Association of Polymorphisms in the Long Non-Coding RNA HOTAIR with Recurrent Pregnancy Loss in a Korean Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Genotyping
2.3. Clinical Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Clinical Characteristics
3.2. Genetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Hachem, H.; Crepaux, V.; May-Panloup, P.; Descamps, P.; Legendre, G.; Bouet, P.E. Recurrent pregnancy loss: Current perspectives. Int. J. Womens Health 2017, 9, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Ford, H.B.; Schust, D.J. Recurrent Pregnancy Loss: Etiology, Diagnosis, and Therapy. Rev. Obstet. Gynecol. 2009, 2, 76–83. [Google Scholar] [PubMed]
- Ryu, C.S.; Sakong, J.H.; Ahn, E.H.; Kim, J.O.; Ko, D.; Kim, J.H.; Lee, W.S.; Kim, N.K. Association study of the three functional polymorphisms (TAS2R46G>A, OR4C16G>A, and OR4X1A>T) with recurrent pregnancy loss. Genes Genom. 2019, 41, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhan, W.; Du, Q.; Wu, L.; Ding, H.; Liu, F.; Yin, A. Variants c.677 C> T, c.1298 A> C in MTHFR, and c.66 A> G in MTRR Affect the Occurrence of Recurrent Pregnancy Loss in Chinese Women. Genet. Test. Mol. Biomark. 2020, 24, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, F.; Li, X.-C.; Shen, F.-J.; Ma, X.-L.; Wu, F.; Zhang, S.-M.; Zeng, W.-H.; Liu, X.-R.; Fan, J.-X.; et al. The YY1-HOTAIR-MMP2 Signaling Axis Controls Trophoblast Invasion at the Maternal-Fetal Interface. Mol. Ther. 2017, 25, 2394–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Clifford, K.; Rai, R.; Watson, H.; Regan, L. Pregnancy: An informative protocol for the investigation of recurrent miscarriage: Preliminary experience of 500 consecutive cases. Hum. Reprod. 1994, 9, 1328–1332. [Google Scholar] [CrossRef]
- Park, H.S.; Ko, K.H.; Kim, J.O.; An, H.J.; Kim, Y.R.; Kim, J.H.; Lee, W.S.; Kim, N.K. Association Study between the Polymorphisms of Matrix Metalloproteinase (MMP) Genes and Idiopathic Recurrent Pregnancy Loss. Genes 2019, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Cao, J. The functional role of long non-coding RNAs and epigenetics. Biol. Proced. Online 2014, 16, 42. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Sukumar, S. The HOX genes and their roles in oncogenesis. Nat. Rev. Cancer 2010, 10, 361–371. [Google Scholar] [CrossRef]
- Liu, K.; Mao, X.; Chen, Y.; Li, T.; Ton, H. Regulatory role of long non-coding RNAs during reproductive disease. Am. J. Transl. Res. 2018, 10, 1–12. [Google Scholar] [PubMed]
