Mechanism of Stone (Hardened Endocarp) Formation in Fruits: An Attempt toward Pitless Fruits, and Its Advantages and Disadvantages
Abstract
:1. Introduction
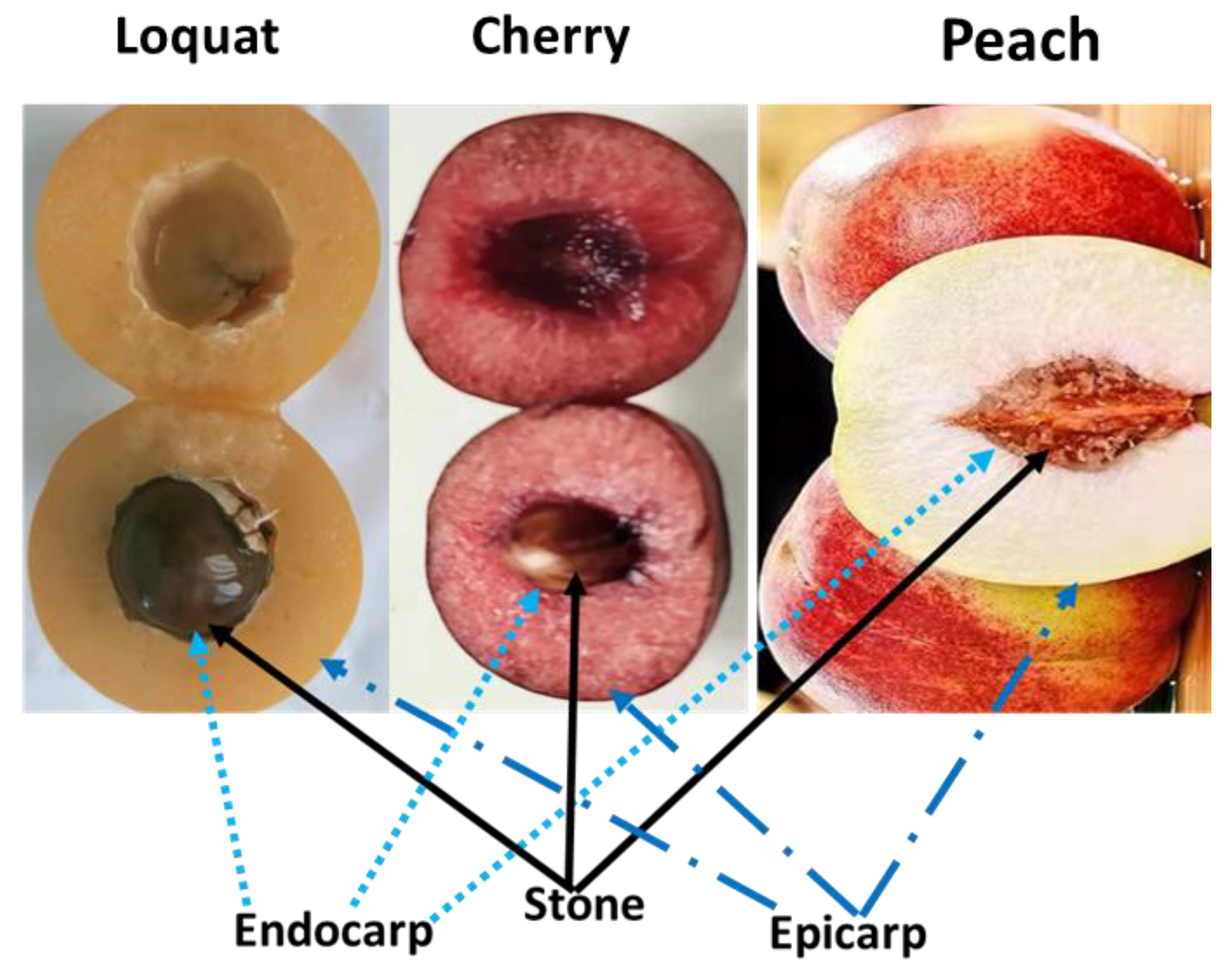
2. Development and Structural Features of the Stone Fruit
3. Molecular Basis for the Configuration of Endocarp
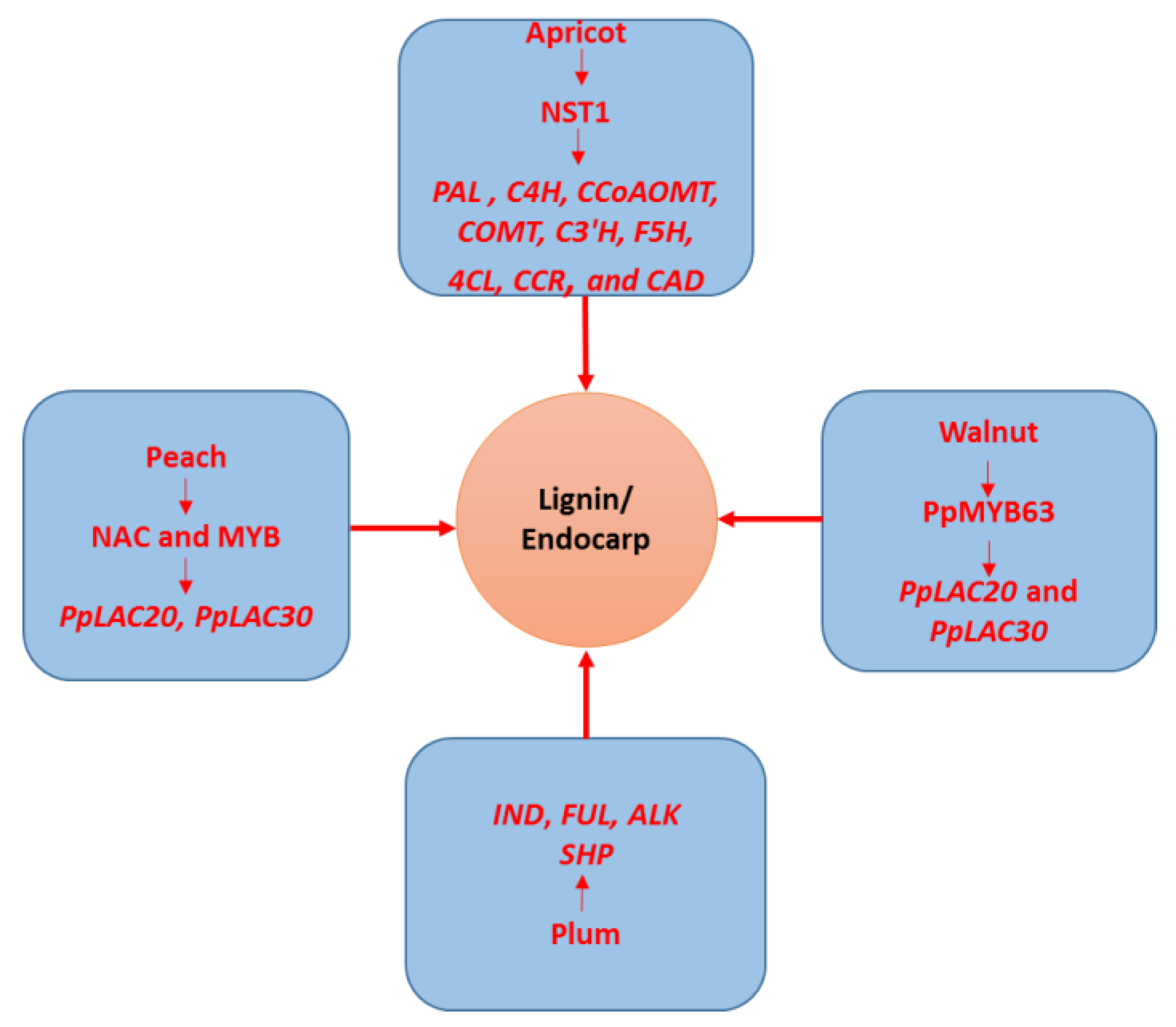
4. Molecular Regulation of Stone Formation in Fruits
4.1. Lignin
4.2. Endocarp
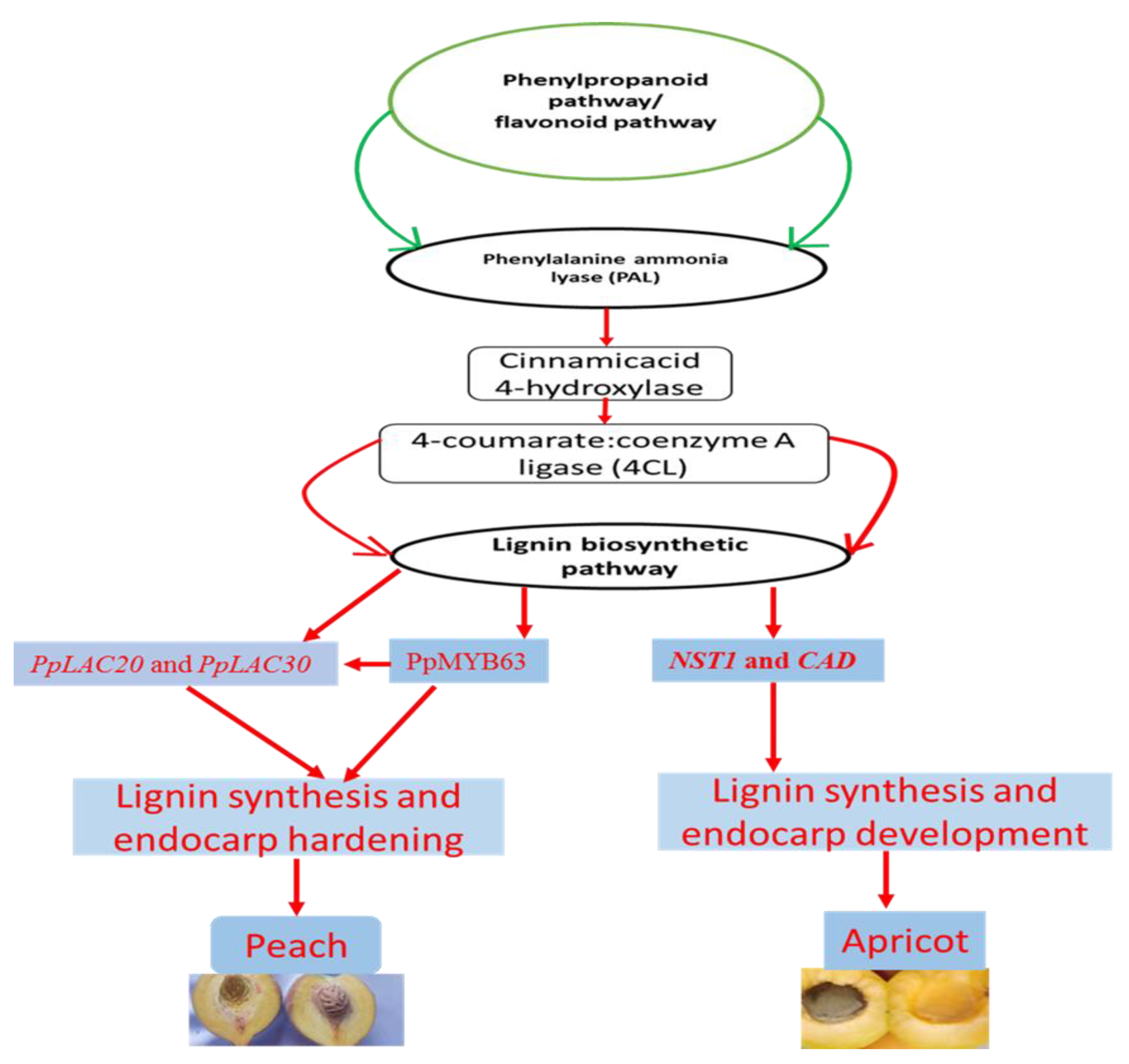
5. Molecular Mechanisms of Endocarp Lignification
6. History of Stoneless Fruit and an Attempt of Making Stoneless or Pitless Fruits
7. Advantages and Disadvantages of Stone in Fruits
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Looney, N.; Jackson, D. Stonefruit. In Temperate and Subtropical Fruit Production; Jackson, D.I., Looney, N., Eds.; CABI Publishing: Oxfordshire, UK, 2006; pp. 171–188. [Google Scholar]
- Famiani, F.; Bonghi, C.; Chen, Z.H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone fruits: Growth and nitrogen and organic acid metabolism in the fruits and seeds—A review. Front. Plant Sci. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Muhammad, N.; Zhao, Z.; Yin, K.; Liu, Z.; Wang, L.; Luo, Z.; Wang, L.; Liu, M. Molecular regulation of fruit size in horticultural plants: A review. Sci. Hortic. 2021, 288, 110353. [Google Scholar] [CrossRef]
- Qui, K.; Zhou, H.; Pan, H.; Sheng, Y.; Yu, H.; Xie, Q.; Chen, H.; Cai, Y.; Zhang, J.; He, J. Genome-wide identification and functional analysis of the peach (P. persica) laccase gene family reveal members potentially involved in endocarp lignification. Trees 2022, 36, 1477–1496. [Google Scholar] [CrossRef]
- Dardick, C.; Callahan, A.M. Evolution of the fruit endocarp: Molecular mechanisms underlying adaptations in seed protection and dispersal strategies. Front. Plant Sci. 2014, 5, 284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, X.; Jin, Q.; Su, X.; Li, M.; Yan, C.; Jiao, X.; Li, D.; Lin, Y.; Cai, Y. Comparison of the transcriptomic analysis between two Chinese white pear (Pyrus bretschneideri Rehd.) genotypes of different stone cells contents. PLoS ONE 2017, 12, e0187114. [Google Scholar] [CrossRef] [Green Version]
