Risk Allele Frequency Analysis and Risk Prediction of Single-Nucleotide Polymorphisms for Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Comparison of PCa SNPs among Global Populations
2.3. Calculation of Polygenic Genetic Risk Scores Using SNPs Related to PCa
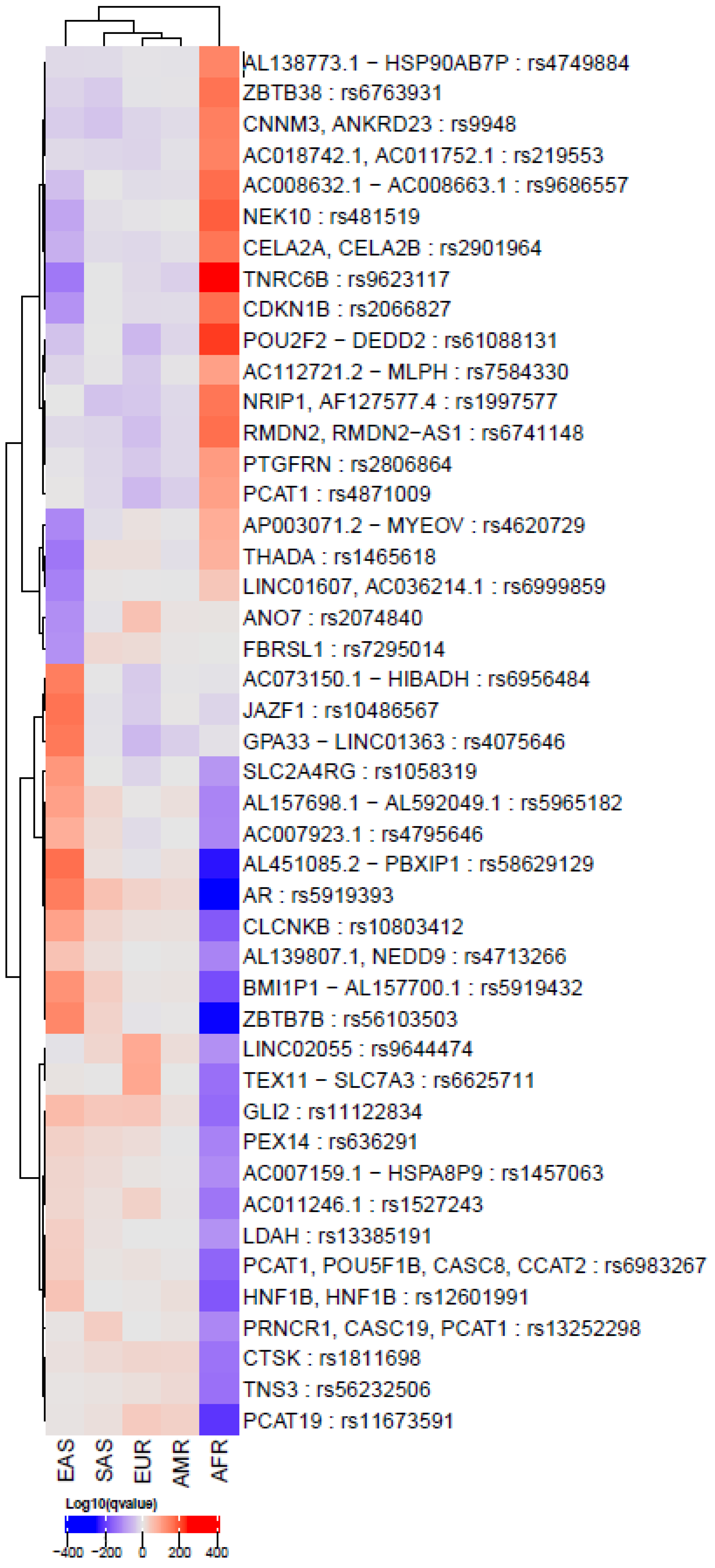
2.4. Comparison of Allele Frequencies
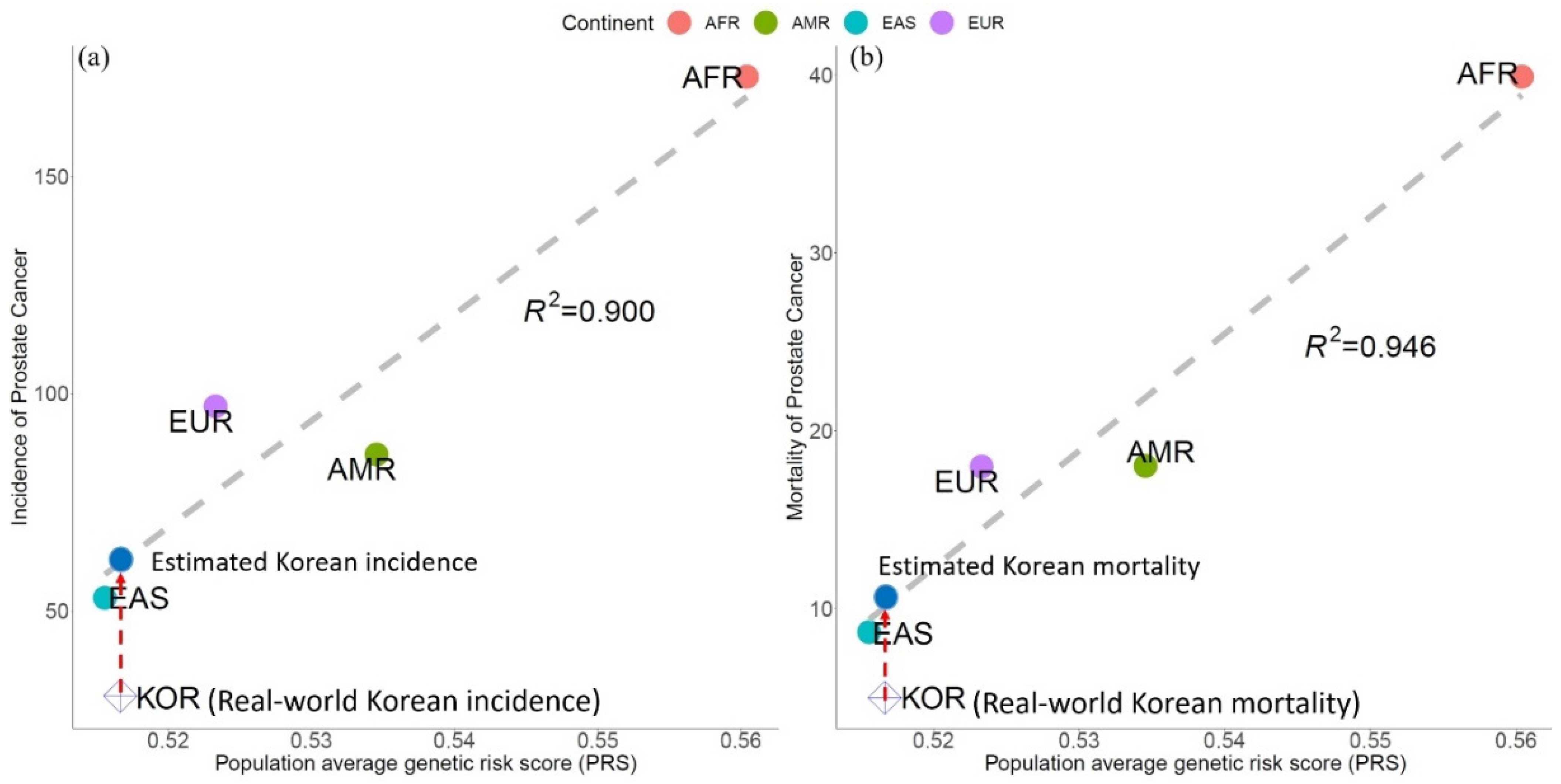
2.5. Validation of PRS Modeling for PCa Epidemiology
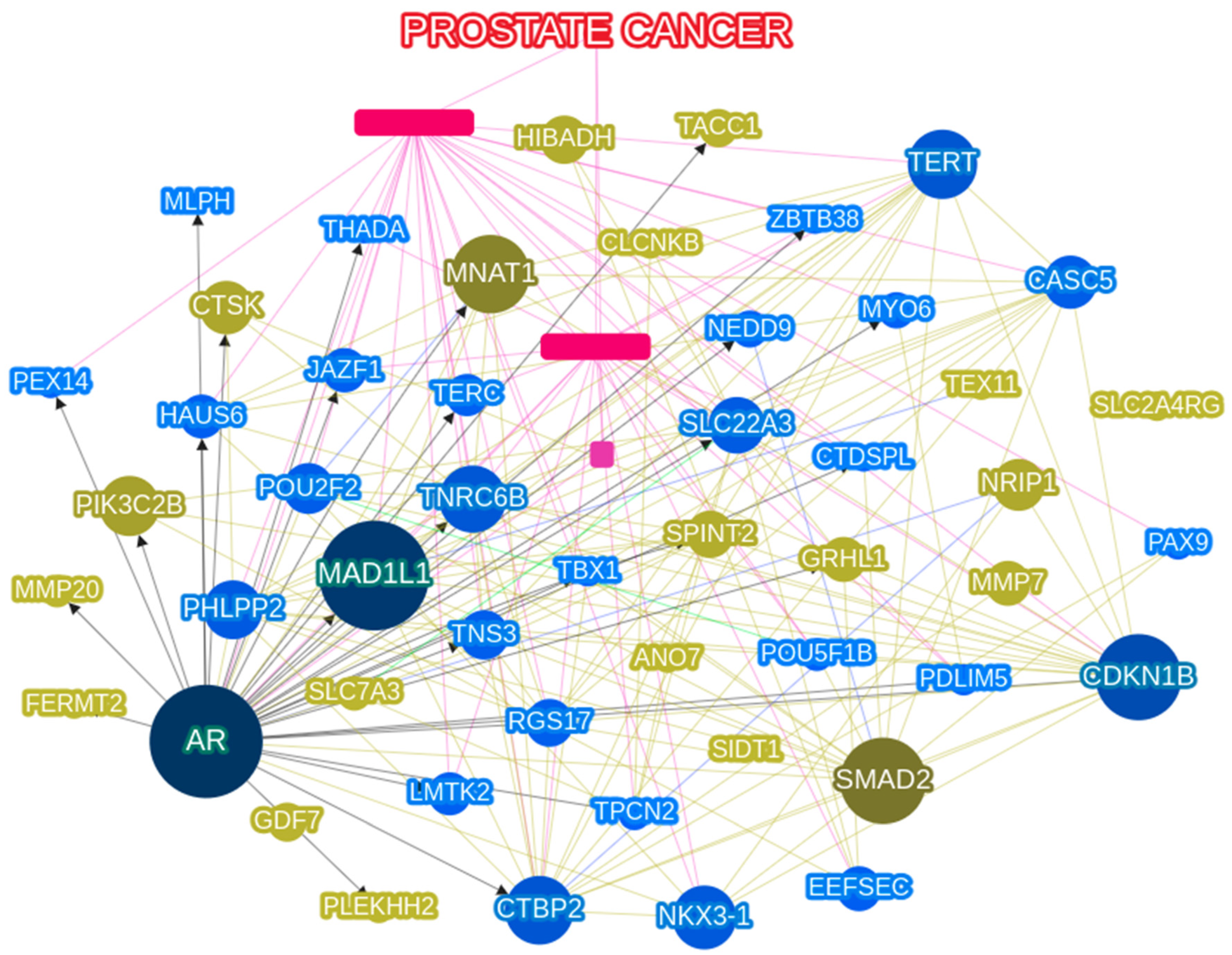
2.6. Network Analysis
2.7. Statistical Analysis
3. Results
3.1. PCa-Related SNPs in the Global Population
3.2. Genetic Risk Scores Calculated using SNPs Related to PCa Incidence & Mortality
3.3. DHT to T Ratios and Vitamin D Related to PCa Incidence & Mortality
3.4. Network Analysis Using PCa Related Gene Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Hall, I.J.; Sabatino, S.A. Racial/Ethnic Disparities in Prostate Cancer Incidence, Distant Stage Diagnosis, and Mortality by U.S. Census Region and Age Group, 2012–2015. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1357–1364. [Google Scholar] [CrossRef]
- Kheirandish, P.; Chinegwundoh, F. Ethnic differences in prostate cancer. Br. J. Cancer 2011, 105, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.; Hounsome, L.; Mehay, A.; Mee, S.; Verne, J.; Cooper, A. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Med. 2015, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.N. Epidemiology of Prostate Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5137–5141. [Google Scholar] [CrossRef]
- Wu, A.H.; Whittemore, A.S.; Kolonel, L.N.; John, E.M.; Gallagher, R.P.; West, D.W.; Hankin, J.; Teh, C.Z.; Dreon, D.M.; Paffenbarger, R.S., Jr. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol. Biomark. Prev. 1995, 4, 735–741. [Google Scholar]