- Liu, Z.; Ouyang, G.; Lu, W.; Zhang, H. Long non-coding RNA HOTAIR promotes hepatocellular carcinoma progression by regulating miR-526b-3p/DHX33 axis. Genes Genom. 2021, 43, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lv, Z.; An, C.; Shi, M.; Pan, W.; Zhou, L.; Yang, W.; Yang, M. Onco-lncRNA HOTAIR and its functional genetic variants in papillary thyroid carcinoma OPEN. Nat. Publ. Gr. 2016, 6, 31969. [Google Scholar] [CrossRef] [Green Version]
- Schorderet, P.; Duboule, D. Structural and Functional Differences in the Long Non-Coding RNA HOTAIR in Mouse and Human. PLoS Genet 2011, 7, 1002071. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Wang, K.; Song, Y.; Li, S.; Yin, H.; Luo, R.; Liao, Z.; Wu, X.; Zhang, Y.; Yang, C. Long non-coding RNA HOTAIR modulates intervertebral disc degenerative changes via Wnt/β-catenin pathway. Arthritis Res. Ther. 2019, 21, 201. [Google Scholar] [CrossRef] [Green Version]
- Unemori, E.N.; Hibbs, M.S.; Amento, E.P. Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. J. Clin. Investig. 1991, 88, 1656–1662. [Google Scholar] [CrossRef]
- Puistola, U.; Rönnberg, L.; Martikainen, H.; Turpeenniemi-Hujanen, T. The human embryo produces basement membrane collagen (type IV collagen)-degrading protease activity. Hum. Reprod. 1989, 4, 309–311. [Google Scholar] [CrossRef]
- Curry, T.E., Jr.; Osteen, K.G. Cyclic Changes in the Matrix Metalloproteinase System in the Ovary and Uterus1. Biol. Reprod. 2001, 64, 1285–1296. [Google Scholar] [CrossRef] [Green Version]
- Nissi, R.; Talvensaari-Mattila, A.; Kotila, V.; Niinimäki, M.; Järvelä, I.; Turpeenniemi-Hujanen, T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod. Biol. Endocrinol. 2013, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.W.; Kim, J.O.; Rah, H.; Kim, J.H.; Kim, Y.R.; Lee, Y.; Lee, W.S.; Kim, N.K. Genetic variants of vascular endothelial growth factor are associated with recurrent implantation failure in Korean women. Reprod. Biomed. Online 2016, 32, 190–196. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.H.; Park, H.W.; Park, H.S.; An, H.J.; Kim, Y.R.; Ahn, E.H.; Lee, W.S.; Kim, N.K. Associations between HOTAIR polymorphisms rs4759314, rs920778, rs1899663, and rs7958904 and risk of primary ovarian insufficiency in Korean women. Maturitas 2021, 144, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Jun, H.H.; Kim, E.J.; Lee, J.Y.; Park, H.S.; Ryu, C.S.; Kim, S.; Oh, D.; Kim, J.W.; Kim, N.K. Genetic Variants of HOTAIR Associated With Colorectal Cancer Susceptibility and Mortality. Front. Oncol. 