- Dardick, C.D.; Callahan, A.M.; Chiozzotto, R.; Schafer, R.J.; Piagnani, M.C.; Scorza, R. Stone formation in peach fruit exhibits spatial coordination of the lignin and flavonoid pathways and similarity to Arabidopsis dehiscence. BMC Biol. 2010, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, H.; Liu, P.; Li, Y.; Liu, K.; An, X.; Zhang, Z.; Zhao, S. The role of JrLACs in the lignification of walnut endocarp. BMC Plant Biol. 2021, 21, 511. [Google Scholar] [CrossRef]
- Callahan, A.M.; Dardick, C.; Scorza, R. Characterization of ‘Stoneless’: A naturally occurring, partially stoneless plum cultivar. J. Am. Soc. Hortic. Sci. 2009, 134, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Tani, E.A.; Polidoros, A.S. Tsaftaris, Characterization and expression analysis of FRUITFULL- and SHATTERPROOF-like genes from peach (Prunus persica) and their role in split-pit formation. Tree Physiol. 2007, 27, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Tani, E.; Tsaballa, A.; Stedel, C.; Kalloniati, C.; Papaefthimiou, D.; Polidoros, A.; Darzentas, N.; Ganopoulos, I.; Flemetakis, E.; Katinakis, P.; et al. The study of a SPATULA-like bHLH transcription factor expressed during peach (Prunus persica) fruit development. Plant Physiol. Biochem. 2011, 49, 654–663. [Google Scholar] [CrossRef]
- Hu, H.; Liu, Y.; Shi, G.L.; Liu, Y.P.; Wu, R.J.; Yang, A.Z.; Wang, Y.M.; Hua, B.G.; Wang, Y.N. Proteomic analysis of peach endocarp and mesocarp during early fruit development. Physiol. Plant. 2011, 142, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Yu, J.; Yang, A.; Liu, Y. caffeoyl shikimate esterase has a role in endocarp lignification in peach (Prunus persica L.) fruit. Hortic. Sci. Technol. 2017, 35, 59–68. [Google Scholar]