- Song, Z.-Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin D level and mortality in prostate cancer patients: A dose–response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Al Olama, A.A.; Berndt, S.I.; Benlloch, S.; Ahmed, M.; Saunders, E.J.; Dadaev, T.; Leongamornlert, D.; Anokian, E.; Cieza-Borrella, C.; et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 2018, 50, 928–936. [Google Scholar] [CrossRef]
- Chau, V.; Madan, R.A.; Figg, W.D. Exploiting defects in homologous recombination repair for metastatic, castration-resistant prostate cancer. Cancer Biol. Ther. 2020, 21, 884–887. [Google Scholar] [CrossRef]
- Lotan, T.L.; Kaur, H.B.; Salles, D.C.; Murali, S.; Schaeffer, E.M.; Lanchbury, J.S.; Isaacs, W.B.; Brown, R.; Richardson, A.L.; Cussenot, O.; et al. Homologous recombination deficiency (HRD) score in germline BRCA2- versus ATM-altered prostate cancer. Mod. Pathol. 2021, 34, 1185–1193. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Rantapero, T.; Wahlfors, T.; Kähler, A.; Hultman, C.; Lindberg, J.; Tammela, T.L.J.; Nykter, M.; Schleutker, J.; Wiklund, F. Inherited DNA Repair Gene Mutations in Men with Lethal Prostate Cancer. Genes 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Pinese, M.; Lacaze, P.; Rath, E.M.; Stone, A.; Brion, M.-J.; Ameur, A.; Nagpal, S.; Puttick, C.; Husson, S.; Degrave, D.; et al. The Medical Genome Reference Bank contains whole genome and phenotype data of 2570 healthy elderly. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-T.; Yoon, B.W.; Seo, J.H. Comparison of risk allele frequencies of single nucleotide polymorphisms associated with age-related macular degeneration in different ethnic groups. BMC Ophthalmol. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-T.; Yoon, B.W.; Seo, J.H. Analysis of risk allele frequencies of single nucleotide polymorphisms related to open-angle glaucoma in different ethnic groups. BMC Med. Genom. 2021, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Fang, Y.; Campbell, M.; Southerland, W.M. Population differentiation in allele frequencies of obesity-associated SNPs. BMC Genom. 2017, 18, 1–16. [Google Scholar] [CrossRef]
- Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013, 9, e1003348. [Google Scholar] [CrossRef]
- Auton, A.; Salcedo, T. The 1000 genomes project. In Assessing Rare Variation in Complex Traits; Springer: New York, NY, USA, 2015; pp. 71–85. [Google Scholar]
- Han, H.H.; Park, J.W.; Na, J.C.; Chung, B.H.; Kim, C.-S.; Ko, W.J. Epidemiology of prostate cancer in South Korea. Prostate Int. 2015, 3, 99–102. [Google Scholar] [CrossRef]
- Litman, H.J.; Bhasin, S.; Link, C.L.; Araujo, A.B.; McKinlay, J.B. Serum Androgen Levels in Black, Hispanic, and White Men. J. Clin. Endocrinol. Metab. 2006, 91, 4326–4334. [Google Scholar] [CrossRef]
- Yoon, Y.-D.; Lee, C.-J.; Chun, E.-H.; Lee, J.-Y. Concentrations of Bioavailable Testosterone and Dihydrotestosterone Determined by Luminescence Immunoassay in Serum. Clin. Exp. Reprod. Med. 1988, 15, 83–92. [Google Scholar]
- Yoon, B.-W.; Shin, H.-T.; Seo, J. Risk Allele Frequency Analysis of Single-Nucleotide Polymorphisms for Vitamin D Concentrations in Different Ethnic Group. Genes 2021, 12, 1530. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Robinson, P.N.; Wang, K. Phenolyzer: Phenotype-based prioritization of candidate genes for human diseases. Nat. Methods 2015, 12, 841–843. [Google Scholar] [CrossRef]
- Conti, D.V.; Darst, B.F.; Moss, L.C.; Saunders, E.J.; Sheng, X.; Chou, A.; Schumacher, F.R.; Al Olama, A.A.; Benlloch, S.; Dadaev, T.; et al. Trans-ancestry genome-wide association meta-analysis of prostate cancer identifies new susceptibility loci and informs genetic risk prediction. Nat. Genet. 