2020, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Hassanzarei, S.; Hashemi, M.; Sattarifard, H.; Hashemi, S.M.; Bahari, G.; Ghavami, S. Genetic polymorphisms of HOTAIR gene are associated with the risk of breast cancer in a sample of southeast Iranian population. Tumor Biol. 2017, 39, 1010428317727539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Liu, Q.; Li, J.; Wang, X.; Wang, Y.; Yuan, Z.; Li, J.; Pei, D.-S. Analysis of the association of HOTAIR single nucleotide polymorphism (rs920778) and risk of cervical cancer. APMIS 2016, 124, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Xavier-Magalhães, A.; Oliveira, A.I.; de Castro, J.V.; Pojo, M.; Gonçalves, C.S.; Lourenço, T.; Viana-Pereira, M.; Costa, S.; Linhares, P.; Vaz, R.; et al. Effects of the functional HOTAIR rs920778 and rs12826786 genetic variants in glioma susceptibility and patient prognosis. J. Neurooncol. 2017, 132, 27–34. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Shi, R.; Jia, J.; Li, X.; Cheng, J. Rs4759314 polymorphism located in HOTAIR is associated with the risk of congenital heart disease by alternating downstream signaling via reducing its expression. J. Cell. Biochem. 2018, 119, 8112–8122. [Google Scholar] [CrossRef]
- Choi, D.-H.; Kim, E.K.; Kim, K.-H.; Lee, K.-A.; Kang, D.-W.; Kim, H.Y.; Bridges, P.; Ko, C. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Hum. Reprod. 2011, 26, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Shahbazian, H.; Nasuri, Y.; Hosseini, S.; Arvandi, S.; Razzaghi, S. A report of the frequency of colorectal carcinoma and involved lymph nodes in South-West Iran. Indian J. Med. Paediatr. Oncol. 2016, 37, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.; Li, C.H.; Man Tong, J.H.; Zheng, D.; He, Q.; Luo, Z.; Lou, U.K.; Wang, J.; To, K.-F.; Chen, Y. The establishment of CDK9/RNA PolII/H3K4me3/DNA methylation feedback promotes HOTAIR expression by RNA elongation enhancement in cancer. Mol. Ther. 2022, 30, 1597–1609. [Google Scholar] [CrossRef]