- Zhu, X.; Jiang, L.; Cai, Y.; Cao, Y. Functional analysis of four Class III peroxidases from Chinese pear fruit: A critical role in lignin polymerization. Physiol. Mol. Biol. Plants 2021, 27, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yan, C.; Qiu, J.; Zhang, N.; Lin, Y.; Cai, Y. Structural characterization and deposition of stone cell lignin in Dangshan Su pear. Sci. Hortic. 2013, 155, 123–130. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhu, J.; Wang, D.Y. Analysis of the content of pear pulp stone cells. J. Jiangsu Agric. Sci. 2013, 46, 173–176. (In Chinese) [Google Scholar]
- Wang, Y.R.; Xing, X.T.; Ren, H.Q.; Yu, Y.; Fei, B.H. Distribution of lignin in Chinese Fir branches determined by ultraviolet microspectrometer. Spectrosc. Spect. Anal. 2012, 6, 1685–1688. [Google Scholar]
- Jiang, T.-D. Lignin; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Vazquez, G.; Antorrena, G.; Gonzalez, J.; Freire, S. FTR 1H and 13C NMR characterization of acetosolv-solubilized Pine and Eucalyptu lignin. Holzforschung 1997, 51, 158–166. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Liu, C.; Liu, Y.; Lu, X.; Du, G.; Lyu, D. Biochemical characterization and expression analysis of lignification in two pear (Pyrus ussuriensis Maxim.) varieties with contrasting stone cell content. Protoplasma 2020, 257, 261–274. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; de Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Ryugo, K. The rate of dry weight accumulation by the peach pit during the hardening process. J. Am. Soc. Hortic. Sci. 1961, 78, 132–137. [Google Scholar]
- Ryugo, K. Changes in methoxyl content in the peach endocarp and some of its soluble phenolic constituents during lignification. J. Am. Soc. Hortic. Sci. 1963, 84, 110–115. [Google Scholar]
