart, P.H.; Zhang, G.B. Cellular and molecular mechanisms of vitamin D in food allergy. J. Cell. Mol. Med. 2018, 22, 3270–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappa, V. Epigenetic alterations and microRNAs: New players in the pathogenesis of myelodysplastic syndromes. Epigenetics 2013, 8, 561–570. [Google Scholar]
- Hong, X.; Wang, X. Epigenetics and development of food allergy (FA) in early childhood. Curr. Allergy Asthma Rep. 2014, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Gerosa, A.; Paladin, F.; Petrocchi, L.; Banchero, S.; Gangemi, S. Vitamin D and Microbiota: Is There a Link with Allergies? Int. J. Mol. Sci. 2021, 22, 4288. [Google Scholar] [CrossRef]
- Bozzetto, S.; Carraro, S.; Giordano, G.; Boner, A.; Baraldi, E. Asthma, allergy and respiratory infections: The vitamin D hypothesis. Allergy 2012, 67, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Fein, S.B.; Labiner-Wolfe, J.; Shealy, K.R.; Li, R.; Chen, J.; Grummer-Strawn, L.M. Infant feeding practices study II: Study methods. Pediatrics 2008, 122 (Suppl. S2), S28–S35. [Google Scholar] [CrossRef] [Green Version]
- Clickner, R.P.; Marker, D.; Viet, S.M.; Rogers, J.; Broene, P. National survey of lead and allergens in housing. Final Rep. 2001, 18, 1. [Google Scholar]
- Shi, J.; Luo, Q.; Chen, F.; Chen, D.; Xu, G.; Li, H. Induction of IL-6 and IL-8 by house dust mite allergen Der p1 in cultured human nasal epithelial cells is associated with PAR/PI3K/NFkappaB signaling. ORL J. Otorhinolaryngol. Relat. Spec. 2010, 72, 256–265. [Google Scholar] [CrossRef]
- Thyagarajan, A.; Burks, A.W. Food allergy: Present and future management. World Allergy Organ. J. 2009, 2, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubiche, T.; Ged, C.; Benard, A.; Léauté-Labrèze, C.; McElreavey, K.; de Verneuil, H.; Taïeb, A.; Boralevi, F. Analysis of SPINK 5, KLK 7 and FLG genotypes in a French atopic dermatitis cohort. Acta Derm. Venereol. 2007, 87, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpathiou, G.; Papoudou-Bai, A.; Ferrand, E.; Dumollard, J.M.; Peoc’h, M. STAT6: A review of a signaling pathway implicated in various diseases with a special emphasis in its usefulness in pathology. Pathol. Res. Pract. 2021, 223, 153477. [Google Scholar] [CrossRef]
- Alfano, D.N.; Klei, L.R.; Klei, H.B.; Trotta, M.; Gough, P.J.; Foley, K.P.; Bertin, J.; Sumpter, T.L.; Lucas, P.C.; McAllister-Lucas, L.M. MALT1 Protease Plays a Dual Role in the Allergic Response by Acting in Both Mast Cells and Endothelial Cells. J. Immunol. 2020, 204, 2337–2348. [Google Scholar] [CrossRef]
- Koury, J.; Ramirez, A.; Xie, C.; Harb, J.; Dong, C.; Maki, C.; Ramos, T.; Izadyar, F.; Clark, D.; Drechsler, Y.; et al. Phosphodiesterase 4D, miR-203 and selected cytokines in the peripheral blood are associated with canine atopic dermatitis. PLoS ONE 2019, 14, e0218670. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Q.; Gu, Y.; Groome, L.J. Up-regulation of miR-203 expression induces endothelial inflammatory response: Potential role in preeclampsia. Am. J. Reprod. Immunol. 2016, 76, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.; Pairet, S.; Álvarez-Larrán, A.; Pons, A.; Ferrer, G.; Longarón, R.; Fernandez-Rodriguez, C.; Camacho, L.; Monzo, M.; Besses, C.; et al. miR-203 and miR-221 regulate SOCS1 and SOCS3 in essential thrombocythemia. Blood Cancer J. 2016, 6, e406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanczyk, J.; Ospelt, C.; Karouzakis, E.; Filer, A.; Raza, K.; Kolling, C.; Gay, R.; Buckley, C.D.; Tak, P.P.; Gay, S.; et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011, 63, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Lacy, P.; Levi-Schaffer, F.; Mahmudi-Azer, S.; Bablitz, B.; Hagen, S.C.; Velazquez, J.; Kay, B.; Moqbel, R. Intracellular Localization of Interleukin-6 in Eosinophils From Atopic Asthmatics and Effects of Interferon γ. Blood 1998, 91, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Moshapa, F.T.; Riches-Suman, K.; Palmer, T.M. Therapeutic Targeting of the Proinflammatory IL-6-JAK/STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus. Cardiol Res Pr. 2019, 2019, 9846312. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signaling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Carow, B.; Rottenberg, M.E. SOCS3, a Major Regulator of Infection and Inflammation. Front. Immunol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, A.; Inoue, H.; Nakano, T.; Matsunaga, Y.; Matsuno, Y.; Matsumoto, T.; Fukuyama, S.; Kan-O, K.; Matsumoto, K.; Tsuda-Eguchi, M.; et al. T cell treatment with small interfering RNA for suppressor of cytokine signaling 3 modulates allergic airway responses in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [PubMed]