- Cao, Z.; Oyang, L.; Luo, X.; Xia, L.; Hu, J.; Lin, J.; Tan, S.; Tang, Y.; Zhou, Y.; Cao, D.; et al. The roles of long non-coding RNAs in lung cancer. J. Cancer 2022, 13, 174–183. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Feng, S. Knockdown of long non-coding RNA HOTAIR promotes bone marrow mesenchymal stem cell differentiation by sponging microRNA miR-378g that inhibits nicotinamide N-methyltransferase. Bioengineered 2021, 12, 12482–12497. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhu, G.; Zhang, C.; Deng, Q.; Katsaros, D.; Mayne, S.T.; Risch, H.A.; Mu, L.; Canuto, E.M.; Gregori, G.; et al. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res. Treat. 2012, 136, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Özeş, A.R.; Miller, D.F.; Özeş, O.N.; Fang, F.; Liu, Y.; Matei, D.; Huang, T.; Nephew, K.P. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 2016, 35, 5350–5361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; An, J.; Wu, M.; Zheng, Q.; Gui, X.; Li, T.; Pu, H.; Lu, D. LncRNA HOTAIR promotes human liver cancer stem cell malignant growth through downregulation of SETD2. Oncotarget 2015, 6, 27847–27864. [Google Scholar] [CrossRef] [PubMed]
- Padam, K.S.R.; Basavarajappa, D.S.; Shenoy, U.S.; Chakrabarty, S.; Kabekkodu, S.P.; Hunter, K.D.; Radhakrishnan, R. In silico interaction of HOX cluster-embedded microRNAs and long non-coding RNAs in oral cancer. J. Oral Pathol. Med. 2022, 51, 18–29. [Google Scholar] [CrossRef]
- Cao, M.; Luo, H.; Li, D.; Wang, S.; Xuan, L.; Sun, L. Research advances on circulating long noncoding RNAs as biomarkers of cardiovascular diseases. Int. J. Cardiol. 2022, 353, 109–117. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Rezaei, N. Long Non-Coding RNAs in Diagnosis, Treatment, Prognosis, and Progression of Glioma: A State-of-the-Art Review. Front. Oncol. 2021, 11, 712786. [Google Scholar] [CrossRef]
- Kim, I.-J.; Lee, J.-Y.; Park, H.-W.; Park, H.-S.; Ko, E.-J.; Sung, J.-H.; Kim, N.-K. Association between HOTAIR lncRNA Polymorphisms and Coronary Artery Disease Susceptibility. J. Pers. Med. 2021, 11, 375. [Google Scholar] [CrossRef]
- Chang, L.; Guo, R.; Yuan, Z.; Shi, H.; Zhang, D. LncRNA HOTAIR Regulates CCND1 and CCND2 Expression by Sponging miR-206 in Ovarian Cancer. Cell. Physiol. Biochem. 2018, 49, 1289–1303. [Google Scholar] [CrossRef]
- Xiong, X.R.; Lan, D.L.; Li, J.; Yin, S.; Xiong, Y.; Zi, X.D. Identification of differential abundances of mRNA transcript in cumulus cells and CCND1 associated with yak oocyte developmental competence. Anim. Reprod. Sci. 2019, 208, 106135. [Google Scholar] [CrossRef]