- Ferrándiz, C.; Fourquin, C. Role of the FUL-SHP network in the evolution of fruit morphology and function. J. Exp. Bot. 2014, 65, 4505–4513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roeder, A.H.K.; Ferrándiz, C.; Yanofsky, M.F. The role of the REPLUMLESS Homeodomain protein in patterning the Arabidopsis fruit. Curr. Biol. 2003, 13, 1630–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genom. Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, L.; Zhang, Q.; Xu, J.; Liu, W.; Dong, W. Comparative transcriptome profiling and morphology provide insights into endocarp cleaving of apricot cultivar (Prunus armeniaca L.). BMC Plant Biol. 2017, 17, 72. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Pérez-López, D.; Hammami, S.B.M.; Agüera, J.; Moriana, A. Fruit pit hardening: Physical measurement during olive fruit growth. Ann. Appl. Biol. 2013, 163, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Bollard, E.G. The physiology and nutrition of developing fruits. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: New York, NY, USA, 1970; Volume 1, pp. 387–425. [Google Scholar]
- Opara, L. Fruit growth measurement and analysis. Hortic. Rev. 2000, 24, 373–431. [Google Scholar] [CrossRef]
- Canton, M.; Drincovich, M.F.; Lara, M.V.; Vizzotto, G.; Walker, R.P.; Famiani, F.; Bonghi, C. Metabolism of stone fruits: Reciprocal contribution between primary metabolism and cell wall. Front. Plant Sci. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Famiani, F.; Casulli, V.; Baldicchi, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Development and metabolism of the fruit and seed of the Japanese plum Ozark premier (Rosaceae). J. Plant Physiol. 2012, 169, 551–560. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Sun, X.; Du, X.; Liu, W.; Dong, W. Differential expression of genes encoding phenylpropanoid enzymes in an apricot cultivar (Prunus armeniaca L.) with cleavable endocarp. Trees 2019, 33, 1695–1710. [Google Scholar] [CrossRef]