2021, 53, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.M.; Gellen, L.P.A.; Leal, D.F.D.V.B.; Pastana, L.F.; Vinagre, L.W.M.S.; Aquino, V.T.; Fernandes, M.R.; de Assumpção, P.P.; Burbano, R.M.R.; dos Santos, S.E.B.; et al. Correlation between Genomic Variants and Worldwide Epidemiology of Prostate Cancer. Genes 2022, 13, 1039. [Google Scholar] [CrossRef]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.J.; Teloken, C.; Tindall, D.J.; et al. Effect of Dutasteride on the Risk of Prostate Cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Bernstein, L.; Pike, M.; Henderson, B.; Lobo, R.; Stanczyk, F.; Shimizu, H. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet 1992, 339, 887–889. [Google Scholar] [CrossRef]
- Lévesque, E.; Laverdière, I.; Lacombe, L.; Caron, P.; Rouleau, M.; Turcotte, V.; Têtu, B.; Fradet, Y.; Guillemette, C. Importance of 5alpha-reductase gene polymorphisms on circulating and intraprostatic androgens in prostate cancer. Clin. Cancer Res. 2014, 20, 576–584. [Google Scholar] [CrossRef]
- Hsing, A.W.; Chen, C.; Chokkalingam, A.P.; Gao, Y.T.; Dightman, A.D.; Nguyen, H.T.; Deng, J.; Cheng, J.; Sesterhenn, A.I.; Mostofi, F.K.; et al. Polymorphic markers in the SRD5A2 gene and prostate cancer risk: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2001, 10, 1077–1082. [Google Scholar]
- Shiota, M.; Fujimoto, N.; Yokomizo, A.; Takeuchi, A.; Itsumi, M.; Inokuchi, J.; Tatsugami, K.; Uchiumi, T.; Naito, S. SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur. J. Cancer 2015, 51, 1962–1969. [Google Scholar] [CrossRef]
- Li, J.; Coates, R.J.; Gwinn, M.; Khoury, M.J. Steroid 5-{alpha}-reductase Type 2 (SRD5a2) gene polymorphisms and risk of prostate cancer: A HuGE review. Am. J. Epidemiol. 2010, 171, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kim, H.J.; Cheong, H.S.; Myung, S.C. The association of 5-alpha reductase type 2 (SRD5A2) gene polymorphisms with prostate cancer in a Korean population. Korean J. Urol. 2015, 56, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Choubey, V.K.; Sankhwar, S.N.; Carlus, S.J.; Singh, A.N.; Dalela, D.; Thangaraj, K.; Rajender, S. SRD5A2 Gene Polymorphisms and the Risk of Benign Prostatic