- Taylor, P.N.; Minassian, C.; Rehman, A.; Iqbal, A.; Draman, M.S.; Hamilton, W.; Dunlop, D.; Robinson, A.; Vaidya, B.; Lazarus, J.H.; et al. TSH Levels and Risk of Miscarriage in Women on Long-Term Levothyroxine: A Community-Based Study. J. Clin. Endocrinol. Metab. 2014, 99, 3895–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhou, L.; Fu, G.; Sun, F.; Shi, J.; Wei, J.; Lu, C.; Zhou, C.; Yuan, Q.; Yang, M. The identification of an ESCC susceptibility SNP rs920778 that regulates the expression of lncRNA HOTAIR via a novel intronic enhancer. Carcinogenesis 2014, 35, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
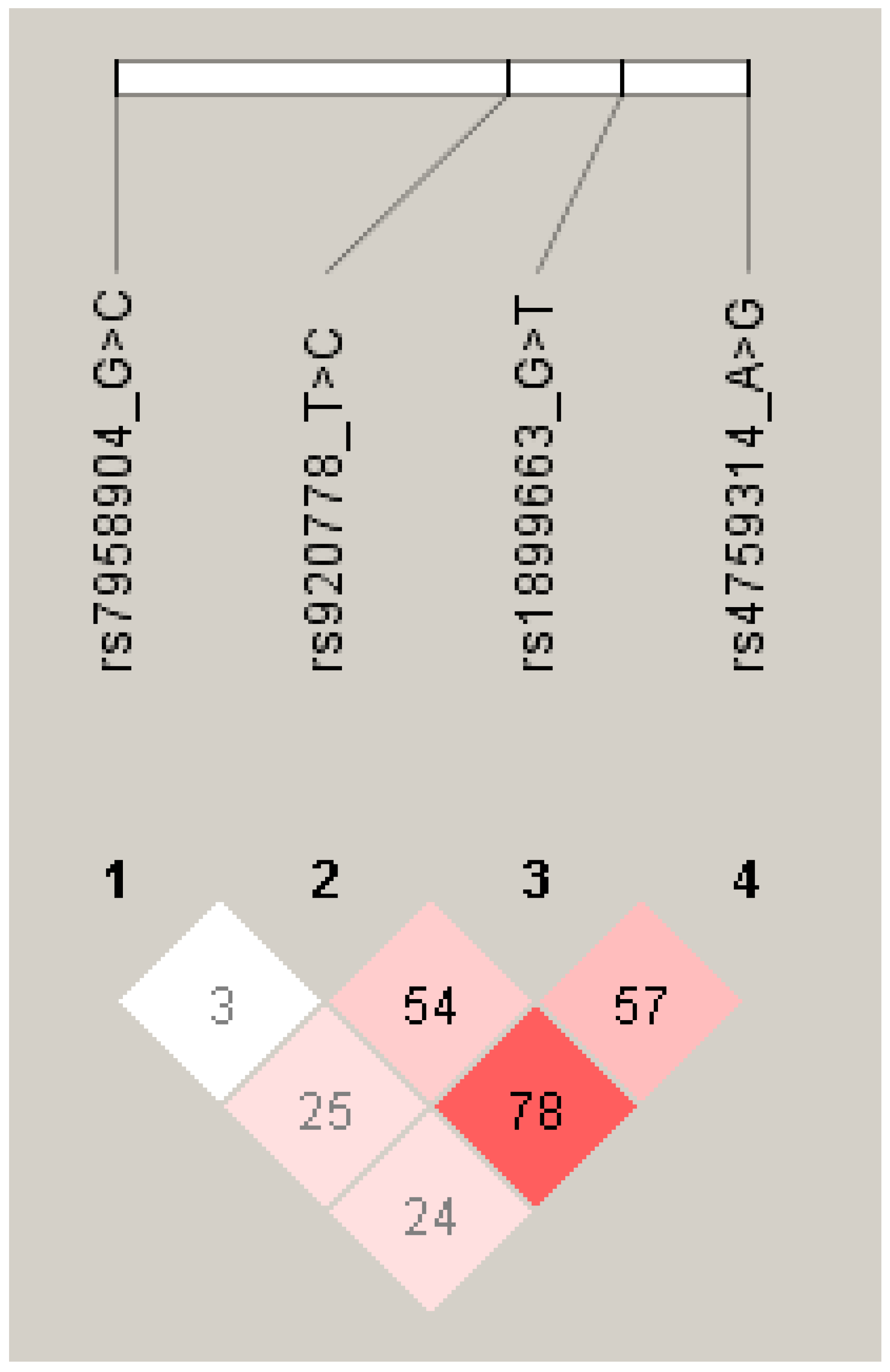
| Characteristics | Controls (n = 383) | RPL (n = 403) | pa |
|---|---|---|---|
| Age (years, mean ± SD) | 32.83 ± 4.17 | 32.93 ± 4.13 | 0.822 |
| BMI (kg/m2) | 21.63 ± 3.26 | 21.48 ± 3.89 | 0.738 b |
| Live birth (n, mean ± SD) | 1.65 ± 0.58 | N/A | |
| Pregnancy loss (n, mean ± SD) | N/A | 2.99 ± 1.48 | |
| Mean gestational age (weeks) | 39.29 ± 1.59 | N/A | |
| PLT (103 /µL) | 239.09 ± 64.05 | 244.84 ± 62.79 | 0.258 |
| PT (s) | 10.78 ± 1.71 | 11.34 ± 1.66 | 0.0002 |
| aPTT (s) | 29.34 ± 3.60 | 31.95 ± 4.36 | <0.0001 b |
| Folate (ng/mL) | 14.07 ± 9.72 | 15.20 ± 14.75 | 0.934 b |
| Homocysteine (µmol/L) | 7.40 ± 5.13 | 6.91 ± 2.07 | 0.858 b |
| Total cholesterol (mg/dL) | 212.15 ± 57.77 | 187.18 ± 46.84 | <0.0001 b |
| Uric acid (mg/dL) | 3.82 ± 1.02 | 3.83 ± 0.87 | 0.742 b |
| BUN (mg/dL) | 8.95 ± 3.04 | 10.37 ± 6.24 | <0.0001 b |
| Creatinine (mg/dL) | 0.64 ± 0.16 | 0.73 ± 0.12 | <0.0001 b |
| FSH (mIU/mL) | 8.12 ± 3.11 | 7.72 ± 11.43 | <0.0001 b |
| LH (mIU/mL) | 3.59 ± 2.55 | 6.31 ± 11.90 | <0.0001 b |
| E2 (pg/mL) | 26.00 ± 14.75 | 52.37 ± 119.94 | <0.0001 b |
| TSH (µIU/mL) | 1.55 ± 1.04 | 2.14 ± 1.44 | <0.0001 b |
| LDL cholesterol (mg/dL) | 145.39 ± 41.65 | 109.62 ± 35.41 | 0.0001 |
| HDL cholesterol (mg/dL) | 84.31 ± 20.32 | 59.94 ± 15.82 | <0.0001 |
| Triglyceride (mg/dL) | 181.26 ± 110.32 | 184.22 ± 145.73 | 0.299b |
| Glucose (mg/dL) | 100.05 ± 24.04 | 96.58 ± 13.54 | 0.893 b |
| FBS (mg/dL) | 6.55 ± 1.96 | 95.07 ± 16.83 | <0.0001 b |
| WBC (103/µL) | 7.99 ± 2.52 | 7.32 ± 2.83 | 0.006 |
| RBC (106/µL) | 4.09 ± 0.39 | 4.22 ± 0.42 | 0.0003 |
| Hemoglobin (g/dL) | 12.21 ± 1.24 | 12.61 ± 1.33 | 0.0006 |
| Hematocrit (%) | 36.80 ± 3.35 | 37.57 ± 3.75 | 0.005 b |
| PAI-1 (ng/mL) | N/A | 11.30 ± 7.81 | |
| CD3 (%) | N/A | 67.33 ± 8.84 | |
| CD4 (%) | N/A | 36.42 ± 7.34 | |
| CD8 (%) | N/A | 27.99 ± 7.61 | |
| CD19 (%) | N/A | 12.54 ± 4.86 | |