- Tani, E.; Polidoros, A.N.; Flemetakis, E.; Stedel, C.; Kalloniati, C.; Demetriou, K.; Katinakis, P.; Tsaftaris, A.S. Characterization and expression analysis of AGAMOUS-like, SEEDSTICK-like, and SEPALLATA-like MADS-box genes in peach (Prunus persica) fruit. Plant Physiol. Biochem. 2009, 47, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Shangqi, Y.U.; Rui, Z.H.A.N.G.; Zhongzhong, G.U.O.; Yan, S.O.N.G.; Jiazhi, F.U.; Pengyu, W.U.; Zhihao, M.A. Dynamic changes of auxin and analysis of differentially expressed genes in walnut endocarp during hardening. Acta Hortic. Sin. 2021, 48, 487. [Google Scholar]
- Wu, X.; Zhang, Z.; Sun, M.; An, X.; Qi, Y.; Zhao, S.; Zhang, Z.; Wang, H. Comparative transcriptome profiling provides insights into endocarp lignification of walnut (Juglans regia L.). Sci. Hortic. 2021, 282, 110030. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Z.; Dong, X.; Cao, F.; Peng, J.; Li, M. Cloning and characterization of a CCoAOMT gene involved in rapid lignification of endocarp in dove tree (Davidia involucrata Baill.). Biotechnol. Biotechnol. Equip. 2018, 32, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Doster, M.A.; Michailides, T.J. Relationship between shell discoloration of pistachio nuts and incidence of fungal decay and insect infestation. Plant Dis. 1999, 83, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Yao, J.L.; Qin, M.F.; Zhang, M.Y.; Allan, A.C.; Wang, D.F.; Wu, J. PbrmiR397a regulates lignification during stone cell development in pear fruit. Plant Biotechnol. J. 2019, 17, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Lee, C.; Zhou, J.; McCarthy, R.L.; Ye, Z.H. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. Plant Cell 2008, 20, 2763–2782. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Richardson, E.A.; Ye, Z.H. Two NAC domain transcription factors, SND1, and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta 2007, 225, 1603–1611. [Google Scholar] [CrossRef]
- Zhou, J.; Lee, C.; Zhong, R.; Ye, Z.H. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 2009, 21, 248–266. [Google Scholar] [CrossRef] [Green Version]