Hyperplasia but not Prostate Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Gagliano, T.; Garland, L.; O’Hanlon, T.; Bortolotti, D.; Gentili, V.; Rizzo, R.; Giamas, G.; Dean, M. Androgen receptor signaling regulates the transcriptome of prostate cancer cells by modulating global alternative splicing. Oncogene 2020, 39, 6172–6189. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef]
- Farashi, S.; Kryza, T.; Clements, J.; Batra, J. Post-GWAS in prostate cancer: From genetic association to biological contribution. Nat. Cancer 2019, 19, 46–59. [Google Scholar] [CrossRef]
- Alrezk, R.; Hannah-Shmouni, F.; Stratakis, C.A. MEN4 and CDKN1B mutations: The latest of the MEN syndromes. Endocr. -Relat. Cancer 2017, 24, T195–T208. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Miller, C.W.; Greenspun, E.; Eshaghian, S.; Kawabata, H.; Fujimoto, T.; Tomonaga, M.; Sawyers, C.; Said, J.W.; Koeffler, H.P. Mutations in the mitotic check point gene, MAD1L1, in human cancers. Oncogene 2001, 20, 3301–3305. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, B.W.; Shin, H.-T.; Seo, J.H. Risk Allele Frequency Analysis and Risk Prediction of Single-Nucleotide Polymorphisms for Prostate Cancer. Genes 2022, 13, 2039. https://doi.org/10.3390/genes13112039
Yoon BW, Shin H-T, Seo JH. Risk Allele Frequency Analysis and Risk Prediction of Single-Nucleotide Polymorphisms for Prostate Cancer. Genes. 2022; 13(11):2039. https://doi.org/10.3390/genes13112039
Chicago/Turabian StyleYoon, Byung Woo, Hyun-Tae Shin, and Je Hyun Seo. 2022. "Risk Allele Frequency Analysis and Risk Prediction of Single-Nucleotide Polymorphisms for Prostate Cancer" Genes 13, no. 11: 2039. https://doi.org/10.3390/genes13112039
APA StyleYoon, B. W., Shin, H.-T., & Seo, J. H. (2022). Risk Allele Frequency Analysis and Risk Prediction of Single-Nucleotide Polymorphisms for Prostate Cancer. Genes, 13(11), 2039. https://doi.org/10.3390/genes13112039