| CD56 NK cells (%) | N/A | 17.66 ± 7.88 |
| Genotypes | Controls (n = 383) | RPL (n = 403) | AOR (95% CI) | p | FDR |
|---|---|---|---|---|---|
| HOTAIR rs4759314 | |||||
| AA | 351 (91.6) | 322 (80.0) | 1.000 (reference) | ||
| AG | 30 (7.8) | 79 (19.6) | 3.054 (1.847-5.050) | <0.0001 | <0.0001 |
| GG | 2 (0.5) | 2 (0.5) | 1.095 (0.098-12.175) | 0.941 | 0.941 |
| Dominant (AA vs. AG+GG) | 2.933 (1.792-4.801) | <0.0001 | <0.0001 | ||
| Recessive (AA+AG vs. GG) | 0.947 (0.085-10.522) | 0.965 | 0.965 | ||
| HWE-P | 0.134 | 0.22 | |||
| A allele | 1.000 (reference) | ||||
| G allele | 2.479 (1.642–3.744) | <0.0001 | |||
| HOTAIR rs920778 | |||||
| TT | 236 (61.6) | 226 (56.1) | 1.000 (reference) | ||
| TC | 134 (35.0) | 149 (37.0) | 1.166 (0.867–1.568) | 0.310 | 0.620 |
| CC | 13 (3.4) | 28 (6.9) | 2.263 (1.140–4.488) | 0.019 | 0.076 |
| Dominant (TT vs. TC+CC) | 1.264 (0.950–1.680) | 0.107 | 0.214 | ||
| Recessive (TT+TC vs. CC) | 2.129 (1.085–4.175) | 0.028 | 0.112 | ||
| HWE-P | 0.251 | 0.612 | |||
| T allele | 1.000 (reference) | ||||
| C allele | 1.297 (1.025–1.641) | 0.031 | |||
| HOTAIR rs1899663 | |||||
| GG | 224 (58.5) | 235 (58.3) | 1.000 (reference) | ||
| GT | 139 (36.3) | 146 (36.2) | 0.921 (0.645–1.316) | 0.651 | 0.756 |
| TT | 20 (5.2) | 22 (5.5) | 0.756 (0.331–1.726) | 0.506 | 0.675 |
| Dominant (GG vs. GT+TT) | 0.902 (0.640–1.273) | 0.558 | 0.558 | ||
| Recessive (GG+GT vs. TT) | 0.800 (0.357–1.794) | 0.589 | 0.589 | ||
| HWE-P | 0.794 | 0.913 | |||
| G allele | 1.000 (reference) | ||||
| T allele | 0.987 (0.788–1.247) | 0.915 | |||
| HOTAIR rs7958904 | |||||
| GG | 210 (54.8) | 232 (57.6) | 1.000 (reference) | ||
| GC | 144 (37.6) | 144 (35.7) | 0.945 (0.662–1.349) | 0.756 | 0.756 |
| CC | 29 (7.6) | 27 (6.7) | 0.678 (0.326–1.413) | 0.300 | 0.600 |
| Dominant (GG vs. GC+CC) | 0.896 (0.638–1.260) | 0.529 | 0.558 | ||
| Recessive (GG+GC vs. CC) | 0.682 (0.333–1.397) | 0.296 | 0.296 | ||
| HWE-P | 0.534 | 0.471 | |||
| G allele | 1.000 (reference) | ||||
| C allele | 0.914 (0.728–1.146) | 0.435 |
| Haplotype | Controls (2n = 766) | Case (2n = 806) | OR (95% CI) | p * |
|---|---|---|---|---|
| rs4759314A>G/rs920778 T>C/rs1899663 G>T/rs7958904 G>C | ||||
| A-T-G-G | 0.689 | 0.577 | 1.000 (reference) | |
| A-T-G-C | 0.007 | 0.039 | 7.040 (2.715–18.260) | <0.0001 |
| A-T-T-G | 0.005 | 0.025 | 5.677 (1.926–16.730) | 0.001 |
| A-C-G-G | 0.025 | 0.071 | 3.406 (1.997–5.811) | <0.0001 |