- Chezem, W.R.; Memon, A.; Li, F.S.; Weng, J.K.; Clay, N.K. SG2-type R2R3-MYB transcription factor MYB15 controls defense-induced lignification and basal immunity in Arabidopsis. Plant Cell 2017, 29, 1907–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wang, X.; Ran, L.; Tian, Q.; Fan, D.; Luo, K. PtoMYB92 is a transcriptional activator of the lignin biosynthetic pathway during secondary cell wall formation in Populus tomentosa. Plant Cell Physiol. 2015, 56, 2436–2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, R.L.; Zhong, R.; Fowler, S.; Lyskowski, D.; Piyasena, H.; Carleton, K.; Spicer, C.; Ye, Z.-H. The poplar MYB transcription factors, PtrMYB3 and PtrMYB20, are involved in the regulation of secondary wall biosynthesis. Plant Cell Physiol. 2010, 51, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Bomal, C.; Bedon, F.; Caron, S.; Mansfield, S.D.; Levasseur, C.; Cooke, J.E.; Blais, S.; Tremblay, L.; Morency, M.-J.; Pavy, N.; et al. Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: A comparative in planta analysis. J. Exp. Bot. 2008, 59, 3925–3939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goicoechea, M.; Lacombe, E.; Legay, S.; Mihaljevic, S.; Rech, P.; Jauneau, A.; Lapierre, C.; Pollet, B.; Verhaegen, D.; Chaubet-Gigot, N.; et al. EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. Plant J. 2005, 43, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Yin, X.R.; Zeng, J.K.; Ge, H.; Song, M.; Xu, C.J.; Li, X.; Ferguson, I.B.; Chen, K.S. Activator- and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J. Exp. Bot. 2014, 65, 4349–4359. [Google Scholar] [CrossRef]
- Xue, C.; Yao, J.L.; Xue, Y.S.; Su, G.Q.; Wang, L.; Lin, L.K.; Allan, A.C.; Zhang, S.L.; Wu, J. PbrMYB169 positively regulates lignification of stone cells in pear fruit. J. Exp. Bot. 2019, 70, 1801–1814. [Google Scholar] [CrossRef]
- Burbank, L. The Stoneless Plum: An Experiment in Teaching a Plant Economy; The Minerva Group, Inc.: Madison, WI, USA, 1914; Available online: http://digicoll.library.wisc.edu/cgi-bin/HistSciTech/HistSciTech-idx?type=article&did=HISTSCITECH.0011.0029.0009&isize=M (accessed on 21 August 2022).