- Łoś-Rycharska, E.; Gołębiewski, M.; Grzybowski, T.; Rogalla-Ładniak, U.; Krogulska, A. The microbiome and its impact on food allergy and atopic dermatitis in children. Adv. Dermatol. Allergol. 2020, 37, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Alves, D.R.; Nzakizwanayo, J.; Dedi, C.; Olympiou, C.; Hanin, A.; Kot, W.; Hansen, L.; Lametsch, R.; Gahan, C.G.M.; Schellenberger, P.; et al. Genomic and Ecogenomic Characterization of Proteus mirabilis Bacteriophages. Front. Microbiol. 2019, 10, 1783. [Google Scholar] [CrossRef] [Green Version]
- Plunkett, C.H.; Nagler, C.R. The Influence of the Microbiome on Allergic Sensitization to Food. J. Immunol. 2017, 198, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ji, Y.; Lan, X.; Gao, X.; Chen, H.D.; Geng, L. miR-203 contributes to IL-17-induced VEGF secretion by targeting SOCS3 in keratinocytes. Mol. Med. Rep. 2017, 16, 8989–8996. [Google Scholar] [CrossRef] [Green Version]
- Lokau, J.; Schoeder, V.; Haybaeck, J.; Garbers, C. Jak-Stat Signaling Induced by Interleukin-6 Family Cytokines in Hepatocellular Carcinoma. Cancers 2019, 11, 1704. [Google Scholar] [CrossRef] [Green Version]
- Babon, J.J.; Varghese, L.N.; Nicola, N.A. Inhibition of IL-6 family cytokines by SOCS3. Semin. Immunol. 2014, 26, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zhang, Y.; Ma, J.; Liu, Y.; Li, W.; Wang, T.; Xu, X.; Wang, Y.; Cheng, K.; Zhuang, R. Interleukin-6 absence triggers intestinal microbiota dysbiosis and mucosal immunity in mice. Cytokine 2022, 153, 155841. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Bosi, A.; Rossetti, C. The Effect of Cyanobacterial LPS Antagonist (CyP) on Cytokines and Micro-RNA Expression Induced by Porphyromonas gingivalis LPS. Toxins 2018, 10, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]



| Characteristic, n (%) | All (n = 164) | Food Allergy (n = 34) | No Food Allergy (n = 130) |
|---|---|---|---|
| Maternal traits | |||
| Age, years; mean (SD) | 30 (4) | 32 (4) | 30 (4) |
| BMI, kg/m2; mean (SD) | 27.5 (6) | 27.5 (6) | 27.5 (6) |
| Maternal food allergies | 14 (8.5) | 8 (23.5) * | 6 (4.6) |
| Maternal asthma | 23 (14.0) | 4 (11.7) | 19 (14.6) |
| Maternal allergic rhinitis | 36 (21.9) | 9 (26.4) | 27 (20.7) |
| Family food allergies in first- or second-degree relative | 15 (9.1) | 6 (17.6) | 9 (6.9) |
| Infant Traits | |||
| Female sex | 96 (58.5) | 22 (64.7) | 74 (56.9) |
| Gestational age, weeks; mean (SD) | 38.9 (1) | 39.0 (1) | 38.9 (1) |
| Race | |||
| African American | 7 (4.3) | 0 (0.0) | 7 (5.4) |
| Asian | 7 (4.3) | 3 (8.8) | 4 (3.1) |
| Bi-racial | 13 (7.9) | 2 (5.9) | 11 (8.5) |
| Other | 11 (6.7) | 1 (2.9) | 10 (7.7) |
| White | 126 (76.8) | 28 (82.4) | 98 (75.4) |
| Birth weight, g; mean (SD) | 3352 (447) | 3373 (416) | 3341 (455) |
| Environmental Exposures | |||
| Vaginal delivery | 132 (80.5) | 26 (76.4) | 106 (81.5) |
| Earliest formula introduction | |||
| None in initial 24 weeks | 67 (43.8) | 11 (34.4) | 56 (46.3) |
| 16–24 weeks | 16 (10.5) | 5 (15.6) | 11 (9.1) |
| 4–16 weeks | 15 (9.8) | 1 (3.1) | 14 (11.6) |
| 0–4 weeks | 55 (35.9) | 15 (46.9) | 40 (33.1) |
| Solid food introduced by 4 months | 22 (13.4) | 5 (14.7) | 17 (13.0) |
| Solid food introduced by 6 months | 101 (80.8) | 19 (55.8) | 82 (63.0) |
| Maternal tobacco use | 22 (13.4) | 3 (8.8) | 19 (14.6) |
| People in household, (range) | 4 (2–9) | 4 (2–9) | 4 (2–9) |
| Salivary factors | |||
| Time of collection, 24 h; mean (SD) | 12 (3) | 12 (3) | 11 (3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beheshti, R.; Stone, S.; Chandran, D.; Hicks, S.D. Multi-Omic Profiles in Infants at Risk for Food Reactions. Genes 2022, 13, 2024. https://doi.org/10.3390/genes13112024
Beheshti R, Stone S, Chandran D, Hicks SD. Multi-Omic Profiles in Infants at Risk for Food Reactions. Genes. 2022; 13(11):2024. https://doi.org/10.3390/genes13112024
Chicago/Turabian StyleBeheshti, Ramin, Shane Stone, Desirae Chandran, and Steven D. Hicks. 2022. "Multi-Omic Profiles in Infants at Risk for Food Reactions" Genes 13, no. 11: 2024. https://doi.org/10.3390/genes13112024