| A-C-G-C | 0.002 | 0.010 | 4.542 (0.959–21.500) | 0.053 |
| A-C-T-G | 0.006 | 0.015 | 3.406 (1.091–10.640) | 0.040 |
| G-T-G-G | 0.009 | 0.036 | 4.704 (2.041–10.840) | <0.0001 |
| G-T-T-G | 0.000 | 0.005 | 10.220 (0.548–190.400) | 0.049 |
| G-T-T-C | 0.001 | 0.012 | 11.350 (1.447–89.080) | 0.004 |
| G-C-T-G | 0.000 | 0.016 | 30.650 (1.816–517.400) | <0.0001 |
| rs4759314A>G/rs920778 T>C/rs1899663 G>T | ||||
| A-T-G | 0.699 | 0.614 | 1.000 (reference) | |
| A-C-G | 0.028 | 0.079 | 3.255 (1.957–5.415) | <0.0001 |
| G-T-G | 0.015 | 0.044 | 3.551 (1.788–7.054) | <0.0001 |
| G-T-T | 0.005 | 0.017 | 3.798 (1.241–11.620) | 0.016 |
| G-C-T | 0.000 | 0.013 | 24.950 (1.466–424.900) | <0.0001 |
| rs4759314A>G/rs920778 T>C/rs7958904 G>C | ||||
| A-T-G | 0.683 | 0.754 | 1.000 (reference) | |
| A-T-C | 0.077 | 0.013 | 0.161 (0.083–0.309) | <0.0001 |
| G-T-C | 0.011 | 0.000 | 0.051 (0.002–0.881) | 0.002 |
| rs4759314A>G/rs1899663 G>T/rs7958904 G>C | ||||
| A-G-G | 0.714 | 0.648 | 1.000 (reference) | |
| A-G-C | 0.009 | 0.048 | 5.827 (2.583–13.150) | <0.0001 |
| A-T-G | 0.011 | 0.041 | 4.314 (1.974–9.429) | <0.0001 |
| G-G-G | 0.011 | 0.043 | 4.067 (1.936–8.546) | <0.0001 |
| G-T-G | 0.000 | 0.021 | 36.600 (2.194–610.700) | <0.0001 |
| G-T-C | 0.002 | 0.014 | 11.500 (1.479–89.470) | 0.003 |
| rs920778 T>C/rs1899663 G>T/rs7958904 G>C | ||||
| T-G-G | 0.697 | 0.613 | 1.000 (reference) | |
| T-G-C | 0.016 | 0.046 | 3.077 (1.616–5.857) | <0.0001 |
| T-T-G | 0.006 | 0.031 | 6.756 (2.334–19.560) | <0.0001 |
| C-G-G | 0.028 | 0.079 | 3.145 (1.908–5.183) | <0.0001 |
| C-T-G | 0.005 | 0.032 | 6.756 (2.334–19.560) | <0.0001 |
| rs4759314A>G/rs920778 T>C | ||||
| A-T | 0.767 | 0.685 | 1.000 (reference) | |
| G-T | 0.024 | 0.061 | 2.742 (1.594–4.718) | <0.0001 |
| G-C | 0.020 | 0.042 | 2.410 (1.298–4.475) | 0.004 |
| rs4759314A>G/rs1899663 G>T | ||||
| A-G | 0.725 | 0.694 | 1.000 (reference) | |
| G-G | 0.042 | 0.071 | 1.769 (1.129–2.770) | 0.012 |
| G-T | 0.003 | 0.032 | 12.910 (3.048–54.660) | <0.0001 |
| rs4759314A>G/rs7958904 G>C | ||||
| A-G | 0.723 | 0.686 | 1.000 (reference) | |
| G-G | 0.013 | 0.069 | 5.510 (2.780–10.920) | <0.0001 |
| rs920778 T>C/rs1899663 G>T | ||||
| T-G | 0.711 | 0.657 | 1.000 (reference) | |
| C-G | 0.055 | 0.108 | 2.134 (1.449–3.144) | <0.0001 |
| rs920778 T>C/rs7958904 G>C | ||||
| T-G | 0.702 | 0.641 | 1.000 (reference) | |
| C-G | 0.034 | 0.113 | 3.642 (2.317–5.726) | <0.0001 |
| rs1899663 G>T/rs7958904 G>C | ||||