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Yang, T.; Chang, S. Anatomy and lignin deposition of stone cell in Camellia oleifera shell during the young stage. Protoplasma 2021, 258, 361–370. [Google Scholar] [CrossRef]
- Cheng, X.; Li, G.; Muhammad, A.; Zhang, J.; Jiang, T.; Jin, Q.; Zhao, H.; Cai, Y.; Lin, Y. Molecular identification, phylogenomic characterization and expression patterns analysis of the LIM (LIN-11, Isl1 and MEC-3 domains) gene family in pear (Pyrus bretschneideri) reveal its potential role in lignin metabolism. Gene 2019, 686, 237–249. [Google Scholar] [CrossRef]
- Nikhontha, K.; Krisanapook, K.; Imsabai, W. Fruit growth, endocarp lignification, and boron and calcium concentrations in Nam Hom (aromatic) coconut during fruit development. J. ISSAAS 2019, 25, 21–31. [Google Scholar]
- Muhammad, N.; Luo, Z.; Yang, M.; Li, X.; Liu, Z.; Liu, M. The joint role of the late anthocyanin biosynthetic UFGT-encoding genes in the flowers and fruits coloration of horticultural plants. Sci. Hortic. 2022, 301, 111110. [Google Scholar] [CrossRef]
| S.No | Plant Species | Parts | Genes | TFs | References |
|---|---|---|---|---|---|
| 1 | Apricots | Endocarp | PAL, C4H, CCoAOMT, COMT, C3’H, F5H, 4CLCCR, and CAD | NST1 | [33] |
| 2 | Peach | PpLAC20, PpLAC30 | NAC and MYB | [4,7] | |
| 3 | E. japonica | Lignin | ----- | EjMYB1 and EjMYB2 | [40,49] |
| 4 | E. grandis | ----- | EgMYB2 | [48] | |
| 5 | Peach | ----- | SHATTERPROOF, SEEDSTCK, and NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1 | [45,46] | |
| 6 | Poplar | ----- | PtoMYB92/PtrMYB3/PtrMYB20 | ||
| 7 | P. taeda | ----- | PtMYB1 and PtMYB8 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.K.U.; Muhammad, N.; Jia, Z.; Peng, J.; Liu, M. Mechanism of Stone (Hardened Endocarp) Formation in Fruits: An Attempt toward Pitless Fruits, and Its Advantages and Disadvantages. Genes 2022, 13, 2123. https://doi.org/10.3390/genes13112123
Khan MKU, Muhammad N, Jia Z, Peng J, Liu M. Mechanism of Stone (Hardened Endocarp) Formation in Fruits: An Attempt toward Pitless Fruits, and Its Advantages and Disadvantages. Genes. 2022; 13(11):2123. https://doi.org/10.3390/genes13112123
Chicago/Turabian StyleKhan, Muhammad Khalil Ullah, Noor Muhammad, Zhuolong Jia, Jianying Peng, and Mengjun Liu. 2022. "Mechanism of Stone (Hardened Endocarp) Formation in Fruits: An Attempt toward Pitless Fruits, and Its Advantages and Disadvantages" Genes 13, no. 11: 2123. https://doi.org/10.3390/genes13112123