| G-G | 0.725 | 0.691 | 1.000 (reference) | |
| G-C | 0.041 | 0.073 | 1.900 (1.211–2.981) | 0.005 |
| T-G | 0.011 | 0.063 | 6.364 (2.992–13.540) | <0.0001 |
| Genotype Combination | Controls (n = 383) | RPL (n = 403) | AOR (95% CI) | p * |
|---|---|---|---|---|
| rs4759314/rs920778 | ||||
| AA/TT | 223 (58.2) | 190 (47.1) | 1.000 (reference) | |
| AA/CC | 10 (2.6) | 20 (5.0) | 2.415 (1.100–5.302) | 0.028 |
| AG/TT | 11 (2.9) | 35 (8.7) | 3.778 (1.865–7.652) | 0.0002 |
| AG/TC | 16 (4.2) | 36 (8.9) | 2.690 (1.445–5.006) | 0.002 |
| rs4759314/rs1899663 | ||||
| AA/GG | 199 (52.0) | 191 (47.4) | 1.000 (reference) | |
| AG/GG | 23 (6.0) | 43 (10.7) | 1.964 (1.139–3.389) | 0.015 |
| AG/GT | 6 (1.6) | 28 (6.9) | 4.798 (1.941–11.861) | 0.0007 |
| AG/TT | 1 (0.3) | 8 (2.0) | 8.529 (1.056–68.916) | 0.044 |
| rs4759314/rs7958904 | ||||
| AA/GG | 203 (53.0) | 188 (46.7) | 1.000 (reference) | |
| AG/GG | 6 (1.6) | 44 (10.9) | 7.874 (3.277–18.920) | <0.0001 |
| rs920778 / rs1899663 | ||||
| TT/GG | 197 (51.4) | 186 (46.2) | 1.000 (reference) | |
| CC/GG | 7 (1.8) | 22 (5.5) | 3.387 (1.411–8.134) | 0.006 |
| rs920778/rs7958904 | ||||
| TT/GG | 197 (51.4) | 183 (45.4) | 1.000 (reference) | |
| TC/GG | 5 (1.3) | 22 (5.5) | 4.792 (1.776–12.933) | 0.002 |
| CC/GG | 8 (2.1) | 27 (6.7) | 3.614 (1.598–8.173) | 0.002 |
| rs1899663/rs7958904 | ||||
| GG/GG | 202 (52.7) | 193 (47.9) | 1.000 (reference) | |
| GG/GC | 21 (5.5) | 35 (8.7) | 1.826 (1.023–3.261) | 0.042 |
| GG/CC | 1 (0.3) | 7 (1.7) | 7.735 (0.939–63.701) | 0.057 |
| GT/GG | 8 (2.1) | 34 (8.4) | 4.293 (1.932–9.538) | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.W.; Kim, Y.R.; Lee, J.Y.; Ko, E.J.; Kwon, M.J.; Kim, J.H.; Kim, N.K. Association of Polymorphisms in the Long Non-Coding RNA HOTAIR with Recurrent Pregnancy Loss in a Korean Population. Genes 2022, 13, 2138. https://doi.org/10.3390/genes13112138
Park HW, Kim YR, Lee JY, Ko EJ, Kwon MJ, Kim JH, Kim NK. Association of Polymorphisms in the Long Non-Coding RNA HOTAIR with Recurrent Pregnancy Loss in a Korean Population. Genes. 2022; 13(11):2138. https://doi.org/10.3390/genes13112138
Chicago/Turabian StylePark, Hyeon Woo, Young Ran Kim, Jeong Yong Lee, Eun Ju Ko, Min Jung Kwon, Ji Hyang Kim, and Nam Keun Kim. 2022. "Association of Polymorphisms in the Long Non-Coding RNA HOTAIR with Recurrent Pregnancy Loss in a Korean Population" Genes 13, no. 11: 2138. https://doi.org/10.3390/genes13112138





